Back to Journals » Clinical Ophthalmology » Volume 16
The REVIVE Study: Long Term Outcomes of a Novel Non-Diffractive Extended Vision IOL versus Monofocal Control IOL
Authors Shafer BM , McCabe C, Reiser H, Newsom TH, Berdahl J
Received 21 October 2022
Accepted for publication 17 November 2022
Published 28 November 2022 Volume 2022:16 Pages 3945—3950
DOI https://doi.org/10.2147/OPTH.S390380
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Brian M Shafer,1 Cathleen McCabe,2 Harvey Reiser,3 T Hunter Newsom,4 John Berdahl5
1Department of Ophthalmology, Chester County Eye Care Associates, Malvern, PA, USA; 2Department of Ophthalmology, The Eye Associates, Sarasota, FL, USA; 3Department of Ophthalmology, Eye Care Specialists, Kingston, PA, USA; 4Department of Ophthalmology, NewsomEye, Sebring, FL, USA; 5Department of Ophthalmology, Vance Thompson Vision, Sioux Falls, SD, USA
Correspondence: Brian M Shafer, Chester County Eye Care Associates, 325 Central Ave, Suite 101, Malvern, PA, 19355, USA, Email [email protected]
Purpose: To evaluate the long-term (> 1 yr) outcomes a non-diffractive extended vision intraocular lens (AcrySof IQ Vivity) compared to monofocal control.
Setting: This was a multicenter trial that took place in 4 separate private ophthalmology practices throughout the United States.
Design: This was a prospective, non-interventional, controlled, multicenter trial. All subjects were enrolled from participants in the Food and Drug Administration (FDA) clinical trial that led to the approval of the AcrySof IQ Vivity.
Methods: Binocular uncorrected distance visual acuity (UCDVA), distance corrected visual acuity (DCVA), uncorrected intermediate visual acuity (UIVA) at 66cm, distance corrected intermediate visual acuity (DCIVA) at 66cm, uncorrected near visual acuity (UNVA) at 40cm, and distance corrected near visual acuity (DCNVA) at 40cm were measured. The binocular defocus curve was measured. A 23-question survey on visual performance including questions on spectacle independence, satisfaction, dysphotopsias, and likelihood of recommending their lens to another person was used administered.
Results: A total of 64 eyes of 32 subjects were enrolled. Seventeen subjects had bilateral implantation of the AcrySof IQ Vivity lens, and 15 subjects had bilateral implantation of the AcrySof IQ Monofocal (SN60WF). Mean follow up time was 1078 days for the study group compared to 1067 days for the control group (p = 0.92). There were no differences in UCVA or DCVA between the two groups. Compared to control, the AcrySof IQ Vivity group had better mean binocular UIVA (logMAR 0.29 vs 0.18; p = 0.09), DCIVA (logMAR 0.33 vs 0.11; p = 0.003), UNVA (logMAR 0.49 vs 0.30, p = 0.01), and DCNVA (logMAR 0.54 vs 0.29; p = 0.001).
Conclusion: The AcrySof IQ Vivity is a novel, non-diffractive extended range of vision intraocular lens that provides long-term, enhanced visual acuity at intermediate and near ranges with high levels of patient satisfaction and minimal dysphotopsias.
Keywords: Vivity, extended depth of focus, defocus curve, cataract surgery
Plain Language Summary
What was known:
- The AcrySof IQ Vivity is a novel, non-diffractive extended range of vision intraocular lens that became commercially available in 2020.
- Given the recency of availability, long-term (>1 year) outcomes are not available for the AcrySof IQ Vivity lens.
What this paper adds:
- The AcrySof IQ Vivity maintains excellent distance and intermediate vision with functional near vision at least 2.5 years following bilateral implantation.
Introduction
The AcrySof IQ Vivity (Alcon Inc., USA) is a recently introduced, novel, non-diffractive, extended range of vision intraocular lens (IOL).1 The goal of this class of IOL is to provide spectacle independence for far and intermediate visions, with minimal negative defocus needed to optimize near vision.2,3 The AcrySof IQ Vivity has an aspheric, biconvex optic that is wavefront shaping utilizing a propriety X-WAVE technology. The IOL material is a hydrophobic acrylate/methacrylate copolymer with UV and blue light filters with an index of refraction of 1.55.4 Because of its recent introduction in the clinical practice in 2020, few data are available regarding the long-term efficacy and tolerability of AcrySof IQ Vivity IOL. The main goal of the present paper is to show the real-life experience regarding long-term outcomes with AcrySof IQ Vivity implantation.
Methods
This was a prospective, non-interventional, controlled, multicenter trial involving 4 different sites and surgeons throughout the United States. All subjects were enrolled from participants in the Food and Drug Administration (FDA) clinical trial that led to the approval of the AcrySof IQ Vivity. All patients signed informed consent before the inclusion into the study. Subjects were informed that the authors do not intend to share individualized patient data. Study documents and data are not intended to be made available at any time point following the completion of the study. This study was Institutional Review Board approved (Aspire IRB) and conducted in accordance with the Declaration of Helsinki.
Patients in the original FDA trial were implanted with either the AcrySof IQ Vivity or the monofocal control (AcrySof IQ Monofocal, SN60WF). Subjects were included in this study if they had been involved in the original trial and had undergone bilateral implantation of an intraocular lens at least 1 year prior to enrollment in this trial. Patients were excluded if they were not in the original trial or underwent subsequent ophthalmic surgery.
All the patients underwent a complete binocular vision evaluation including binocular uncorrected distance visual acuity (UCVA), binocular uncorrected intermediate visual acuity at 66cm (UIVA), binocular uncorrected near visual acuity at 40cm (UCNVA), binocular distance corrected intermediate visual acuity (DCIVA) at 66cm, binocular distance corrected near visual acuity (DCNVA) at 40cm, and binocular corrected distance visual acuity (CDVA).
Defocus curves were generated by placing the patient’s manifest refraction into a trial lens set and checking logMAR visual acuity after −0.5 D was sequentially added until a defocus step of −3.0 D. The same was then completed with a +0.5 D placed over the patient’s manifest refraction.
Subjects completed a 23-question survey on visual performance including questions on spectacle independence, satisfaction, dysphotopsias, and likelihood of recommending their lens to another person.
Snellen visual acuities were all converted to logMAR visual acuity for analysis. Then, a t-test was used to analyze the strength of difference in visual acuity between AcrySof IQ Vivity and the monofocal control lens. Analysis with a non-parametric method (Kruskal–Wallis) was performed for confirmation. The sample size was chosen for a margin of error of 5% and a confidence interval of 95%. All the analyses were performed by Ektropia Solutions LLC (Laguna Beach, CA, USA). Statistical significance was set to p < 0.05.
Results
A total of 64 eyes of 32 subjects were enrolled. Seventeen subjects had bilateral implantation of the AcrySof IQ Vivity lens and 15 subjects had bilateral implantation of the AcrySof IQ Monofocal (SN60WF), all of whom were targeted for emmetropia. There were no statistically significant differences in baseline demographics or mean follow-up times between the two groups (Table 1). Mean spherical equivalent was −0.34 for the AcrySof IQ Vivity group and −0.31 for the AcrySof IQ monofocal (p = 0.85).
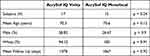 |
Table 1 Demographics |
Mean binocular visual acuities can be found in Table 2. There were no statistically significant differences in binocular UCVA or binocular DCVA in either group. There was statistically significantly better binocular UIVA, DCIVA, UNVA, and DCNVA for the AcrySof IQ Vivity lens compared to monofocal control. 100% of AcrySof IQ Vivity subjects had binocular DCIVA 20/40 or better compared to 46.6% of control (p < 0.001). 70.6% of AcrySof IQ Vivity subjects had binocular DCNVA 20/40 or better compared to 26.6% of control (p < 0.001).
 |
Table 2 Mean Binocular Visual Acuities |
Figure 1 shows the cumulative percentage of patients achieving various levels of binocular visual acuity at all measured distances for the study and control groups. Figure 2 shows the mean binocular defocus curve for the AcrySof IQ Vivity lens compared to the monofocal control.
 |
Figure 1 Percentage of patients achieving uncorrected and distance corrected distance, intermediate, and near visual acuities. |
Figure 3 shows the results of the visual satisfaction questionnaire. Visual satisfaction was high for both groups with 88.2% of AcrySof IQ Vivity patients expressing that they would both have the same lenses implanted again compared to 73.3% of control patients, though this was not statistically significant (p = 0.53). Subjects in the AcrySof IQ Vivity group reported greater levels of spectacle independence at near ranges, particularly in low lit scenarios, compared to the monofocal equivalent (p = 0.013). Starbursts, glare, and halos were more common in the control group (34% vs 7%, p = 0.023). No patients had negative dysphotopsias.
 |
Figure 3 Results of 23-part visual satisfaction questionnaire. *Statistical significance. |
No patients in either group underwent YAG capsulotomy at any time point during the study. There were no incidences of retinal detachment, cystoid macular edema, or lens dislocation.
Discussion
The AcrySof IQ Vivity is the first non-diffractive, extended range of vision IOL available in the United States.1,4 Since it was launched in late 2020 in US, there is minimal data on the long-term outcomes. This study represents the longest follow-up time for patients with the AcrySof IQ Vivity lens. This is the first study to show data demonstrating stable vision from six months to three years following implantation of this lens.
The current advanced technology IOL (AT-IOL) market is evolving to meet the demands of patients. Currently, diffractive trifocal technology provides for the most extended range of vision, but there are inherent limitations.5,6 Diffraction splits light to set foci; in doing so, there are photons projected to extrafoveal portions of the retina. As such, glare and halos are common.7–9 While they are noticeable by many patients, most are not bothered by them.8 However, there are patients that are highly averse to any disruptions in visual quality that a trifocal lens may provide. Conversely, the AcrySof IQ Vivity utilizes non-diffractive wavefront shaping technology that provides a dysphotopsia profile comparable to a monofocal. Therefore, this represents a novel option for patients to experience increased levels of spectacle independence with a monofocal-like visual disturbance profile.1
Our study is bound by certain limitations. With the relatively small sample size, the standard deviations are larger than ideal. Additionally, visual satisfaction was obtained via a questionnaire which bases its evaluation on the subjective feelings of the subjects. Despite standardization within the protocol, having four separate clinical trial sites may lead to minor differences in surgical technique and, therefore, results.
In conclusion, the AcrySof IQ Vivity is a novel, non-diffractive extended range of vision intraocular lens that provides long-term, enhanced spectacle independence at intermediate and near ranges with high levels of patient satisfaction and minimal dysphotopsias.
Funding
Financial support for this manuscript provided by Alcon. This paper/a seminar based on this paper was presented at the American Society of Cataract and Refractive Surgeons (ASCRS) Annual Meeting in both 2020 and 2021 as a presentation with interim findings. There was no publication from this presentation.
Disclosure
Drs. Shafer, McCabe, Reiser, Newsom, and Berdahl are all consultants for Alcon. Dr Newsom also reports study research for Rxsight, Inc., Premier Health Care, Allergan, and Lenstec, outside the submitted work. Dr Berdahl also reports personal fees for consulting from Bausch and Lomb, Johnson and Johnson Surgical Vision, Zeiss, during the conduct of the study; personal fees for consulting and/or owndership from Abbvie, Aerie, Aerpio, Aldeyra, Aurea Medical, Aurion Biotech/Cornea Gen, Dakota Lions Eye Bank, Elios Vision INC, Equinox Ophthalmic, Expert Opinion, Glaukos, Gore, Imprimis/Harrow Health, iRenix, Kala, Kedalion, MELT Pharmaceuticals, MicroOptx, New World Medical, Ocular Surgical Data, Ocular Theraputix, Omega Ophthalmic, Orasis, Oyster Point, RxSight, Santen, Sight Sciences, Surface, Tarsus, Tear Clear, Vertex Ventures, Vialase, Vittamed, Vance Thompson Vision, Verana Health, Versea Biologics, Visionary Ventures, and Visus, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Arrigo A, Gambaro G, Fasce F, Aragona E, Figini I, Bandello F. Extended depth-of-focus (EDOF) AcrySof IQ Vivity intraocular lens implant: a real-life experience. Graefes Arch Clin Exp Ophthalmol. 2021;259(9):2717–2722. doi:10.1007/s00417-021-05245-6
2. Akella SS, Juthani VV. Extended depth of focus intraocular lenses for presbyopia. Curr Opin Ophthalmol. 2018;29(4):318–322. doi:10.1097/ICU.0000000000000490
3. Kanclerz P, Toto F, Grzybowski A, Alio JL. Extended depth-of-field intraocular lenses: an update. Asia-Pac J Ophthalmol. 2020;9(3):194–202. doi:10.1097/APO.0000000000000296
4. Health C for D and R. AcrySof IQ Vivity Extended Vision Intraocular Lens (IOL) (Model DFT015), AcrySof IQ Vivity Toric Extended Vision IOLs (DFT315, DFT415, DFT515), AcrySof IQ Vivity Extended Vision UV Absorbing IOL (DAT015), and AcrySof IQ Vivity Toric Extended Vision UV absorbing IOLs (DAT315, DAT415, DAT515) - P930014/S126. FDA; 2020. https://www.fda.gov/medical-devices/recently-approved-devices/acrysof-iq-vivity-extended-vision-intraocular-lens-iol-model-dft015-acrysof-iq-vivity-toric.
5. Shafer BM, Greenwood M. Presbyopia correction at the time of cataract surgery. Curr Ophthalmol Rep. 2020;8(3):79–87. doi:10.1007/s40135-020-00236-y
6. Schallhorn JM. Multifocal and extended depth of focus intraocular lenses: a comparison of data from the United States Food and Drug Administration premarket approval trials. J Refract Surg Thorofare. 2021;37(2):98–104. doi:10.3928/1081597X-20201111-02
7. Mencucci R, Favuzza E, Caporossi O, Savastano A, Rizzo S. Comparative analysis of visual outcomes, reading skills, contrast sensitivity, and patient satisfaction with two models of trifocal diffractive intraocular lenses and an extended range of vision intraocular lens. Graefes Arch Clin Exp Ophthalmol. 2018;256(10):1913–1922. doi:10.1007/s00417-018-4052-3
8. Sudhir RR, Dey A, Bhattacharrya S, Bahulayan A. AcrySof IQ PanOptix intraocular lens versus extended depth of focus intraocular lens and trifocal intraocular lens: a clinical overview. Asia-Pac J Ophthalmol. 2019;8(4):335–349. doi:10.1097/APO.0000000000000253
9. García-Pérez JL, Gros-Otero J, Sánchez-Ramos C, Blázquez V, Contreras I. Short term visual outcomes of a new trifocal intraocular lens. BMC Ophthalmol. 2017;17(1):72. doi:10.1186/s12886-017-0462-y
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

