Back to Journals » OncoTargets and Therapy » Volume 12
The reversal of MRP1 expression induced by low-frequency and low-intensity ultrasound and curcumin mediated by VEGF in brain glioma
Received 19 November 2018
Accepted for publication 1 March 2019
Published 13 May 2019 Volume 2019:12 Pages 3581—3593
DOI https://doi.org/10.2147/OTT.S195205
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gaetano Romano
Lei Yao, Zhen Zhang
Department of ultrasound, First Affiliated Hospital of China Medical University, Shenyang City, Liaoning Province, People’s Republic of China
Purpose: To explore the effect of curcumin and low-frequency and low-intensity ultrasound (LFLIU) on C6 and U87 cell, and whether LFLIU could inhibit multidrug resistance protein 1 (MRP1) by increasing the sensitivity of curcumin via vascular epithelial growth factor (VEGF)/PI3K/Akt signaling pathway targeting.
Methods: C6 and U87 cells were treated with various doses of curcumin and/or different intensities of LFLIU for 60 s. After 24 hrs, the effects of curcumin and/or LFLIU on the proliferation of C6 and U87 cells were examined. Real-time PCR and western blot analysis were used to detect the expression of VEGF and MRP1 at both mRNA and protein levels. The expression of MRP1 in C6 and U87 cells was also determined following stimulation with recombinant human VEGF and/or LY294002.
Results: Curcumin and LFLIU inhibited the proliferation of glioma cells in an intensity- or dose-dependent manner. Furthermore, survivin was significant after combined treatment compares with that of curcumin or LFLIU treatment alone. VEGF and MRP1 were highly expressed in C6 and U87 cells, curcumin and LFLIU alone or in combination could decrease the expression of both VEGF and MRP1. MRP1 expression was down-regulated following LY294002 treatment, which blocked after exposure to VEGF.
Conclusion: The synergistic effects, such as a higher inhibition rate, and lower expressions of MRP1 and VEGF, of combined curcumin and LFLIU against glioma was much better than that of a single treatment. The down-regulation of MRP1 may be related with the VEGF/PI3K/Akt pathway in glioma.
Keywords: LFLIU, curcumin, glioma cells, MRP1, VEGF
Introduction
Glioma is the most common and aggressive types of primary brain tumor. Although major improvements have been made in the standardized treatment of glioma, the outcome is still very poor, with a median survival of ~1 year only after diagnosis.1 The glioma, one of the most lethal and ascular tumors, cannot be treated with extensive resection, because specific areas confined to the brain are unable to be compensated by brain tissue from other regions. Several studies have shown that angiogenesis is essential for glioma growth and metastases.2–5 Vascular epithelial growth factor (VEGF) is the most important stimulant factor in regulating angiogenesis,3 and plays an important role in the formation, development and recurrence of glioma.4 In particular, VEGF is highly expressed in cells with high malignancy and in the serum of glioma patients correlating with poor prognosis of the disease.2,5 Therefore, antiangiogenic treatment targeting the VEGF signaling pathway has recently become an integral part of modern cancer therapy. Many angiogenesis inhibitors have been approved by the US FDA for various cancers including brain glioma.6
However, drug resistance develops following long-term application of chemotherapeutic drugs, with multidrug resistance (MDR) as the main form of drug resistance in glioma.7,8 MDR is closely related to the over-expression of drug efflux pumps such as multidrug resistance protein 1 (MRP1), an MDR protein whose transmembrane domain forms the pore of the drug transport system. VEGF has been related to the over-expression of MRP1 and MDR in glioblastomas,9–11 providing a possible explanation for the failure of the routine use of the anti-VEGF antibody in glioma treatment. Investigating the MDR-related genes regulated by VEGF may identify a new therapeutic target to reduce or prevent MDR in gliomas.
Curcumin, a natural polyphenolic compound, isolated from the rhizome of the plant Curcuma longa,12,13 can effectively inhibit VEGF in a dose-dependent manner in VEGF-overexpressing tumors.14 More importantly, curcumin can penetrate through the blood-brain barrier and selectively target on tumor cells, leaving normal neural cells intact.15,16 However, the clinical application of curcumin is limited due to its poor aqueous solubility in aqueous solution, low biological availability and being rapidly metabolized once inside the body.
Ultrasound has been used as a safe diagnostic tool since the 1960s. In recent years, studies have shown that low-frequency low-intensity ultrasound (LFLIU) can enhance the efficacy of curcumin, induce apoptosis in human breast cancer cells, and reduce the expression of MRP1.10,17 The tumor cells are more sensitive to LFLIU than normal cells. LFLIU increases the permeability of the cell membrane,17 which plays an important role in tumor treatment. The purpose of this study is to investigate the efficacy of curcumin and/or LFLIU or the two in combination in the treatment of glioma. We examined the expression of VEGF and MRP1 in glioma cells after treatment with curcumin and/or LFLIU and demonstrated that the VEGF/PI3K/Akt signaling pathway may be involved in the effects of MRP1.
Materials and reagents
Curcumin (95% purity) [(E, E) −1, 7-bis (4-hydroxy-3-methoxyphenyl) −1, 6-heptadiene-3, 5-dione] was purchased from Sigma–Aldrich (St. Louis, MO, USA), and was stored as a 100 mM stock solution in dimethyl sulfoxide (DMSO) at −20°C until use.
Dulbecco’ s modification of Eagle’ s Medium (DMEM) and fetal bovine serum (FBS) used for cell culture and treatments were purchased from Gibco (Grand Island, NK, USA).
Cell cytotoxicity was determined using cell counting kit-8 (CCK-8, Dojindo Molecular Technologies, Japan), whereas the BCA kit from Shanghai Biyuntian Biotechnology Company (Binyuntian, Shanghai, China). Recombinant human vascular epithelial growth factor (rhVEGF) was purchased from PeproTech (Rocky Hill, USA). The PI3K/Akt pathway inhibitor, LY294002, was purchased from Sigma (St Louis, MO, USA).
Cell lines and cell culture
Rat C6 glioma cells and human U87 glioma cells kindly provided by the Department of Neurosurgery, the First Hospital, China Medical University were purchased from a typical cell culture collection Committee of the Chinese Academy of Sciences Library. Cells were cultured in DMEM and 10% FBS at 37°C in a humidified atmosphere containing 5% CO2. The cells were cultured and then harvested for in vitro experiments.
Treatment of cells
To investigate the efficacy of curcumin and LFLIU in the treatment of glioma, the glioma cells were randomly divided into the control groups and treatment groups.
Control groups contain negative control (cells cultured in DMEM and 10% FBS) and 0.3% DMSO group. Solutions of curcumin were prepared in DMSO, and final concentrations of DMSO used in the experiments were below 0.03%.
Treatment groups contain the curcumin group and LFLIU groups. Curcumin groups contain: 5 μmol/L (C5), 10 μmol/L (C10), 15 μmol/L (C15), and 20 μmol/L (C20), and the LFLIU groups contain: 3 mW/cm2 (U3), 50.4 mW/cm2 (U50.4), 83.4 mW/cm2 (U83.4), 142 mW/cm2 (U142), 290 mW/cm2 (U290) and 474 mW/cm2 (U474).
To investigate the effects of a combination of curcumin and LFLIU on glioma cells, and on the expression of VEGF and MRP1, the glioma cells were divided into negative control group, DMSO control group, U142, C10, C10+U142, C15 and C15+U142 groups.
To further explore the relationship between VEGF and MRP1, we used rhVEGF (20 ng/mL) and/or LY294002 (50 mmol/L) incubated for 24 hrs, with groups divided to rhVEGF group, LY294002 group and rh VEGF + LY294002 group.
Sensitivity of glioma cells treated by curcumin and LFLIU alone or in combination
C6 and U87 cells were seeded in 96-well plate at a concentration of 5×103 cells/well, respectively, and were incubated for 24 hrs, before treated by curcumin in different concentrations for 1 day. Ten ml CCK-8 was then added into the wells and incubated for an additional 2 hrs. Finally, optical density (OD) for each well was obtained at 450 nm using a microplate reader (BioTek, Winooski, VT, USA). Relative inhibitory rate of C6 and U87 cells was calculated according to the following equation: Relative inhibitory rate (%,RI)=(Ac−As) (Ac−Ab)−1×100%, whereas denotes the absorption rate in experimental wells (including negative control, CCK-8 post-sonication and post-curcumin), Ac denotes the absorption rate in control wells (including C6 cells, U87 cell, DMEM, CCK-8 without sonication) and Ab denotes the absorption rate in blank wells (CCK-8 and DMEM only without C6 or U87 cells).
Concomitant treatment of glioma cells with curcumin and LFLIU
C6 and U87 cells were seeded in a 96-well plate at a concentration of 5×103 cells/well, respectively, and were cultured for 24 hrs before treatment. Groups were divided according to the concentrations of curcumin (C10 and C15) and LFLIU at the intensity of 142 mW/cm2 for 60 s alone or in combination with the following experiments. As described above, 10-mL CCK-8 was then added into the wells and the culture plate was incubated for 2 hrs before measurement. RI of C6 and U87 cells was calculated according to the formula listed above. C6 and U87 cells were treated with curcumin and/or LFLIU for 24 hrs, the expression of survivin was measured by western blot. Light microscopy was used to determine the effect of curcumin and/or LFLIU on glioma cell morphology.
RNA extraction and quantitative real-time PCR
Cells were lysed on the ice, and total RNA was extracted from cells using TRizol reagent (Invitrogen, Grand Island, NY, USA) following the protocol recommended by the manufacturer. The concentration of RNA was determined by measuring the absorbance at 260 nm using nano-drop (BioSpec-nano). PrimeScriptRT Regent Kit with gDNA Eraser (Takara, Shiga, Japan) was used to obtain cDNA according to the manufacturer’s protocols. The sequence of MRP1 and VEGF primers (Shanghai, China) were as follows:
hMRP1-forward-5ʹ-GTACATTAACATGATCTGGTC-3ʹ, reverse-5ʹ-GGTTCATCAGCTTGATCCGAT-3ʹ, hVEGF-forward-5ʹ-CTTGCCTTGCTGCTCTACC-3ʹ, reverse-5ʹ-CACACAGGATGGCTTGAAG-3ʹ. Real-time PCR was carried out in a 20 μL reaction mix, containing 0.5 μmol/L forward primer, 0.5 μmol/L reverse primer, 0.2 μg cDNA template and 10 μl of 2× SYBR Premix Ex TaqTM (Takara, Shiga, Japan), using a protocol of 30 s at 95°C,followed by 45 cycles at 95°C for 5 s, at 60°C for 30 s, and a final 1 min at 60°C. Glyceraldehyde-3-phosphate dehydrogenase(GAPDH) was used as an internal reference. A standard curve and a melting curve were created automatically when the reactions completed, and comparative CT method (2−ΔΔCT) was adopted for quantification.
Western blot analysis
Cells were lysed in the ice-cold RIPA lysis buffer (Beyotime, P0013B). The protein concentration of the lysate was determined by BCA kits (Beyotime, P0010S). The protein samples (20 µg per lane) from the C6 and U87 cells were separated on 10% polyacrylamide gels using Mini Protean III electrophoresis tanks (Bio-Rad, Hercules, CA). After electrophoresis, the proteins were transferred to PVDF membranes according to standard procedures. The polyvinylidene fluoride (PVDF) membranes were blocked for 1 h with 5% nonfat dry milk in TBST buffer (20 mM Tris-HCl, pH 7.4; 150 mM NaCl; 0.1% Tween-20), followed by incubating with primary antibodies an overnight at 4°C. After washing the membranes three times with TBST, the blots were incubated with corresponding secondary antibodies (Proteintech, US) for 2 hrs at room temperature. Finally, specific proteins were visualized using enhanced chemiluminescence (BIO-RAD, USA) and detected using ImageJ software. We utilized the following primary antibodies: anti-VEGF (ab1316;1:200), anti-MRP1 (ab3368;1:1,000), anti-p-Akt (ab38449;1:1,000) and anti-survivin (ab182132;1:1,000), all purchased from Abcam (Cambridge, UK), anti-GAPDH antibody and all of secondary antibody, all purchased from Proteintech Group (Inc., Rosemont, IL, USA).
Statistics
Data were expressed as the mean ± standard deviation (SD), and all analyses were performed using SPSS21.0 software (IBM SPSS, Armonk, NY, USA). Data analysis was carried out using one-way ANOVA followed by Tukey`s posthoc test for multiple comparisons and an independent student t-test for simple comparisons. P<0.05 was considered being statistically significant. All experiments were repeated at least three times.
Results
Curcumin inhibited C6 and U87 cells proliferation in a dose-dependent manner
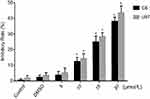
C6 cells and U87 cells were treated by curcumin in different concentrations, followed by CCK-8 to determine the inhibitory effect of curcumin on malignant glioma. No statistical difference was observed between the two control group, negative control and 0.3% DMSO control (C6 cells were 2.3±1.3, U87 cells were 3.4±1.7, P>0.05) indicating DMSO itself does not affect the viability of the cells. When exposed to C5, C10, C15, C20, the relative inhibitory rate of glioma increased significantly with the increasing curcumin concentrations. The relative inhibitory rate of C6 cells were 3.7±2.3, 12.4±2.6, 25.0±3.7, 38.2±2.6, respectively (≥10 μmol/L, P<0.05, Figure 1); the relative inhibitory rate of U87 cells were 5.2±3.4, 14.3±3.1, 28.3±2.6, 43.6±4.4, respectively (≥10 μmol/l, P<0.05, Figure 1). This result indicates that curcumin inhibits the growth of C6 and U87 cells in a dose-dependent manner. Curcumin at concentrations of 10 and 15 μmol/L have significant inhibitory effects on glioma cells proliferation. (P<0.05). Therefore, we chose these two concentrations to further investigate in the following experiments.
LFLIU inhibited C6 and U87 cells proliferation in an intensity-dependent manner
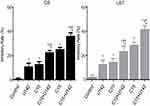
To determine whether LFLIU had an inhibitory effect on malignant glioma, C6 and U87 cells were exposed to different intensities of LFLIU for 60 s, followed by CCK-8 assays was performed. When exposed to U3, U50.4, U83.4, U142, U290 and U474, the relative inhibitory rate of glioma increased significantly with the increasing LFLIU intensity. The relative inhibitory rate of C6 cells were 2.2±1.3, 3.5±1.7, 4.6±2.4, 10.7±3.0, 15.6±2.3, 29.7±2.2, respectively (≥ U142, P<0.05, Figure 2); the relative inhibitory rate of U87 cells were 4.1±3.7, 5.4±4.5, 10.5±3.6, 12.3±3.8, 18.5±4.0, 33.3±3.9, respectively (≥U83.4, P<0.05, Figure 2). In order to achieve a significant inhibitory effect and avoid unnecessary damage to normal cells, the first significant intensity of 142.0 mW/cm2 was used in the following experiments.
LFLIU and curcumin in combination inhibited C6 and U87 cells proliferation in a synergistic manner
As described above, glioma cells (C6 and U87 cells) were treated with 10 μmol/L or 15 μmol/L of curcumin in the presence or absence of LFLIU (intensity of 142.0 mW/cm2, duration of 60 s) for 24 hrs. The relative inhibitory rate of C6 cells after the combination treatment of LFLIU (U142) and curcumin (C10 and C15) were 22.8±1.2, 34.8±1.8, respectively. The relative inhibitory rate of U87 cells following combination treatment of LFLIU (U142) and curcumin (C10 or C15) were 26.9±2.0, 38.1±1.6, respectively. This suggested that curcumin and LFLIU in combination may decrease the proliferation of glioma cells in a synergistic manner (Figure 3).
Curcumin and/or LFLIU down-regulated expressions of surviving
The effect of curcumin and LFLIU alone or in combination on glioma cell apoptosis was evaluated by anti-apoptotic protein survivin. Curcumin (C10 and C15) and/or LFLIU (U142) alone down-regulated survivin expression. The combination of curcumin and LFLIU, further decreased the expressions of survivin in glioma cells compared with curcumin (C10 and C15) and alone LFLIU (U142) (P<0.05, Figure 4).
Treated by curcumin and LFLIU alone or in combination led to morphological changes in C6 and U87 cells
Morphological change is one of the most direct indicators for cell health. In bright field images, normal glioma cells in control groups showed a long fusiform shape, spindle or polygonal, clear cell boundaries. And have a reticular connection with surrounding cells. Compared with the corresponding time points in control groups, curcumin (C10 and C15) and/or LFLIU (U142) inhibited glioma cell proliferation. The cells exhibited more rounded shape, with decreased cell synapses, decreased cell size and the widened intercellular space (Figure 5).
Curcumin and/or LFLIU down-regulates the expressions of VEGF and MRP1 at mRNA and protein level
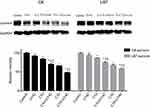
Both VEGF and MRP1 were highly expressed in C6 and U87 cells in the control group (Figure 6). mRNA expressions of VEGF and MRP1 reduced significantly following with the treatment of curcumin and LFLIU alone or in combination (P<0.05). Combined treatment with curcumin (C10, C15) and LFLIU (U142), further decreased the expressions of VEGF and MRP1 compared with control, curcumin and LFLIU alone groups (P<0.05).
Consistent with the transcript analysis VEGF and MRP1 proteins were significantly down-regulated significantly in experimental groups 24 hrs post curcumin or LFLIU incubation (P<0.05). The combination of curcumin and LFLIU further decreased the expressions of VEGF and MRP1 in glioma cells compared with a single treatment (P<0.05 Figure 7).
These results indicate a synergistic effect of curcumin and LFLIU in regulating VEGF and MRP1 expressions.
The VEGF/PI3K/Akt pathway is involved in the regulation of MRP1 expression
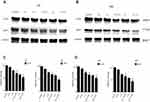
The protein expressions of MRP1 and p-Akt were significantly up-regulated following treatment with exogenous VEGF in both cell lines compared with the control group (P<0.05, Figure 8). In contrast, the expression of MRP1 and p-Akt was markedly decreased in the presence of an inhibitor of VEGF/PI3K/Akt signaling pathway, LY294002. Combine treatment with exogenous VEGF and LY294002 reversed the expression of MRP1 and p-Akt to the control level. These findings suggested a potential mechanism by which VEGF regulates MRP1 expression in glioma cells and that suppression of the VEGF/PI3k/Akt signaling pathway may contribute to the reduction of MRP1 expression and subsequentially reduce MDR in glioma cells.
Discussion
We report that curcumin and LFLIU reduced the proliferation of C6 and U87 glioma cells in a synergistic manner. Curcumin and LFLIU reduced the expression of VEGF and MRP1. VEGF up-regulated MRP1 expression, which is reversed by an inhibitor of VEGF/PI3K/Akt Pathway, LY294002.
It is well known that one of the advantages of ultrasound is that it is safer than other drug analogs that are more toxic to healthy cells. Ultrasound can be selectively targeted on tumor cells using focused ultrasound beams and ultrasound may potentially be more toxic to tumor cells due to the higher metabolic rate in glioma cells.10,18 Previous studies showed that ultrasound inhibits cell proliferation in osteosarcoma cells through inhibiting cell viability, mitochondrial membrane potential, reducing phosphorylated mitogen-activated protein kinase 7 and so on.19 In this study, LFLIU inhibited the proliferation of C6 and U87 cells in an intensity-dependent manner, indicates it seems to be an effective and safe procedure in the treatment of glioma.
Curcumin is a synergistic drug used in chemotherapies in cancer and has exhibited promising results for cancer treatment in various tissues.20–24 Curcumin has multiple therapeutic targets in cancer therapy, such as inhibiting cancer cell proliferation, migration and invasion, promoting cancer cell cycle arrest (at the G2/M phase) and inducing apoptosis in cancer cells.25–27 In this study, curcumin markedly inhibited the proliferation of C6 cells and U87 cells in a dose-dependent manner. The selections of the concentrations with curcumin in our study were based on the previous report from the literature,25 which showed that the curcumin (15 and 20 µmol/L) inhibited glioma lines of A1207 and SNB19 cells growth. We chose a lower concentration of curcumin when combined with LFLIU against glioma cells to minimize the off-target effect.
Furthermore, we found that using curcumin and LFLIU in combination significantly inhibited glioma cells proliferation in a synergistic manner. On one hand, curcumin can potentiate the antitumor activity of ionizing radiation, and may be used as a radiosensitizer for the clinical cancer treatment.28 Curcumin also targets various inflammatory mediators could attenuate the release of proinflammatory and profibrotic cytokines, and suppressing chronic production of free radicals, which culminates in the amelioration of normal tissue toxicity.29
On the other hand, ultrasound selectively enhance the antitumor efficacy by enhancing permeation retention effect.30 In addition, different intensities of ultrasound may increase the permeability of tight junctions, which allows agents and/or antibodies to pass the blood-tumor barrier.31,32 This altogether indicates that ultrasound can be used as an accessory tool to deliver curcumin in brain glioma therapy.
Survivin, an inhibitor of apoptosis protein, prevents cancer cell apoptosis by counteracting the G2/M phase,33 and is associated with the degree of differentiation and the advancement of clinical stage of tumors.34 In this study, the proteins of survivin were over-expressed in glioma cells, which is inhibited by treatments with LFLIU and/or curcumin. Morphologically, more tumor cells were observed with a disorganized pattern in the control group. Curcumin and LFLIU treatment decreased the amount of tumor cell and improved cell morphology. These results indicate that curcumin and LFLIU could accelerate the apoptosis of glioma cells.
Low-frequency ultrasound induces anti-tumor immunity by decreasing the expression of VEGF.35 LFLIU in certain radiation parameters, also affect the VEGF expression levels.36 In this study, we combined a lower concentration of curcumin, and a lower intensity of LFLIU compared with previous studies and observed a synergistic down-regulation of VEGF expression (Figure 6). The combined use of low level of tumor regulators may potentially reduce off-target effect and serve as a safer tumor treatment clinically.
Curcumin and LFLIU have been proven to be beneficial for MDR tumors. However, the underlying mechanisms remain unclear. The VEGF/PI3K/Akt pathway is one of the most important signal pathways in cancers, which is vital to angiogenesis, proliferation, invasion, enhancing cell repair motility and migration ability, and inhibition of apoptosis in cancer cells.37–40 VEGFR2, VEGF receptor, its downstream targets the PI3K/Akt pathway.
To explore the effect of VEGF on the expression of MRP1 in glioma, we used recombinant human VEGF and LY294002 to stimulate C6 and U87 cells. Western blot analysis showed that VEGF increased the expression of MRP1 at a protein level consistent with previous reports.11,41 This increase in MRP1 is reversed by application of LY294002, an inhibitor of VEGF/PI3K/Akt pathway. And the combination of curcumin and LFLIU could significantly decrease the expressions of MRP1 and VEGF in a synergistic manner. Notably, our findings showed that VEGF up-regulated the protein expression of MRP1, but LY294002 could partially block this up-regulation as indicated in Figure 8. Furthermore, NF-κB, a contributor to chemoresistance, is the downstream target of the PI3K/Akt pathway.42 For refractory acute myeloid leukemia cells,PI3K/AKT/NF-kB pathway mediates resistance to radiation.43 Curcumin down-regulates NF-κB and NF-κB-regulated gene products involved in apoptosis, matrix degradation, and inflammation,whose effects are mediated through down-regulation of PI3K/Akt signaling.44
And Muthusamy G, et al, found some drug contributes to the reversal of the MDR via the inhibition of the signaling pathway.45
Therefore, the down-regulation of MRP1 following treatment with curcumin and LFLIU is potentially mediated by the VEGF/PI3K/Akt/NF-kB signal pathway (Figure 9).
Conclusion
In summary, the results in this study indicate that curcumin and LFLIU alone or in combination may attenuate the proliferation of glioma. Further, curcumin and LFLIU can act synergistically in their antitumor effects by down-regulating the expressions of MRP1 and the VEGF/PI3K/Akt signaling pathway may play a role in this regulation. These findings provide evidence that low dose curcumin combined with low-level LFLIU has the potential to be an effective treatment method for glioma and may potentially be used for treatment in human glioma.
Abbreviations list
VEGF, vascular epithelial growth factor; MRP1, multidrug resistance protein 1; LFLIU, low-frequency and low-intensity ultrasound; MDR, multidrug resistance; PBS, phosphate-buffered saline; DMSO, dimethyl sulfoxide; DMEM, dulbecco’ s modification of Eagle’ s Medium; CCK-8, cell counting kit-8; PI3K, phosphatidylinositol-3-kinase; Akt, protein kinase B; p-Akt, phospho-protein kinase B; NF-κB, nuclear factor κB; rhVEGF, recombinant human vascular epithelial growth factor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PVDF, polyvinylidene fluoride.
Acknowledgments
Fund number when manuscript submitted (81471809, National Natural Science Foundation of China).
Disclosure
The authors report no conflicts of interest in this work.
References
1.
2. Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. doi:10.1016/j.ccr.2006.02.019
3. Vajkoczy P, Farhadi M, Gaumann A, et al. Microtumor growth initiates angiogenic sprouting with simultaneous expression of VEGF, VEGF receptor-2, and angiopoietin-2. J Clin Invest. 2002;109(6):777–785. doi:10.1172/JCI14105
4. Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3(6):401–410. doi:10.1038/nrc1093
5. Lamszus K, Ulbricht U, Matschke J, et al. Levels of soluble vascular endothelial growth factor (VEGF) receptor 1 in astrocytic tumors and its relation to malignancy, vascularity, and VEGF-A. Clin Cancer Res. 2003;9(4):1399–1405.
6. Lu KV, Bergers G. Mechanisms of evasive resistance to anti-VEGF therapy in glioblastoma. CNS Oncol. 2013;2(1):49–65. doi:10.2217/cns.12.36
7. Duhem C, Ries F, Dicato M. What Does Multidrug Resistance (MDR) expression mean in the clinic. Oncologist. 1996;1(3):151–158.
8. Mohri M, Nitta H, Yamashita J. Expression of multidrug resistance-associated protein (MRP) in human gliomas. J Neurooncol. 2000;49(2):105–115.
9. DeLay M, Jahangiri A, Carbonell WS, et al. Microarray analysis verifies two distinct phenotypes of glioblastomas resistant to antiangiogenic therapy. Clin Cancer Res. 2012;18(10):2930–2942. doi:10.1158/1078-0432.CCR-11-2390
10. Zhang Z, Xu K, Bi Y, et al. Low intensity ultrasound promotes the sensitivity of rat brain glioma to Doxorubicin by down-regulating the expressions of p-glucoprotein and multidrug resistance protein 1 in vitro and in vivo. PLoS One. 2013;8(8):e70685. doi:10.1371/journal.pone.0070685
11. Li J, Wu X, Gong J, et al. Vascular endothelial growth factor induces multidrug resistance-associated protein 1 overexpression through phosphatidylinositol-3-kinase/protein kinase B signaling pathway and transcription factor specificity protein 1 in BGC823 cell line. Acta Biochim Biophys Sin (Shanghai). 2013;45(8):656–663. doi:10.1093/abbs/gmt062
12. Milacic V, Banerjee S, Landis-Piwowar KR, et al. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68(18):7283–7292. doi:10.1158/0008-5472.CAN-07-6246
13. Dhillon N, Aggarwal BB, Newman RA, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14(14):4491–4499. doi:10.1158/1078-0432.CCR-08-0024
14. Shen ZY, Shen E, Zhang JZ, et al. Effects of low-frequency ultrasound and microbubbles on angiogenesis-associated proteins in subcutaneous tumors of nude mice. Oncol Rep. 2013;30(2):842–850. doi:10.3892/or.2013.2492
15. Zanotto-Filho A, Braganhol E, Edelweiss MI, et al. The curry spice curcumin selectively inhibits cancer cells growth in vitro and in preclinical model of glioblastoma. J Nutr Biochem. 2012;23(6):591–601. doi:10.1016/j.jnutbio.2011.02.015
16. Wu F, Shao ZY, Zhai BJ, et al. Ultrasound reverses multidrug resistance in human cancer cells by altering gene expression of ABC transporter proteins and Bax protein. Ultrasound Med Biol. 2011;37(1):151–159. doi:10.1016/j.ultrasmedbio.2010.10.009
17. Li Y, Wang P, Chen X, et al. Activation of microbubbles by low-intensity pulsed ultrasound enhances the cytotoxicity of curcumin involving apoptosis induction and cell motility inhibition in human breast cancer MDA-MB-231 cells. Ultrason Sonochem. 2016;33:26–36. doi:10.1016/j.ultsonch.2016.04.012
18. Ibsen S, Benchimol M, Simberg D, et al. A novel nested liposome drug delivery vehicle capable of ultrasound triggered release of its payload. J Control Release. 2011;155(3):358–366. doi:10.1016/j.jconrel.2011.06.032
19. Matsuo T, Sato K, Matsui T, et al. Inhibitory effects of low-intensity pulsed ultrasound sonication on the proliferation of osteosarcoma cells. Oncol Lett. 2017;14(3):3071–3076. doi:10.3892/ol.2017.6472
20. Xu S, Yang Z, Fan Y, et al. Curcumin enhances temsirolimus-induced apoptosis in human renal carcinoma cells through upregulation of YAP/p53. Oncol Lett. 2016;12(6):4999–5006. doi:10.3892/ol.2016.5376
21. He B, Wei W, Liu J, et al. Synergistic anticancer effect of curcumin and chemotherapy regimen FP in human gastric cancer MGC-803 cells. Oncol Lett. 2017;14(3):3387–3394. doi:10.3892/ol.2017.6627
22. He GL, Liu Y, Li M, et al. The amelioration of phagocytic ability in microglial cells by curcumin through the inhibition of EMF-induced pro-inflammatory responses. J Neuroinflammation. 2014;11:49. doi:10.1186/s12974-014-0139-x
23. Palange AL, Di MD, Carallo C, et al. Lipid-polymer nanoparticles encapsulating curcumin for modulating the vascular deposition of breast cancer cells. Nanomedicine. 2014;10(5):991–1002. doi:10.1016/j.nano.2014.02.004
24. Cheng C, Jiao JT, Qian Y, et al. Curcumin induces G2/M arrest and triggers apoptosis via FoxO1 signaling in U87 human glioma cells. Mol Med Rep. 2016;13(5):3763–3770. doi:10.3892/mmr.2016.5037
25. Lopes-Rodrigues V, Oliveira A, Correia-da-Silva M, et al. A novel curcumin derivative which inhibits P-glycoprotein, arrests cell cycle and induces apoptosis in multidrug resistance cells. Bioorg Med Chem. 2017;25(2):581–596. doi:10.1016/j.bmc.2016.11.023
26. Shi J, Zhang X, Shi T, et al. Antitumor effects of curcumin in human bladder cancer in vitro. Oncol Lett. 2017;14(1):1157–1161. doi:10.3892/ol.2017.6205
27. Zhu GH, Dai HP, Shen Q, et al. Curcumin induces apoptosis and suppresses invasion through MAPK and MMP signaling in human monocytic leukemia SHI-1 cells. Pharm Biol. 2016;54(8):1303–1311. doi:10.3109/13880209.2015.1060508
28. Orr WS, Denbo JW, Saab KR, et al. Curcumin potentiates rhabdomyosarcoma radiosensitivity by suppressing NF-κB activity. PLoS One. 2013;8(2):e51309. doi:10.1371/journal.pone.0051309
29. Farhood B, Mortezaee K, Goradel NH, et al. Curcumin as an anti-inflammatory agent: implications to radiotherapy and chemotherapy. J Cell Physiol. 2019;234(5):5728–5740.
30. Cha JM, You DG, Choi EJ, et al. Improvement of antitumor efficacy by combination of thermosensitive liposome with high-intensity focused ultrasound. J Biomed Nanotechnol. 2016;12(9):1724. doi:10.1166/jbn.2016.2272
31. Zhang Z, Xia C, Xue Y, et al. Synergistic effect of low-frequency ultrasound and low-dose bradykinin on increasing permeability of the blood-tumor barrier by opening tight junction. J Neurosci Res. 2009;87(10):2282–2289. doi:10.1002/jnr.22061
32. Shang X, Wang P, Liu Y, et al. Mechanism of low-frequency ultrasound in opening blood-tumor barrier by tight junction. J Mol Neurosci. 2011;43(3):364–369. doi:10.1007/s12031-010-9451-9
33. Abe A, Kokuba H. Harmol induces autophagy and subsequent apoptosis in U251MG human glioma cells through the downregulation of survivin. Oncol Rep. 2013;29(4):1333–1342. doi:10.3892/or.2013.2242
34. Gao Y, Li L, Song L. Expression of p16 and Survivin in gliomas and their correlation with cell proliferation. Oncol Lett. 2015;10(1):301–306. doi:10.3892/ol.2015.3180
35. Zhang W, Shou WD, Xu YJ, et al. Low-frequency ultrasound-induced VEGF suppression and synergy with dendritic cell-mediated anti-tumor immunity in murine prostate cancer cells in vitro. Sci Rep. 2017;7(1):5778. doi:10.1038/s41598-017-06242-8
36. Hou R, Xu Y, Lu Q, et al. Effect of low-frequency low-intensity ultrasound with microbubbles on prostate cancer hypoxia. Tumour Biol. 2017;39(10):1010428317719275. doi:10.1177/1010428317719275
37. Liao XZ, Tao LT, Liu JH, et al. Matrine combined with cisplatin synergistically inhibited urothelial bladder cancer cells via down-regulating VEGF/PI3K/Akt signaling pathway. Cancer Cell Int. 2017;17:124. doi:10.1186/s12935-017-0495-6
38. Ma X, Yao H, Yang Y, et al. miR-195 suppresses abdominal aortic aneurysm through the TNF-α/NF-κB and VEGF/PI3K/Akt pathway. Int J Mol Med. 2018;41(4):2350–2358. doi:10.3892/ijmm.2018.3426
39. Peng N, Gao S, Guo X, et al. Silencing of VEGF inhibits human osteosarcoma angiogenesis and promotes cell apoptosis via VEGF/PI3K/Akt signaling pathway. Am J Transl Res. 2016;8(2):1005–1015.
40. Xie J, Liu JH, Liu H, et al. Tanshinone IIA combined with adriamycin inhibited malignant biological behaviors of NSCLC A549 cell line in a synergistic way. BMC Cancer. 2016;16(1):899. doi:10.1186/s12885-016-2921-x
41. Zhu X, Li H, Long L, et al. miR-126 enhances the sensitivity of non-small cell lung cancer cells to anticancer agents by targeting vascular endothelial growth factor A. Acta Biochim Biophys Sin (Shanghai). 2012;44(6):519–526. doi:10.1093/abbs/gms026
42. Mao L, Liang RF, Xiang W, et al. BKM120 sensitizes C6 glioma cells to temozolomide via suppression of the PI3K/Akt/NF-κB/MGMT signaling pathway. Oncol Lett. 2017;14(6):6597–6603. doi:10.3892/ol.2017.7034
43. Li X, Chen F, Zhu Q, et al. Gli-1/PI3K/Akt/NF-kB pathway mediates resistance to radiation and is a target for reversion of responses in refractory acute myeloid leukemia cells. Oncotarget. 2016;22(7):33004–33015.
44. Buhrmann C, Mobasheri A, Busch F, et al. Curcumin modulates nuclear factor κB (NF-κB)-mediated inflammation in human tenocytes in vitro. J Biol Chem. 2011;286. doi:10.1074/jbc.M111.256180
45. Muthusamy G, Gunaseelan S, Prasad NR. Ferulic acid reverses P-glycoprotein mediated multidrug resistance via inhibition of PI3K/Akt/NF-κB signaling pathway. J Nutr Biochem. 2019;63(1):62–71.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.