Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
The relationship between serum-free insulin-like growth factor-1 and metabolic syndrome in school adolescents of northeast China
Authors Xie S , Jiang R , Xu W , Chen Y , Tang L, Li L , Li P
Received 22 November 2018
Accepted for publication 30 January 2019
Published 5 March 2019 Volume 2019:12 Pages 305—313
DOI https://doi.org/10.2147/DMSO.S195625
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Shuang Xie,1,2 Ranhua Jiang,3 Wanfeng Xu,4 Yu Chen,4 Lei Tang,1 Ling Li,1 Ping Li4
1Department of Endocrinology, Shengjing Hospital of China Medical University, Shenyang, Liaoning Province, P.R. China; 2Department of General Medicine (VIP ward), Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute, Shenyang, Liaoning Province, P.R. China; 3Department of Endocrinology, Liaoyang Diabetes Hospital, Liaoyang, Liaoning Province, P.R. China; 4Department of Endocrinology, Shengjing Hospital of China Medical University, Shenyang, Liaoning Province, P.R. China
Purpose: Free insulin-like growth factor-1 (IGF-1) ratio (the ratio of IGF-1/insulin-like growth factor binding protein-3 [IGFBP-3]) was shown to be negatively correlated with metabolic syndrome (MetS) in adults, but it was unknown in Chinese adolescents.
Patients and methods: The cross-sectional study enrolled 701 healthy school students (aged 12–16 years, 46.1% females) and 93 of them (18–22 years old, 46.2% females) were followed after 5 years.
Results: In the cross-sectional study, the IGF-1/IGFBP-3 ratios were found correlated with low-density lipoprotein cholesterol (LDL-C; r= -0.071, P<0.05) and diastolic blood pressure (r= -0.077, P=0.034). A lower IGF-1/IGFBP-3 ratio was an independent risk factor for MetS (OR =2.348, 95% CI: 1.040–5.303), hypertension (OR=1.729, 95% CI: 1.040–5.303), and increased LDL-C (OR=1.841, 95% CI: 1.230–2.755). In the follow-up study, all the participants were >18 years old. We found a lower baseline ratio of IGF-1/IGFBP-3 in adolescence was an independent risk factor for MetS in adulthood (OR=10.724, 95% CI: 1.032–11.403) and also indicated a higher body mass index (β=-1.361, 95% CI: -2.513 to -0.208) after 5 years.
Conclusion: The lower IGF-1/IGFBP-3 ratio was an independent risk factor for MetS, hypertension, and high LDL-C in adolescents of northeast China and was also a predictive marker for MetS and increased body mass index in the adulthood.
Keywords: IGF-1, IGFBP-3, adolescent, metabolic syndrome
Introduction
In recent years, along with China’s economic development and improvements in living standards, the incidence of metabolic syndrome (MetS) has increased not only in adults but also in adolescents.1,2 However, the adolescent population who are in the growth and development period must be different from adults in terms of metabolic characteristics.3 Identification of serum-specific markers that are closely related to adolescent MetS is significant in the prevention of MetS disease and determination of pathogenesis. Insulin-like growth factor-1 (IGF-1) is a polypeptide hormone possessing structural and functional homology with proinsulin and is widely involved in inflammation, glucose and lipid metabolism.4 Ninety-nine percent of circulating IGF-1 exists in the form that binds to insulin-like growth factor binding proteins (IGFBPs), of which at least 75% of IGF-1 constitutes a stable trimer structure with insulin-like growth factor binding protein-3 (IGFBP-3) and an acid-labile subunit to extend the half-life of IGF-1 and regulate transport of IGF-1 between intravascular and extravascular spaces.5–8 It is widely accepted that the IGF-I/IGFBP-3 ratio can be used as a marker for the accurate assessment of free IGF-1 levels.9 Studies in adults have found that free IGF-1 levels are negatively correlated with MetS;10 however, a correlation between free IGF-1 levels in the adolescent population and MetS has not yet been reported.11 In this study, adolescents aged 12–16 years in Liaoyang, a city in northeast China, were selected and observed for 5 years to analyze the correlations between adolescent levels of IGF-1, MetS, and its components as well as other cardiovascular disease risk factors. This will contribute not only to a deeper understanding of the pathophysiological function of IGF-1/IGFBP-3 but also to knowledge on serum-specific markers of MetS in adolescents, thereby providing data to establish control strategies for the disease.
Materials and methods
Study population
From December 2010 to January 2011, junior and senior high school students in Liaoyang, a city with a medium-sized economic development level in northeast China, were selected using the stratified cluster sampling method. Questionnaires were assigned to 947 students with informed consent signed by their guardian. In addition, fasting blood samples were collected and the levels of IGF-1, IGFBP-3, fasting plasma glucose (FPG), fasting insulin (FINS), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), and serum uric acid were determined. In total, 701 students (12–16 years old, 46.1% females) with complete data were included in the statistical analysis. These subjects had no history of anemia, diabetes, and hypertension or drug therapy. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of Shengjing Hospital, China Medical University.
Height, weight, waist circumference (WC), hip circumference, and blood pressure (systolic blood pressure [SBP] and diastolic blood pressure [DBP]) were measured by a trained physician before blood sample collection. The subjects then sat quietly for more than 10 minutes, and blood pressure was measured twice in the sitting position using a desktop mercury sphygmomanometer with a 2-minute interval between measurements. The average systolic and DBP was recorded. All subjects fasted for≥10 hours at night and fasting venous blood was obtained at 07:00–09:00 the next morning. All blood samples were immediately transported to the laboratory in Liaoyang Diabetes Hospital and centrifuged (within 1 hour). FPG (Glucose Oxidase method, Olympus 400; Olympus Optical Company, Tokyo, Japan) was determined within 2 hours after centrifugation. Serum LDL-C, HDL-C, TG, and uric acid were determined by routine enzymatic methods. Some of the plasma was stored at -80°C and FINS was measured by radioimmunoassay (China Institute of Atomic Energy, Beijing, P.R. China). Serum total IGF-1 and IGFBP-3 were measured by enzyme-linked immunosorbent assay. Body mass index (BMI) was calculated by body weight/height2 (kg/m2). Steady-state model homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the following formula: HOMA-IR= fasting blood glucose (mmol/L) × FINS (μU/mL) /22.5.
The subjects were followed-up 5 years later (July 2016). As most of the students went to universities in various cities, only 93 subjects (18–22 years old, 46.2% females) were recruited in the follow-up. Methods of investigation and personnel, as well as the clinical and biochemical parameters measured, were the same as in the baseline study. None of the 93 subjects had new chronic diseases or long-term medication use during the previous 5 years.
Diagnostic criteria and definition
Metabolic syndrome for adolescents
MetS was defined according to 2007 guidelines provided by International Diabetes Federation (IDF).12 These guidelines state that for an adolescent to be diagnosed as MetS, the individual must have central obesity as defined by ethnic-, gender-, and age-specific values (≥90th percentile, criteria proposed by the Capital Institute of Pediatrics),13 plus any two of the following four components: hypertriglyceridemia (TG ≥1.7 mmol/L), low HDL-C (<1.03 mmol/L for individuals 10–15 years of age and boys ≥16 years of age or <1.29 mmol/L for girls ≥16 years of age), hypertension (SBP ≥130 or DBP ≥85 mmHg), and fasting hyperglycemia (FPG ≥5.6 mmol/L).
Metabolic syndrome for adults
After the 5-year follow-up, the 93 subjects were all >18 years old. The IDF definition of MetS in adult was applied to this cohort14 as follows. The IDF defines MetS as having an increased WC, with ethnic-specific WC cut points (for our study population, the IDF indicates the cut points for South-Asian populations, which are ≥90 cm in men and ≥80 cm in women), plus any two of the following: 1) TG >150 mg/dL or specific treatment for this lipid abnormality; 2) HDL-C <40 mg/dL in men, <50 mg/dL in women, or specific treatment for this lipid abnormality; 3) SBP ≥130 mmHg, DBP ≥85 mmHg, or treatment for previously diagnosed hypertension; 4) FPG ≥100 mg/dL, or previously diagnosed type 2 diabetes.
Overweight/Obesity
We adopted the age- and gender-specific criteria for obesity and overweight proposed by the Group of China Obesity Task Force15 in which obesity and overweight were defined as BMI ≥95th and ≥85th percentiles, respectively.
High LDL-C hyperlipidemia
There were no diagnostic criteria for high LDL-C developed for children and adolescents in China yet. In this study, we defined LDL-C ≥95th percentile as high LDL-C.
Statistical analysis
All analyses were carried out using the statistical program SPSS for Windows (version 22.0; SPSS, Inc., Chicago, IL, USA). A normal distribution was evaluated with the Kolmogorov–Smirnov test. Nonnormally distributed variables were ln-transformed to normality before analysis. Normally distributed continuous variables are presented as mean ± standard error and nonnormally distributed variables as the median (interquartile range). Categorical data are shown as numbers (percentage) and were analyzed using a chi-square test. The between-group differences in means were tested using Kruskal–Wallis H test or ANOVA. Comparisons were conducted after adjusting for confounding factors using one-way generalized linear models. Correlations of IGF-1/IGFBP-3 levels with MetS and other cardiovascular disease (CVD)-related risk factors were evaluated after adjusting for confounding factors with partial correlation analysis. Logistic multivariate regression analysis was used to assess associations of IGF-1/IGFBP-3 levels with MetS and risk factors of CVD. The linear regression analysis was used to analyze the relationship between different IGF-1/IGFBP-3 levels and the BMI levels after 5 years. P<0.05 was considered significant for each of these analyses.
Results
The cross-sectional study
All the subjects in this study were of Han nationality. The IGF-1 (495.39±17.42 ng/mL) and IGFBP-3 (6,489.94±334.37 ng/mL) levels were well correlated (r=0.449, P<0.01). The ratios of IGF-1/IGFBP-3 in men and women were 0.147 (95% CI: 0.127–0.168) and 0.156 (95% CI: 0.133–0.178), respectively, without significant difference (Figure 1). The baseline men had a higher level of SBP than the women (Table 1). As shown in Table 2, the subjects were divided into three groups according to the tertile range values of IGF-1/IGFBP-3 ratios from low to high (group T1, T2, and T3 with age 12–16 years in each group), the IGF-1/IGFBP-3 ratio being highest in group T3. Table 2 shows that the adolescents in group T3 were older than the other two groups. After adjusting for sex and age, the levels of LDL-C decreased significantly (P<0.05) with increased IGF-1/IGFBP-3 ratios. The FPG level was lowest in group T3 (P<0.05).
  | Figure 1 Serum IGF-1/IGFBP-3 levels in men (A) and women (B). Abbreviations: (n), number of people; IGF-1, insulin-like growth factor-1; IGFBP-3, insulin-like growth factor binding protein-3. |
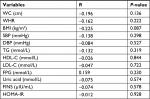
Partial correlation analysis, after age and sex were adjusted, showed that IGF-1/IGFBP-3 ratios were significantly negatively correlated with LDL-C and DBP (the correlation coefficient r was -0.071 and -0.077, respectively, all P<0.05; Table 3).
Logistic regression analysis was performed with group T3 with highest IGF-1/IGFBP-3 ratio as the reference (Table 4). The risk of MetS clearly increased with a decrease in IGF-1/IGFBP-3 ratios (P<0.05). This higher risk still existed statistically after all other factors such as age, sex, family history, high-sensitivity C-reactive protein (hs-CRP), alanine transaminase, and adiponectin were corrected, and the risk of MetS in the lowest IGF-1/IGFBP-3 group was 2.348 times that in the reference group (95% CI: 1.040–5.303). Of all the MetS components, hypertension was most closely related to the decrease in IGF-1/IGFBP-3 ratios, and the risk of hypertension in the lowest IGF-1/IGFBP-3 group was 1.729 times (95% CI: 1.040–5.303) that in the reference group. For cardiovascular disease risk factors other than MetS components, we analyzed the associations of different IGF-1/IGFBP-3 ratios with LDL-C elevation and overweight/obesity, and found that the reduction of IGF-1/IGFBP-3 ratios was closely associated with increased LDL-C levels (odds ratio [OR]=1.841, 95% CI: 1.230–2.755), but not overweight/obesity.
The follow-up study
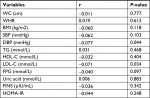
There were no significant differences in basic clinical characteristics and baseline serum biochemical markers between the subjects who participated in the follow-up (n=93) and the subjects who did not participate in the follow-up survey (n=701; Table S1), suggesting that the follow-up subjects were representative of the overall baseline population. WC, SBP, and HDL-C were significantly higher in the follow-up men than in women (Table 1). Adult MetS diagnostic criteria were used in these subjects, which was completely different from the MetS diagnostic criteria for adolescents used 5 years previously.
It was observed that BMI levels in the three groups statistically decreased with increased IGF-1/IGFBP-3 ratios (Table 5). Correlation analysis showed that the baseline IGF-1/IGFBP-3 ratios in adolescents were not correlated with any cardiovascular risk factors after 5 years in adulthood (all P>0.05; Table S2). Logistic multivariate regression analysis showed that the risk of MetS in the baseline lowest IGF-1/IGFBP-3 group was 10.724 times (OR =10.724, CI: 1.032–11.403) that in the baseline highest IGF-1/IGFBP-3 group after 5 years, and there were no statistically significant differences between the three groups in terms of any single MetS component (Table 6). We used BMI after 5 years as the dependent variable and the three IGF-1/IGFBP-3 ratio groups as the independent variables in the linear regression analysis and found that a lower ratio of IGF-1/IGFBP-3 in adolescence indicated a significantly higher BMI in adulthood (β=-1.361, 95% CI: -2.513 to -0.208, P=0.021).
Discussion
Both the cross-sectional and the longitudinal data in the present study showed that IGF-1/IGFBP-3 ratios in Han adolescents in Liaoyang China were independently associated with the risk of MetS, and lower baseline IGF-1/IGFBP-3 ratios predicted a significantly higher risk of MetS when the subjects became adults 5 years later. We also found that the ratios of IGF-1/IGFBP-3 were negatively correlated with DBP and LDL-C, although this correlation was not observed 5 years later. In addition, a negative association between the baseline ratios of IGF-1/IGFBP-3 in adolescence with BMI 5 years later was revealed.
The IGF system includes three ligands (IGF-1, IGF-2, and insulin), their surface receptors, six binding proteins (IGFBP-1–6) and their proteases (eg, PAPP-A). IGF-1 enhances insulin sensitivity, promotes glucose uptake, improves lipid metabolism, and has anti-inflammatory activity.16–20 The IGFBP family in serum functions to regulate the endocrine actions of IGF-1 by regulating the amount of bioavailable IGF-1, and the major binding protein (>75%) for IGF-1 is IGFBP-3. A study of IGF-1 and IGFBP-3 in children and adolescents showed that the trend in IGF-1 and IGF-1/IGFBP-3 ratio changes was the same.21 Furthermore, the IGF-1/IGFBP-3 ratio had a stronger association with all of the MetS components than either IGF-1 or IGFBP-3 alone, also represents a balance between cardiac and metabolic risk.22 Thus, in this study, the ratio was used to determine the correlations between IGF-1, MetS, and cardiovascular disease risk factors.
In terms of whether IGF-1/IGFBP-3 ratios are associated with the risk of MetS, in recent years, studies of different populations have not been consistent. A low ratio of IGF-1/IGFBP-3 was associated with an increased prevalence of MetS, according to a cross-sectional study of the German adult population by Friedrich et al.23 In contrast, Yeap et al found that the IGF-1/IGFBP-3 ratio was independent of MetS based on the data obtained from 3,980 males >70 years old in Australia.24 These studies suggest that the relationship between the IGF-1/IGFBP-3 ratio and MetS may be different due to age or race. Numerous epidemiological and clinical studies have shown that MetS is a complex phenotype, and abnormal glucose and lipid metabolism, insulin resistance, central obesity, and the inflammatory reaction are leading factors in the development of MetS.25,26 Thus, in addition to IGF-1, population-specific factors, diet, physical activity environment affect the onset of MetS, which explain the contradicting results in the literature.
In this study, the correlation between IGF-1/IGFBP-3 ratio and MetS in the adolescent population in northeastern China was determined, and a 5-year follow-up study was performed to further investigate whether this correlation changed with age. In the cross-sectional investigation, we found that there was a clear association between the IGF-1/IGFBP-3 ratios and the high risk of MetS, and this association was driven by hypertension, which was shown to be the most closely correlated component. In the follow-up study, we also found a strong correlation between baseline IGF-1/IGFBP-3 ratios in adolescents and the presence of MetS in early adulthood, although different diagnostic criteria were used at different stages, while no correlation between baseline IGF-1/IGFBP-3 ratio and any MetS components in the adult period was found. As to this inconsistency, we speculated that the effects from various MetS components showed the same trend; thus, the accumulated effects led to the significant correlation between baseline IGF-1/IGFBP-3 ratios and overall risk of MetS after 5 years. Our present study was the first longitudinal one to reveal the prospective effects of IGF-1/IGFBP-3 for MetS. Also, it was a novel finding that despite changes in growth and development from adolescence to adulthood, adolescent IGF-1/IGFBP-3 ratios were still indicative of adult metabolic diseases.
In addition to MetS components, LDL-C was also considered as an important cardiovascular risk factor. We found a significant negative correlation between IGF-1/IGFBP-3 and LDL-C levels, and a decrease in IGF-1/IGFBP-3 ratio was an independent risk factor for high LDL-C. In contrast, TG and HDL-C, which are closely related to MetS, did not show any independent association with IGF-1/IGFBP-3 in this study. Malík et al also demonstrated this difference in their study, as the IGF-1/IGFBP-3 ratio was reduced in patients with hypercholesterolemia alone, but elevated in the mixed hyperlipidemia group.27 Therefore, the relationship between IGF-1 or IGFBP-3 and various lipid metabolism components may not be the same. As the evaluation of obesity, BMI was not associated with IGF-1/IGFBP-3 ratio in the adolescent cross-sectional data. However, in the follow-up study, when the 93 adolescents became adults, the group with the lowest ratio of IGF-1/IGFBP-3 in adolescence showed the highest level of BMI in adulthood, which was consistent with the increased risk of MetS. It was reported that the association of IGF-1 with MetS was affected by adiponectin, hs-CRP, and abnormal liver function,10,24,28 and that is why we adjusted these potentially confounding factors in the analysis to show an independent relationship. Some national and international studies showed that the IGF-1/IGFBP-3 ratio was negatively correlated with the number of MetS components,12,21,29 which was not found in this study (results not shown).
The results of our cross-sectional and prospective studies were not completely the same, and this was presumably due to the following reasons. First, the subjects experienced adolescence and adulthood, and definitions of cardiovascular disease risk factors such as MetS components and overweight/obesity in the two periods were very different; thus, even in the same disease state, the degree of consistency of diagnosis for subjects before and after was significantly reduced due to different criteria. Second, the correlation between IGF-1/IGFBP-3 ratio and metabolic diseases was affected by factors such as age, growth, and development, which could partly explain why the results of published studies in different age groups were often contradictory. For example, a study of young people aged 11–18 years in the United States showed a clear correlation between IGF-1/IGFBP-3 ratio and adolescent blood pressure,30 while another study carried out in young people aged 20–34 years failed to find this correlation.31 Third, as most of the subjects had gone to universities in different cities, the follow-up sample size in the present study was small, which was an obvious flaw of this study. Therefore, expanding the follow-up sample size and prolonging the follow-up period would help to further elucidate the causal relationships between IGF-1/IGFBP-3 ratio, adolescent MetS, and various cardiovascular disease risk factors.
Conclusion
Using cross-sectional and prospective methods, we determined the ratios of IGF-1/IGFBP-3 in Han adolescents aged 12–16 years residing in Liaoyang, northeastern China, and showed that a decrease in IGF-1/IGFBP-3 ratios in adolescents had a predictive effect on the high risk of MetS in adulthood. The prevalence of obesity, type 2 diabetes, and other metabolic diseases in Chinese adolescents is increasing.32 The IGF-1/IGFBP-3 ratio would be useful for epidemiological screening as a simple and effective serum marker of MetS, especially its predictive ability for future MetS and higher BMI in adulthood, which is of great practical value.
Acknowledgments
The authors are grateful to all children and their parents for participating in this study. This work was supported by Fund for young scientists of the National Natural Science Foundation of China (grant number 81600644).
Disclosure
The authors report no conflicts of interest in this work.
References
Xi B, He D, Hu Y, Zhou D. Prevalence of metabolic syndrome and its influencing factors among the Chinese adults: the China health and Nutrition Survey in 2009. Prev Med. 2013;57(6):867–871. | ||
Ye P, Yan Y, Ding W, et al. 中国儿童青少年代谢综合征患病率Meta分析 [Prevalence of metabolic syndrome in Chinese children and adolescents: a meta-analysis]. Zhonghua Liu Xing Bing Xue Za Zhi. 2015;36(8):884–888. Chinese. | ||
Agirbasli M, Tanrikulu AM, Berenson GS. Metabolic syndrome: bridging the gap from childhood to adulthood. Cardiovasc Ther. 2016;34(1):30–36. | ||
Clemmons DR. Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol Metab Clin North Am. 2012;41(2):425–443. | ||
Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J Endocrinol. 2002;175(1):19–31. | ||
Clemmons DR. Role of IGF binding proteins in regulating metabolism. Trends Endocrinol Metab. 2016;27(6):375–391. | ||
Rajpathak SN, Gunter MJ, Wylie-Rosett J, et al. The role of insulin-like growth factor-I and its binding proteins in glucose homeostasis and type 2 diabetes. Diabetes Metab Res Rev. 2009;25(1):3–12. | ||
Rosenfeld RG, Hwa V, Wilson E, Plymate SR, Oh Y. The insulin-like growth factor-binding protein superfamily. Growth Horm IGF Res. 2000;10(Suppl A):S16–S17. | ||
Juul A, Dalgaard P, Blum WF, et al. Serum levels of insulin-like growth factor (IGF)-binding protein-3 (IGFBP-3) in healthy infants, children, and adolescents: the relation to IGF-I, IGF-II, IGFBP-1, IGFBP-2, age, sex, body mass index, and pubertal maturation. J Clin Endocrinol Metab. 1995;80(8):2534–2542. | ||
Koegelenberg AS, Schutte R, Smith W, Schutte AE. Bioavailable IGF-1 and its relation to the metabolic syndrome in a bi-ethnic population of men and women. Horm Metab Res. 2016;48(2):130–136. | ||
Liang S, Hu Y, Liu C, Qi J, Li G. Low insulin-like growth factor 1 is associated with low high-density lipoprotein cholesterol and metabolic syndrome in Chinese nondiabetic obese children and adolescents: a cross-sectional study. Lipids Health Dis. 2016;15(1):112. | ||
Zimmet P, Alberti G, Kaufman F, et al. The metabolic syndrome in children and adolescents. Lancet. 2007;369(9579):2059–2061. | ||
Guansheng MA, Chengye Ji, Jun MA, et al. Waist circumference reference values for screening cardiovascular risk factors in Chinese children and adolescents aged 7-18 years. Chin J Epidemiol. 2010;31:609–615. | ||
Herrera-Enriquez K, Narvaez-Guerra O. Discordance of metabolic syndrome and abdominal obesity prevalence according to different criteria in Andean highlanders: a community-based study. Diabetes Metab Syndr. 2017;11(Suppl 1):S359–S364. | ||
Group of China Obesity Task Force. The classification standard of body mass index for overweight and obesity in Chinese school-age children and adolescents. Chin J Epidemiol. 2004;25:97–102. | ||
Knacke H, Pietzner M, Do KT, et al. Metabolic fingerprints of circulating IGF-1 and the IGF-1/IGFBP-3 ratio: a multifluid metabolomics study. J Clin Endocrinol Metab. 2016;101(12):4730–4742. | ||
Conti E, Andreotti F, Sestito A, et al. Reduced levels of insulin-like growth factor-1 in patients with angina pectoris, positive exercise stress test, and angiographically normal epicardial coronary arteries. Am J Cardiol. 2002;89(8):973–975. | ||
Dimmeler S, Zeiher AM. Exercise and cardiovascular health: get active to “AKTivate” your endothelial nitric oxide synthase. Circulation. 2003;107(25):3118–3120. | ||
Malik J, Stulc T, Ceska R. Unraveling Reaven’s syndrome X: serum insulin-like growth factor-I and cardiovascular disease. Circulation. 2003;107(20):190–192. | ||
Spies M, Nesic O, Barrow RE, Perez-Polo JR, Herndon DN. Liposomal IGF-1 gene transfer modulates pro- and anti-inflammatory cytokine mRNA expression in the burn wound. Gene Ther. 2001;8(18):1409–1415. | ||
Kong APS, Wong GWK, Choi K-C, et al. Reference values for serum levels of insulin-like growth factor (IGF-1) and IGF-binding protein 3 (IGFBP-3) and their ratio in Chinese adolescents. Clin Biochem. 2007;40(15):1093–1099. | ||
Sierra-Johnson J, Romero-Corral A, Somers VK, et al. IGF-I/IGFBP-3 ratio: a mechanistic insight into the metabolic syndrome. Clin Sci. 2009;116(6):507–512. | ||
Friedrich N, Nauck M, Schipf S, et al. Cross-sectional and longitudinal associations between insulin-like growth factor I and metabolic syndrome: a general population study in German adults. Diabetes Metab Res Rev. 2013;29(6):452–462. | ||
Yeap BB, Chubb SA, Ho KK, et al. IGF1 and its binding proteins 3 and 1 are differentially associated with metabolic syndrome in older men. Eur J Endocrinol. 2010;162(2):249–257. | ||
Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev. 2008;29(7):777–822. | ||
Leroith D. Pathophysiology of the metabolic syndrome: implications for the cardiometabolic risks associated with type 2 diabetes. Am J Med Sci. 2012;343(1):13–16. | ||
Malík J, Stulc T, Wichterle D, et al. Hyperlipidemia is associated with altered levels of insulin-like growth factor-I. Physiol Res. 2008;57(6):919–925. | ||
Oh J, Kim JY, Park S, et al. The relationship between insulin-like growth factor-1 and metabolic syndrome, independent of adiponectin. Clinica Chimica Acta. 2012;413(3–4):506–510. | ||
Parekh N, Roberts CB, Vadiveloo M, et al. Lifestyle, anthropometric, and obesity-related physiologic determinants of insulin-like growth factor-1 in the third National Health and Nutrition Examination Survey (1988–1994). Ann Epidemiol. 2010;20(3):182–193. | ||
Jiang X, Srinivasan SR, Dalferes ER, Berenson GS. Plasma insulin-like growth factor 1 distribution and its relation to blood pressure in adolescents: the Bogalusa heart study. Am J Hypertens. 1997;10(7Pt 1):714–719. | ||
Colangelo LA, Liu K, Gapstur SM, CARDIA Male Hormone Study. Insulin-like growth factor-1, insulin-like growth factor binding protein-3, and cardiovascular disease risk factors in young black men and white men: the CARDIA Male Hormone Study. Am J Epidemiol. 2004;160(8):750–757. | ||
Viner R, White B, Christie D. Type 2 diabetes in adolescents: a severe phenotype posing major clinical challenges and public health burden. Lancet. 2017;389(10085):2252–2260. |
Supplementary material
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.