Back to Journals » Cancer Management and Research » Volume 11
The relationship between pancreatic cancer and type 2 diabetes: cause and consequence
Authors Li Y , Bian X, Wei S , He M, Yang Y
Received 12 April 2019
Accepted for publication 23 August 2019
Published 9 September 2019 Volume 2019:11 Pages 8257—8268
DOI https://doi.org/10.2147/CMAR.S211972
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Yan Li1,2,*, Xiaohui Bian2,*, Shuyi Wei2, Meizhi He2, Yuelian Yang1
1Department of Gerontology, Zhujiang Hospital, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China; 2The Second School of Clinical Medicine, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuelian Yang
Department of Gerontology, Zhujiang Hospital, Southern Medical University, No. 253, Industrial Avenue, Haizhu District, Guangzhou, Guangdong 510280, People’s Republic of China
Tel +86 1 366 057 4670
Email [email protected]
Abstract: Pancreatic cancer (PC) is a devastating and lethal malignant disease and it is well known that there is a complex bidirectional relationship between PC and type 2 diabetes mellitus (T2DM). In order to more deeply summarize the relationship between them, this article summarizes the epidemiological data on the relationship between PC and T2DM in the past 5 years, and further explains the mechanism of interaction between them. Meanwhile, it also summed up the effects of drug therapy for T2DM on PC and the impact of T2DM on surgical resection of PC. Epidemiological studies clearly indicate that the risk of PC is increased in patients with T2DM. But increasing epidemiological data points out that PC also acts as a cause of T2DM and new-onset T2DM is sign and consequence of PC. Insulin resistance, hyperinsulinemia, hyperglycemia, and chronic inflammation are the mechanisms of T2DM-Associated PC. Metformin decreases the risk of PC, while insulin therapy increases the risk of PC. Besides, studies have shown that T2DM decreases the survival in patients with PC resection.
Keywords: pancreatic cancer, type 2 diabetes, bidirectional relationship
Introduction
Pancreatic cancer (PC) is one of the most devastating and lethal malignant diseases because of its high rate of advanced-stage disease at diagnosis and its resistance to therapy.1 Worldwide, the estimated incidence and mortality of PC in the general population are nearly 8/100,000 person-years and 7/100,000 person-years, which are significantly higher in the United States than in the rest of the world.2 In the United States, PC is the fourth most common cause of death in both men and women.3 The American Cancer Society estimates that in 2018, about 55,440 people consisting of 29,200 males and 26,240 females will be diagnosed with PC, and about 44,330 people will die from it in the United States this year.3
PC was associated with a very poor prognosis with a 5-year survival rate of less than 10%,4 meanwhile, it was 3% in the United States.3 Due to the high recurrence rate even after potential curative resection, the 5-year survival of completely resected patients was only up to 25%.5
Current treatment modalities were surgical resection, adjuvant therapy, neoadjuvant therapy, palliative therapy, targeted therapy, and novel therapies.6,7 Among them, surgery resection was the mainstay of treatment and the only potentially curative therapy for PC,1 however, merely 15–20% of the patients were considered as appropriate for surgical resection.8 Adjuvant therapy included chemotherapy and radiation therapy was able to reduce the risk of distant metastases and locoregional failure, but the role of radiation therapy was still controversial.1,9,10
Diabetes mellitus (DM) is a group of metabolic disorders of multiple etiologies characterized by chronic hyperglycemia with disturbances of carbohydrate, fat, and protein metabolism. DM occurs when the body cannot produce any or enough of the hormone insulin or use insulin effectively.11 DM is reaching an epidemic stage in the world with International Diabetes Federation estimating that 1 in 11 adults aged 20–79 years (425 million adults) had DMglobally in 2017 and more than 90% of whom had T2DM, with a projected increase to 629 million by 2045.11 The characteristics of T2DM were increased hyperinsulinemia, insulin resistance (IR) in target organs, and pancreatic β-cell dysfunction.12
The relationships between PC and T2DM
Many recent studies have proved that the relationship between PC and T2DM is complex and bidirectional.13 Pancreatic ductal adenocarcinoma (PDAC), the most common form of PC, has been thought to be more related to T2DM.14 As American Cancer Society’s Cancer Facts & Figures 2013 stated, about 25% of the patients had T2DM at the time of diagnosis of PC, and roughly another 40% had pre-diabetes.15 Besides, a total of 50% increased risk was observed in PC patients with long-term (≥5 years) T2DM, consequently, PC can result in T2DM. Moreover, it also stated that T2DM was an early sign of the tumor. Therefore, the relationships between PC and T2DM are both cause and consequence.16,17
T2DM as a risk factor of PC
Epidemiological studies have demonstrated that T2DM is a risk factor of PC.18–24 And many studies in the past 5 years have also proved that T2DM is the cause of PC. Risk associations between temporal pattern of risk and duration of T2DM given in five reports (25, 26, 28, 29, 30, 42, 44, 45) are plotted in Figure 1. It clearly shows that the shorter the duration of T2DM is, the higher the average risk associations between PC and T2DM are. Even if T2DM has lasted for 20 years, the hazard ratios (HR) or odds ratios (OR) between them are still greater than 1, thus proving that T2DM is a risk factor of PC.
 |
Figure 1 Hazard ratio, odds ratio, or relative risk of pancreatic cancer by the duration of type 2 diabetes. Abbreviations: HR, hazard ratio; OR, odds ratio; RR, relative risk. |
Bosetti C et al, analyzed individual-level data from 15 case–control studies within the Pancreatic Cancer Case-Control Consortium, including 1155 cases and 1087 controls that were diagnosed of T2DM 2 or more years before PC diagnosis, corresponding to an OR of 1.90 (95% CI=1.72–2.09).25 And the study also demonstrated that the duration of T2DM was associated with decreased risk of PC, but there was still a significant excess risk between them 20 or more years after diabetes diagnosis (OR=1.30, 95% CI=1.03–1.63), therefore supporting that T2DM could be a risk factor of PC.
A nested case–control study concerning the association between PC and T2DM was performed by Lu Y et al, in 2015.26 It was initiated from the Health Improvement Network in the UK from 1996 to 2010, including 529 PC cases and 5000 controls. Increased OR of PC (OR=2.16, 95% CI=1.72–2.72) was found in T2DM patients and changed HbA1c levels in T2DM patients (OR=5.06, 95% CI=1.52–16.87). Consequently, it was suggested that T2DM and T2DM with rising HbA1c were likely to be independent risk factors for PC. Similarly, Haugvik S P et al, made a meta-analysis including five studies evaluating four individual populations and involving 827 cases and 2407 controls.27 It concluded that a pooled adjusted OR was 2.74 (95% CI=1.63–4.62; P<0.01) in T2DM patients with pancreatic neuroendocrine tumors, proving T2DM to be a risk factor of pancreatic neuroendocrine tumors once again.
In 2016, Antwi S O et al, investigated independent association between inflammatory potential of diet, cigarette smoking, and long-standing T2DM (≥5 years) in relation to risk of PC.28 The study was composed of 817 cases and 1756 controls. It revealed a 3.09-fold increase (OR=3.09, 95% CI=2.02–4.72) in risk of PC associated with long-standing T2DM compared to non-diabetics, and a 2.54-fold increase (OR=2.54, 95% CI=1.87–3.46) with a more pro-inflammatory diet and a 3.40- fold increase (OR=3.40, 95% CI=2.28–5.07) with current smokers. Besides, joint associations were observed for the combined effects of being a current smoker (OR=4.79, 95% CI=3.00–7.65) or having long-standing diabetes (OR=6.03, 95% CI=3.41–10.85), concluding that long-standing T2DM and cigarette smoking were risk factors of PC, whereas a pro-inflammatory diet might act as cofactor with cigarette smoking and diabetes.
In 2017, Pang Y and his colleagues undertook a study to find out the association between T2DM and PC in China.29 The prospective study recruited 512,000 adults aged 30–79 years from 10 diverse areas of China from 2004 to 2008, which was further meta-analyzed with 22 published prospective studies. It revealed a 1.87-fold increase in the risk of PC associated with T2DM (adjusted HR=1.87, 95% CI=1.48–2.37), and it was with excess higher risk in those with longer duration since diagnosis (P=0.01). Moreover, previously diagnosed T2DM was found with a 52% excess risk and with 1.52 increased risk of PC (adjusted HR=1.52, 95% CI=1.43–1.63) in meta-analysis of China Kadoorie Biobank and 22 other studies, consequently, T2DM was associated with a higher risk of PC in Chinese populations.
In 2018, Setiawan VW et al, examined a study concerning the relationships between recent-onset diabetes and PC incidence in African Americans and Latinos in the Multiethnic Cohort, which included 48,995 African Americans and Latinos without prior T2DM and PC.30 2.39-fold increase was revealed in the risk of PC associated with T2DM (HRage75=2.39, 95% CI=1.91–2.98) in African Americans and Latinos, suggesting that T2DM was a risk factor for PC.
Therefore, the evidence clearly shows that T2DM is a risk factor for PC, so T2DM can be translated to improved morbidity and mortality for patients accursed with PC, which may lead to improvement of PC associated survival by preventing and treating T2DM.
T2DM as sign and consequence of PC, and PC as a cause of T2DM
Clinicians realize that new-onset T2DM can be a sign of PC from their clinical experience.31 Patients with new-onset T2DM have more possibilities to be diagnosed with PC within 3 years, suggesting that new-onset T2DM is as an early sign of PC.32 There were many epidemiological studies that can support that point.32–39 What is more, lots of cohort and case–control studies of patients diagnosed with PC show that 25–50% of the patients will have developed T2DM within 1–3 years before their diagnosis of PC and it is indicated that 85% of the patients diagnosed with PC have impaired glucose tolerance or frank T2DM.20,32,35,36,40 Also, it was clearly demonstrated that loss of glycemic control and T2DM could result from PC and could precede the diagnosis of PC by a few weeks, to a few months, to 2–3 years.41 These findings implied that recent-onset T2DM was caused by PC. That was to say, recent-onset T2DM was an early sign of PC.
In the past 5 years, there have been multitudinous studies that proved T2DM to be sign and consequence of PC. In 2014, Munigala S et al, made a retrospective analysis of VA database including 452,804 patients from 1998 to 2007.42 They observed that 73,811 (16.3%) of subjects developed T2DM from 2000 to 2007. Among that, 147 (0.2%) patients with new-onset T2DM were diagnosed with PC within 2 years of diagnosis. While the annual incidence of PC in the controls was 0.04% (realtive risk (RR)=2.58, 95% CI=2.12–3.15, P<0.0001), which was much lower than 0.2%. Hence, new-onset T2DM was considered as an early manifestation of PC. In another study of 2014, Singh J et al, intended to figure out the association between new-onset T2DM and the stage of resectable PC from 181 patients.43 They found 56.9% (n=82) of resectable cancer stage had new-onset T2DM (within 3 years) and 65 of 82 had new-onset T2DM within 1 year of the PC diagnosis. These findings suggested that the new-onset T2DM could be regarded as an initial surrogate marker of resectable PC stage, and be able to capture a significant number of PC patients still at the resectable stage.
In 2015, there was a study in which 183 of 73,811 new-onset T2DM patients (0.25%) were diagnosed with PC within 3 years.44 While 434 of 378,993 remaining patients (0.11%) developed PC in 3 years (RR=2.27, 95% CI=1.96–2.63, P<0.0001), which provided strong support for new-onset T2DM as an early sign of PC. A meta-analysis including 29 studies to evaluate the effect of T2DM on survival in patients with PC was performed by Mao Y et al45. They concluded that patients with new-onset T2DM (≤2 years of diabetes duration) were with an increased HR (HR=1.52, 95% CI=1.20–1.93, while HR was 1.22 (0.83–1.80) for those with long-standing T2DM (>2 years). Therefore, they believed that new-onset T2DM was a significantly independent association with higher mortality overall in patients with PC and the overall survival (OS) was associated with the duration of T2DM.
In 2016, Boursi B et al, screened individuals with new-onset T2DM to allow earlier diagnosis of PC.46 They analyzed data from 109,385 patients with new-onset T2DM of which 390 (0.4%) were diagnosed with PC within 3 years. And they developed a risk model which had 44.7% sensitivity, 94.0% specificity, and a positive predictive value of 2.6% based on widely available clinical parameters including age, body mass index, change in body mass index, smoking, use of proton pump inhibitors and antidiabetic medications, as well as levels of HbA1C, cholesterol, hemoglobin, creatinine, and alkaline phosphatase. Therefore, it was helpful to identify patients with new-onset T2DM who might benefit from PC screening.
A new recent study was made by Setiawan V W et al, in 2018 to illuminate the relationship between recent-onset T2DM and PC in African Americans and Latinos.30 It demonstrated that the HRage75 for recent-onset T2DM was 4.08 (95% CI=2.76–6.03) in Latinos and 3.38 (95% CI=2.30–4.98) in African Americans. Additionally, the authors found that recent-onset T2DM was associated with a nearly 2.3-fold greater increase in the risk of PC than long-standing T2DM in all groups, which significantly supported the hypothesis that long-standing T2DM was a risk factor for PC, while recent-onset T2DM was a manifestation of PC in African Americans and Latinos.
Hence, there is clear evidence to demonstrate that new-onset T2DM is an early sign of PC, and T2DM can be a screening tool to identify and surveillance patients at high-risk for PC to determine the treatment modality and prognosis.
Mechanisms of T2DM-associated PC
The mechanisms of the association between DM and PC were complex, which included IR, hyperinsulinemia, hyperglycemia, and chronic inflammation.39,47–51 In a study made by Stolzenberg-Solomon R Z et al, of 29,133 male Finnish smokers followed for over 10 years, the authors found that hyperglycemia (HR=2.16, 95% CI=1.05–4.42, P=0.02), hyperinsulinemia (HR=2.90, 95% CI=1.22–6.92, P=0.005), and IR (HR=2.71, 95% CI=1.19–6.18, P=0.006) were each significantly associated with increased risk of PC.22
IR and hyperinsulinemia
The characteristics in patients with T2DM are IR with ensuing hyperinsulinemia and high levels of insulin-like growth factor-1 (IGF-1).14,52,53 In T2DM, IR can result in hyperinsulinemia by means of serine phosphorylation of insulin receptor substrate proteins, which can activate some kinases such as PKCζand mammalian target of rapamycin (mToR) complex/S6K, thus involved in the down-regulation of insulin signaling.54 And insulin reduces the hepatic production of IGFBP-1 and −2 (IGF binding protein), whose affinity was high for both IGF-1 and IGF-2, thus increasing levels of free circulating physiologically active IGF-1.55,56
The majority of cancer cells express insulin and insulin-like growth factor-1 receptors (IGF-1R), which are members of the tyrosine kinase class of membrane receptors, and are homologous to oncogenes of the tyrosine kinase class.57 Both the IGF-1R and the insulin receptor are complex molecules. Each gene product is processed extensively and finally forms glycosylated α-chains and β-chains that associates to form a “half” receptor, then two half receptors associate to form a holoreceptor. And heterodimer hybrid receptors are composed of a half insulin receptor and a half IGF-1R.58,59 Besides, the insulin receptor exists in two splice variant isoforms, the “B” isoform recognizes only insulin, while the “A” isoform recognizes both insulin and IGFII, and it is the isoform most commonly expressed by tumors.58 When insulin and IGF-1 are combined with their receptors, they can mediate the initiation of signal transduction that activates important intracellular signal pathways, including the Ras/Raf/mitogen-activated protein kinase (MAPK) and phosphoinositide-3 kinase (PI3K)/Akt/mTOR pathways, which contribute to the development of PC.55
In addition, insulin itself is a growth-promoting hormone that augments cell proliferation and glucose use.60,61 Insulin may promote specific IGF-1R signaling pathways to mediate cell proliferation, inhibition of apoptosis, and growth by binding to hybrid receptors.48 Chronic hyperinsulinemia effects can result in a chain of metabolic responses, including changes in IGFBP that make tissue availability of both IGF-1 and -2 increase.60 And it is interesting that IR does not inhibit activation of the mitogenic pathway.62
The mitogenic and anti-apoptotic activities of IGF-1 have a more potent effect than those of insulin and may be considered as growth stimuli in cells expressing insulin and the IGF-1R.50 In addition, it was demonstrated that dose-dependent increases in neoplastic cell proliferation were with increasing IGF-1 concentration in vitro experiments.63 IGF-1-mediated signaling transduction results in the increasement of proliferation, invasion, and expression of angiogenesis mediators and the decreasement of apoptosis in PC cells.64
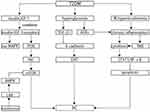
Overall, patients with T2DM are with hyperinsulinemia and high levels of IGF-1. As shown in Figure 2, insulin and IGF-1 binding to their receptors activate the MAPK and PI3K/Akt/mTOR pathways, thus promoting cell proliferation and decreasing of apoptosis.
Hyperglycemia
In patients with T2DM, the occurrence of hyperglycemia is not only as a result of promoting IR but also because of decreasing insulin output via pancreatic β-cells.56 Prospective cohort and case–control studies showed that hyperglycemia was associated with increased free radical formation and might result in the development of advanced glycation end products (AGEs) that could increase inflammation.65 AGEs are composed of a heterogeneous group of compounds that accumulate in tissues of aging individuals and, at an accelerated rate, in diabetic subjects,59 which is due to the mechanisms including increased carbohydrate and lipid substrate availability, oxidative, and non-oxidative conditions favoring the glycation process, and impaired detoxification.66,67 Exogenous AGE administration to PC-prone mice led to the up-regulation of AGEs receptor (RAGE) in pancreatic intraepithelial neoplasias and markedly stimulated progression to invasive PC.68
In a cohort study to find out the tumor-promoting role of the AGE Nε-carboxymethyllysine (CML) which was a major AGE found in vivo and a known RAGE ligand in human PDAC cell lines in 2018, the authors discovered that PC was observed in 8 of 11 (72.7%) CML-treated vs 1 of 11 (9.1%) vehicle-treated mice.68 Meanwhile, CML promoted PDA cell growth and led to the up-regulation of RAGE expression, by a concentration- and time-dependent manner, and activated downstream tumorigenic signaling pathways, thus markedly stimulating progression to invasive PC. It was interesting that RAGE antagonist peptidedelayed pancreatic intraepithelial neoplasias development in vehicle-treated mice but failed to prevent PC development in CML-treated mice. This because of competition with soluble RAGE for binding to AGEs and/or compensatory up-regulation of the RAGE homolog CD166/activated leukocyte cell adhesion molecule, which also was propitious to tumor spread. These findings implied that AGEs modulated the development and progression of PC through receptor-mediated mechanisms. Consequently, it could be a good way to prevent PC by reducing AGEs.
What is more, hyperglycemia can overproduction of reactive oxygen species (ROS), which is mitogenic and capable of stimulating cell proliferation.69 Besides, hyperglycemia can attenuate antioxidant enzyme activity.70
Study of Rahn S et al, in 2018 investigated whether hyperglycemia promotes malignant pancreatic ductal epithelial cells (PDEC) and cancer stem cells (CSC) that are essential for initiation and maintenance of tumors and epithelial-mesenchymal-transition (EMT) linked to the acquisition of CSC-features in premalignant.71 They demonstrated that hyperglycemia (25 mm D-glucose) did not influence the mesenchymal phenotype of Panc1 cells, but CSC-properties were aggravatedly exemplified by increased Nanog expression and Nanog-dependent formation of holo- and meroclones. In addition, in H6c7-kras cells, high glucose stimulates transforming growth factor-β1 (TGF-β1) signaling and TGF-β1, which is mostly secreted by lymphoid cells and also largely exists in platelets and bones, as well as circulates in the plasma.71,72 Besides, it decreased E-cadherin expression, while increased Nestin expression and the number of meroclones in TGF-β1-dependent manner. They also found that reduced E-cadherin was detected in pancreatic ducts of hyperglycemic but not normoglycemic mice. These findings indicated that hyperglycemia advanced the acquisition of mesenchymal and CSC-properties in PDEC by activating TGF-β signaling and might explain how T2DM facilitated PC, which is concluded in Figure 2.
Chronic inflammation
Inflammatory responses may be a way to increase the risk of PC in T2DM patients.39 In T2DM patients, common with hyperinsulinemia and IR, it is prone to many adipose tissue and abundant inflammatory cells, thus promoting systemic inflammation and resulting in a tumorigenic environment.73 Inflammatory states also promote a cellular environment, which can support the development of genomic aberrations and the initiation of carcinogenesis.74,75
Moreover, glucose and fat intake may lead to inflammation by increasing oxidative stress and the activating transcriptional factors such as nuclear factor kappa B (NF-κB), activating protein-1, early growth response-1 and so on.76–78
In addition, adipose tissue is an active endocrine organ recognized as a low-grade inflammatory state to regulate the release of fatty acids, hormones, and pro-inflammatory cytokines like leptin, adiponectin, tumor necrosis factor alpha, and interleukin-6, which are not only key molecules involved in innate immunity, inflammation, apoptosis, metabolism, and development, but also can increase IR and subclinical inflammation.79–82 Besides, pro-inflammatory cytokines, known as adipocytokines, may play an etiologic role in regulating malignant transformation or cancer progression and can promote angiogenesis, tumor progression, and metastasis.80–82
Inflammatory cytokines, ROS, and mediators of inflammatory pathways, such as cyclooxygenase-2 and NF-κB are strongly linked with the signal transduction of signal transducer and activator of transcription 3 (STAT3) pathway.83 The STAT3 and NF-κB signaling suppress apoptosis and the promote cell cycle progression.83,84 And they also induce EMT by downregulating the expression of E-cadherin.84 In addition, Inflammation exerts great effects on the composition of the tumor microenvironment, where the immune cells release cytokines and growth factors, thus directly promoting tumor growth and progression.85 All of these plotted in Figure 2 may facilitate PC.70,86
Furthermore, altered levels or functions of several molecules previously associated with diabetes, such as leptin,87 IGF-1,88 and peroxisome proliferator-activated receptor-g,89 may promote PC development by impairing immune function.80,86–89
Relationship between drug therapy for T2DM and PC
Current medical treatments for T2DM include insulin or insulin analogs, insulin secretagogues, glucagon-like peptide-1 agonists, and dipeptidyl peptidase IV inhibitors, drugs that reverse IR such as biguanides, and other drugs such as α-glucosidase inhibitors.90
Metformin therapy
Metformin is a cornerstone in the treatment of T2DM. Retrospective studies have concluded a survival benefit in diabetic patients with PC treated with metformin.91 And lots of studies and researches have been made to illuminate the magnitude of metformin risk reduction of PC in the past 5 years.
In 2014, Bosetti C et al, analyzed 15 case–control studies, including 8305 cases and 13,987 controls to illustrate the risk relationship between antidiabetic medications and PC.25 It was shown that long duration of oral antidiabetic use was an independent association with a reduced PC risk (OR=0.31, 95% CI=0.14–0.69, for ≥15 years) among T2DM patients, thus supporting that oral antidiabetics might decrease the risk of PC.
In 2015, a study was made to investigate the effect of metformin use on survival in PC patients with curative resection and T2DM, including 764 subjects with T2DM and PC with curative resection, 530 of which were exposed to metformin.92 The authors found that the adjusted risk for PC specific mortality of metformin user was lower than that of metformin non-user (HR=0.73, 95% CI=0.61–0.87, P<0.001) and also significantly lower in medication possession ratio (MPR) of more than 80% compared with that of MPR of less than 80% (HR=0.60, 95% CI=0.47–0.76, P<0.001) in multivariable analysis. These findings concluded that metformin use was significantly associated with increased survival in T2DM patients with PC, which might provide a theory for further prospective study that the use of metformin was as an adjunct to the standard of care in the treatment of PC. However, there was not a significantly reduced risk in PC patients treated with metformin (OR 1.46, 95% CI=0.85–2.52) in another study in 2015.26
In 2018, a population-based study was performed to evaluate the effects of T2DM and antidiabetic medications on the risk of PC by Lee D Y et al.93 It was shown that among antidiabetic medications, metformin was an independent association with a reduced risk for future PC (HR=0.86, 95% CI=0.77–0.96) and subjects with dual exposure history to metformin plus thiazolidinedione or metformin plus dipeptidyl peptidase-4 inhibitor had a lower risk of PC compared to metformin-only treated subjects, which implicated that metformin could decrease the risk of PC. Pusceddu S et al, made a study in 2018 to figure out the association between metformin use and PC.94 They found that progression-free survival (PFS) of patients treated with metformin was significantly longer than for patients without T2DM (HR=0.45, 95% CI=0.32–0.62, P<0.00001) and was longer than for patients with T2DM receiving other treatments (HR=0.49, 95% CI=0.34–0.69, P<0.0001). In multivariable analysis, metformin was also significantly associated with longer PFS after adjusting for other factors (P=0.004). Metformin was also associated with higher PFS of patients receiving somatostatin analogs and in those receiving everolimus. Therefore, metformin use was significantly associated with longer PFS, implying that Metformin could decrease the risk of PC.
Although there is definitely contradictory evidence in the literature but it seems clear that, in some patients, metformin can decrease the risk of PC. Consequently, metformin may prevent the development of malignant lesions and holds promise to be an anticancer agent.
The current consensus of the mechanism that metformin can decrease the risk of PC as metformin activates the liver kinase B1 (LKB1)-adenosine monophosphate protein-activated kinase (AMPK) pathway, which can not only facilitate cellular energy production and restrain hepatic glucose production but also inhibit the signaling mechanisms regulating cellular proliferation.95,96 As a known tumor suppressor, LKB1 can activate AMPK that is a potent inhibitor of mTOR complex 1 and disrupt cross-talk between insulin/IGF-1 receptors and G protein-coupled receptors, which regulate protein synthesis and replication.97–99 What is more, metformin may play a role in PC stem cells through the mTOR pathway, which is evidenced by decreasing cancer stem cell markers such as CD44, CD133, aldehyde dehydrogenase type 1, epithelial adhesion molecule and so on.100 In one study to evaluate the effect of metformin on PC, the authors found that cancer stem cell (Alk4, Nodal, Activin, and Smad2) and pluripotency-associated (Nanog, Oct4, and Sox2) messenger RNA (mRNA) and protein expression were significantly altered after treatment with metformin through a mTOR/AMPK-independent pathway, and it might possibly through reduced form of nicotinamide-adenine dinucleotide dehydrogenase inhibition, free ROS production, thus causing direct cancer stem cell damage.101
These studies indicated that metformin can decrease the risk of PC, activating the LKB1/AMPK pathway, thus inhibiting mTOR to regulate cellular proliferation, which is showed in Figure 2. Therefore, metformin is promising to become an anticancer agent for PC.
Insulin therapy
Insulin therapy is often necessary for long-standing T2DM. And there were abundant studies and research showing that insulin therapy was associated with higher risk of PC because insulin itself may directly increase PC risk.90,91,102,103
In 2014, Bosetti C et al, analyzed 15 case–control studies including 8305 cases and 13,987 controls to illustrate the risk relationship between antidiabetic medications and PC.25 The authors found that insulin use was independently associated with a higher PC risk in the short term (OR=5.60, 95% CI=3.75–8.35, for <5 years), but not for longer duration of use (OR=0.95, 95% CI=0.53–1.70, for ≥15 years), whereas long duration of oral antidiabetic use was an independent association with a reduced PC risk (OR=0.31, 95% CI=0.14–0.69, for ≥15 years) among T2DM, thus illustrating that insulin use showed an inconsistent duration–risk relationship.
In 2015, Lu Y et al, made a study aiming to clarify associations between antidiabetic medications and PC.26 It included 1,574,768 persons of follow-up, of which 529 PC cases and 5000 controls were identified. The results showed that insulin users were with a higher risk of PC (OR=25.57, 95% CI=11.55–56.60), but metformin users were not associated with PC (OR=1.46, 95% CI=0.85–2.52) compared with no use of any antidiabetic medications among the antidiabetic medications in patients with T2DM. These results indicated that different antidiabetic medications had a different relation with PC, with the highest risk among users of insulin.
In 2018, Lee D Y et al, undertook a population-based study to find out the effects of T2DM and antidiabetic medications on the risk of PC.93 It was concluded that among antidiabetic medications, insulin exposure was with an increased risk of PC (HR=2.86, 95% CI=1.43–5.74) compared to subjects with no drug exposure, which implicated that insulin could increase the risk of PC.
Ding et al, found that physiologic concentrations of insulin promoted PC cell proliferation as well as glucose utilization by activating MAPK, PI3K, and enhancing IGF-1 expression.103 Insulin has potential mitogenic and anti-apoptotic effects on cultured cancer cells by activating of the IGF-1 pathway; moreover, insulin induces phosphorylation of ERK and Akt, which suggests that insulin can stimulate the ras-raf-MAPK and PI3K/Akt pathways.104–106
Overall, as summarized in Figure 2, insulin use is independently associated with a higher PC risk by promoting PC cell proliferation and activating the MAPK and PI3K/Akt/mTOR pathways, indicating that the patients at high-risk for PC cannot benefit from insulin use to treat T2DM.
Effect of T2DM on surgical resection of PC
Surgical resection is the mainstay of treatment of PC, including pancreaticoduodenectomy, distal pancreatectomy, central pancreatectomy, and total pancreatectomy.107 In general, T2DM has an adverse effect on surgical resection of PC, including not only operative morbidity and mortality, but also survival and operative complications in patients undergoing resection for PC.
Chu C K et al, conducted a study in 2010 to find out the impact of T2DM on perioperative morbidity and mortality after PC resection.108 There were 116 (46%) patients had preoperative T2DM of 251 PC resection cases. It was showed that 60-day mortality was 3.6%, delayed gastric emptying occurred in 40.1% of the patients and pancreatic fistulas developed in 17 (6.8%) patients. Besides, T2DM patients had a higher possibility of developing fistulas (T2DM =10.3%, non-T2DM =3.7%, P<0.04) and developing acute kidney injury (T2DM =23.3%, non-T2DM =12.6%, P<0.03). Moreover, T2DM remained independently associated with fistula formation (OR=4.3, 95% CI=1.18–15.8, P≤0.027) after controlling for age, comorbidities, body mass index, preoperative albumin level, operation type, operative time, and pancreatic quality. These findings indicated that T2DM increased operative complications following pancreatic surgery. Furthermore, the authors made another study in 2010 to assess clinicopathologic features and postresection survival of T2DM-associated PC.109 They found that median survival was reduced in patients with T2DM compared with non-T2DM (15 vs 17 months, P=0.015), and multivariate analysis indicated that T2DM was independently correlatedly with decreased survival (HR=1.55, 95% CI=1.02–2.35). Hence, they concluded that pre-existing T2DM was associated with decreased survival in patients with PC resection.
In 2012, Cannon and colleagues undertook a survey to determine whether preoperative T2DM had any predictive value for survival of patients with resection for PC.110 The study included 509 patients with PC resection, and 31.2% had T2DM. It was demonstrated that preoperative T2DM was significantly associated with OS and disease-free in both univariate analysis and cox multivariate analysis with P<0.001, which implied that preoperative T2DM reduced both OS and disease-free for PC operative patients.
The effect of T2DM on survival in patients with PC resection in 2015 was evaluated in a systematic review and meta-analysis by Mao Y et al.45 Subgroup analyses showed that T2DM was an independently related to poor survival in patients with resectable disease (HR=1.37, 95% CI=1.15–1.63) but not in those with unresectable disease (HR=1.07, 95% CI=0.89–1.29), thus supporting that T2DM was associated with higher mortality overall in patients with surgery for PC. Similarly, Kleeff J et al, performed a study to analyze the prognostic impact of diabetes on the outcome of PC following resection and adjuvant chemotherapy.29 They found that patients with T2DM had an increased risk of death (HR=1.19, 95% CI=1.01–1.40, P=0.034) in multivariable analysis after adjustment, which showed T2DM’s independent association with decreased survival following PC resection and adjuvant chemotherapy.
Reviewing the studies on the impact of diabetes preoperatively in PC, T2DM was found to shorten the survival in patients with PC resection and the better survival rate, and good prognosis was reported on non-T2DM compared to diabetic. What is more, preoperative diabetes status provides useful information to stratify, surveillance, and manage patients with PC resection.
Conclusion
PC is highly aggressive and lethal malignant, and T2DM has a wide range of morbidity. Epidemiological studies prove that the relationships between PC and T2DM are complex. For one thing, T2DM is a risk factor for PC. For another, PC is a cause of T2DM, which can act as sign and consequence of PC. In other words, new-onset T2DM is an early sign of PC. So T2DM can be translated to improved morbidity and mortality for patients accursed with PC, and it can be a screening tool in high-risk case of PC to determine the treatment modality and prognosis. The mechanisms of the association between DM and PC include IR, hyperinsulinemia, hyperglycemia, and chronic inflammation. Metformin and insulin are the main medical treatments for T2DM. Studies have shown that metformin decreases the risk of PC, while insulin therapy is associated with higher risk of PC; therefore,, metformin can be a treatment to prevent the development of malignant lesions and holds promise as an anticancer agent. The potential curative treatment for PC is surgical resection, but it is indicated that T2DM is associated with decreased survival and is a significant comorbidity predicting worse outcomes in patients with PC resection.
Abbreviations
PC, pancreatic cancer; T2DM, type 2 diabetes mellitus; DM, diabetes mellitus; IDF, International Diabetes Federation; IR, insulin resistance; PDAC, pancreatic ductal adenocarcinoma; HR, hazard ratio; OR, odds ratio; CI, confidence interval; CKB, China Kadoorie Biobank; RR, relative risk; OS, overall survival; IGF-1, insulin-like growth factor-1; IRS, insulin receptor substrate; mTORC, mammalian target of rapamycin complex; IGFBP, IGF binding protein; IGF-1R, insulin-like growth factor-1 receptor; MAPK, mitogen-activated protein kinase; PI3K, phosphatidyl inositol-3 kinase; mTOR, mammalian target of rapamycin; AGEs, advanced glycation end products; RAGE, AGEs receptor; CML, carboxymethyllysine; RAP, RAGE antagonist peptide; ROS, reactive oxygen species; PDEC, pancreatic ductal epithelial cells; CSC, cancer stem cells; EMT, epithelial-mesenchymal-transition; TGF-β1, transforming growth factor-β1; NF-κB, nuclear factor kappa B; JAK, Janus kinase; STAT3, signal transducer and activator of transcription 3; COX-2, cyclooxygenase-2; TME, tumor microenvironment; GLP-1, glucagon-like peptide-1; MPR, medication possession ratio; PFS, progression-free survival; LKB1, liver kinase B1; AMPK, adenosine monophosphate protein-activated kinase; mRNA, messenger RNA; NADH, reduced form of nicotinamide-adenine dinucleotide; DGE, delayed gastric emptying.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039–1049. doi:10.1056/NEJMra1404198
2. Xiao AY, Tan ML, Wu LM, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1(1):45–55. doi:10.1016/S2468-1253(16)30004-8
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
4. Maruthappu M, Watkins J, Noor AM, et al. Economic downturns, universal health coverage, and cancer mortality in high-income and middle-income countries, 1990–2010: a longitudinal analysis. Lancet. 2016;388(10045):684–695. doi:10.1016/S0140-6736(16)00577-8
5. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi:10.3322/caac.21208
6. Werner J, Combs SE, Springfeld C, Hartwig W, Hackert T, Büchler MW. Advanced-stage pancreatic cancer: therapy options. Nat Rev Clin Oncol. 2013;10(6):323–333. doi:10.1038/nrclinonc.2013.66
7. Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388(10039):73–85. doi:10.1016/S0140-6736(16)00141-0
8. Konstantinidis IT, Warshaw AL, Allen JN, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg. 2013;257(4):731–736. doi:10.1097/SLA.0b013e318263da2f
9. Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 2000;232(5):776.
10. Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi:10.1056/NEJMoa032295
11. International Diabetes Federation. IDF diabetes atlas—8th edition. DiabetesAtlas. 2018. Available from: http://www.diabetesatlas.org/.
12. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389(10085):2239–2251. doi:10.1016/S0140-6736(17)30058-2
13. Li J, Cao G, Ma Q, Liu H, Li W, Han L. The bidirectional interation between pancreatic cancer and diabetes. World J Surg Oncol. 2012;10:171. doi:10.1186/1477-7819-10-198
14. Rozengurt E. Mechanistic target of rapamycin (mTOR): a point of convergence in the action of insulin/IGF-1 and G protein-coupled receptor agonists in pancreatic cancer cells. Front Physiol. 2014;5:357. doi:10.3389/fphys.2014.00268
15. American Cancer Society. Cancer Facts & Figures 2013. Atlanta: American Cancer Society; 2013.
16. Wang F, Gupta S, Holly EA. Diabetes mellitus and pancreatic cancer in a population-based case-control study in the San Francisco Bay Area, California. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1458–1463. doi:10.1158/1055-9965.EPI-06-0188
17. Amin S, Mhango G, Lin J, et al. Metformin improves survival in patients with pancreatic ductal adenocarcinoma and pre-existing diabetes: a propensity score analysis. Am J Gastroenterol. 2016;111(9):1350–1357. doi:10.1038/ajg.2016.288
18. Wideroff L, Gridley G, Mellemkjaer L, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89(18):1360–1365. doi:10.1093/jnci/89.18.1360
19. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi:10.1056/NEJMoa021423
20. Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92(11):2076–2083. doi:10.1038/sj.bjc.6602619
21. Saydah SH, Platz EA, Rifai N, Pollak MN, Brancati FL, Helzlsouer KJ. Association of markers of insulin and glucose control with subsequent colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2003;12(5):412–418.
22. Stolzenberg-Solomon RZ, Graubard BI, Chari S, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA. 2005;294(22):2872–2878. doi:10.1001/jama.294.22.2872
23. Li D, Tang H, Hassan MM, Holly EA, Bracci PM, Silverman DT. Diabetes and risk of pancreatic cancer: a pooled analysis of three large case-control studies. Cancer Causes Control. 2011;22(2):189–1897. doi:10.1007/s10552-010-9686-3
24. Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer. 2011;47(13):1928–1937. doi:10.1016/j.ejca.2011.03.003
25. Bosetti C, Rosato V, Li D, et al. Diabetes, antidiabetic medications, and pancreatic cancer risk: an analysis from the International Pancreatic Cancer Case-Control Consortium. Ann Oncol. 2014;25(10):2065–2072. doi:10.1093/annonc/mdu276
26. Lu Y, García Rodríguez LA, Malgerud L, et al. New-onset type 2 diabetes, elevated HbA1c, anti-diabetic medications, and risk of pancreatic cancer. Br J Cancer. 2015;113(11):1607–1614. doi:10.1038/bjc.2015.353
27. Haugvik SP, Hedenström P, Korsæth E, et al. Diabetes, smoking, alcohol use, and family history of cancer as risk factors for pancreatic neuroendocrine tumors: a systematic review and meta-analysis. Neuroendocrinology. 2015;101(2):133–1342. doi:10.1159/000375164
28. Antwi SO, Oberg AL, Shivappa N, et al. Pancreatic cancer: associations of inflammatory potential of diet, cigarette smoking and long-standing diabetes. Carcinogenesis. 2016;37(5):481–490. doi:10.1093/carcin/bgw022
29. Pang Y, Kartsonaki C, Guo Y, et al. Diabetes, plasma glucose and incidence of pancreatic cancer: A prospective study of 0.5 million Chinese adults and a meta‐analysis of 22 cohort studies. Int J Cancer. 2017;140(8):1781–1788. doi:10.1002/ijc.30599
30. Setiawan VW, Stram DO, Porcel J, et al. Pancreatic cancer following incident diabetes in African Americans and Latinos: the multiethnic cohort. J Natl Cancer Inst. 2019;111(1):27–33. doi:10.1093/jnci/djy090
31. Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1252–1261. doi:10.1053/j.gastro.2013.01.068
32. Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: a populationbased study. Gastroenterology. 2005;129(2):504–511. doi:10.1053/j.gastro.2005.05.017
33. Gupta S, Vittinghoff E, Bertenthal D, et al. New-onset diabetes and pancreatic cancer. Clin Gastroenterol Hepatol. 2006;4(11):1366–1372. doi:10.1016/j.cgh.2006.06.024
34. Aggarwal G, Kamada P, Chari ST. Prevalence of diabetes mellitus in pancreatic cancer compared to common cancers. Pancreas. 2013;42(2):198–201. doi:10.1097/MPA.0b013e3182592c96
35. Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134(4):981–987. doi:10.1053/j.gastro.2008.01.074
36. Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134(1):95–101. doi:10.1053/j.gastro.2008.01.074
37. Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10(1):88–95. doi:10.1016/S1470-2045(09)70234-7
38. Schwarts SS, Zeidler A, Moossa AR, Kuku SF, Rubenstein AH. A prospective study of glucose tolerance, insulin, C-peptide, and glucagon responses in patients with pancreatic carcinoma. Am J Dig Dis. 1978;23(12):1107–1114. doi:10.1007/BF01072886
39. Li D. Diabetes and pancreatic cancer. Mol Carcinog. 2012;51(1):64–74. doi:10.1002/mc.v51.1
40. Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA. 1995;273(20):1605–1609.
41. Sah RP, Nagpal SJS, Mukhopadhyay D, Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol. 2013;10(7):423–433. doi:10.1038/nrgastro.2013.49
42. Munigala S, Singh A, Gelrud A, Agarwal B. Higher pancreatic cancer risk following new onset of diabetes mellitus in non-obese patients with chronic pancreatitis. Gastrointest Endosc. 2014;79(5):AB191–AB192. doi:10.1016/j.gie.2014.02.241
43. Singh J, D A P, Urayama S. Potential for new-onset diabetes mellitus as a marker of resectable pancreatic cancer from EUS registry. Gastroenterology. 2014;146(5):
44. Munigala S, Singh A, Gelrud A, Agarwal B. Predictors for pancreatic cancer diagnosis following new-onset diabetes mellitus. Clin Transl Gastroenterol. 2015;6(10):e118. doi:10.1038/ctg.2015.44
45. Mao Y, Tao M, Jia X, et al. Effect of diabetes mellitus on survival in patients with pancreatic cancer: a systematic review and meta-analysis. Sci Rep. 2015;5:17102. doi:10.1038/srep17102
46. Boursi B, Finkelman B, Giantonio BJ, et al. A clinical prediction model to assess risk for pancreatic cancer among patients with new-onset diabetes. Gastroenterology. 2017;152(4):840–850.e3. doi:10.1053/j.gastro.2016.11.046
47. Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60(4):207–221. doi:10.3322/caac.20078
48. Cui Y, Andersen DK. Diabetes and pancreatic cancer. Endocr Relat Cancer. 2012;19(5):F9–F26. doi:10.1530/ERC-12-0105
49. Magruder JT, Elahi D, Andersen DK. Diabetes and pancreatic cancer: chicken or egg? Pancreas. 2011;40(3):339–351. doi:10.1097/MPA.0b013e318209e05d
50. Biadgo B, Type AM. 2 diabetes mellitus and its association with the risk of pancreatic carcinogenesis: a review. Korean J Gastroenterol. 2016;67(4):168–177. doi:10.4166/kjg.2016.67.4.168
51. Andersen DK, Korc M, Petersen GM, et al. Diabetes, pancreatogenic diabetes, and pancreatic cancer. Diabetes. 2017;66(5):1103–1110. doi:10.2337/db16-1477
52. Ireland L, Santos A, Ahmed MS, et al. Chemoresistance in pancreatic cancer is driven by stroma-derived insulin-like growth factors. Cancer Res. 2016;76(23):6851–6863. doi:10.1158/0008-5472.CAN-16-1201
53. Jang WI, Kim MS, Kang SH, et al. Association between metformin use and mortality in patients with type 2 diabetes mellitus and localized resectable pancreatic cancer: a nationwide populationbased study in Korea. Oncotarget. 2017;8(6):9587–9596. doi:10.18632/oncotarget.14525
54. Tanti JF, Jager J. Cellular mechanisms of insulin resistance: role of stress-regulated serine kinases and insulin receptor substrates (IRS) serine phosphorylation. Curr Opin Pharmacol. 2009;9(6):753–762. doi:10.1016/j.coph.2009.07.004
55. Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8(12):915–928. doi:10.1038/nrc2536
56. Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12(3):159–169. doi:10.1038/nrc3215
57. Ullrich A, Bell JR, Chen EY, et al. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature. 1985;313(6005):756–761. doi:10.1038/313803a0
58. Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30(6):586–623. doi:10.1210/er.2008-0047
59. Benyoucef S, Surinya KH, Hadaschik D, Siddle K. Characterization of insulin/IGF hybrid receptors: contributions of the insulin receptor L2 and Fn1 domains and the alternatively spliced exon 11 sequence to ligand binding and receptor activation. Biochem J. 2007;403(3):603–613. doi:10.1042/BJ20061709
60. Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363(9418):1346–1353. doi:10.1016/S0140-6736(04)16044-3
61. Goodwin PJ. Insulin in the adjuvant breast cancer setting: a novel therapeutic target for lifestyle and pharmacologic interventions? J Clin Oncol. 2008;26(6):833–834. doi:10.1200/JCO.2007.14.7132
62. Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18(2):139–143. doi:10.1097/MED.0b013e3283444b09
63. Myal Y, Shiu RP, Bhaumick B, Bala M. Receptor binding and growth-promoting activity of insulin-like growth factors in human breast cancer cells (T-47D) in culture. Cancer Res. 1984;44:5486–5490.
64. Denley A, Carroll JM, Brierley GV, et al. Differential activation of insulin receptor substrates 1 and 2 by insulin-like growth factor-activated insulin receptors. Mol Cell Biol. 2007;27(10):3569–3577. doi:10.1128/MCB.01447-06
65. Grote VA, Rohrmann S, Nieters A, et al. Diabetes mellitus, glycated haemoglobin and C-peptide levels in relation to pancreatic cancer risk: a study within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Diabetologia. 2011;54(12):3037–3046. doi:10.1007/s00125-011-2316-0
66. Wautier JL, Schmidt AM. Protein glycation: a firm link to endothelial cell dysfunction. Circ Res. 2004;95(3):233–238. doi:10.1161/01.RES.0000137876.28454.64
67. Iacobini C, Menini S, Oddi G, et al. Galectin-3/AGE-receptor 3 knockout mice show accelerated AGE-induced glomerular injury: evidencefor a protective role of galectin-3 as an AGE receptor. Faseb J. 2004;18(14):1773–1775. doi:10.1096/fj.03-0678fje
68. Menini S, Iacobini C, de Latouliere L, et al. The advanced glycation end-product N -carboxymethyllysine promotes progression of pancreatic cancer: implications for diabetes-associated risk and its prevention. J Pathol. 2018;245(2):197–208. doi:10.1002/path.5072
69. Li W, Ma Q, Li J, et al. Hyperglycemia enhances the invasive and migratory activity of pancreatic cancer cells via hydrogen peroxide. Oncol Rep. 2011;25(5):1279–1287. doi:10.3892/or.2011.1150
70. Sindhu RK, Koo JR, Roberts CK, Vaziri ND. Dysregulation of hepatic superoxide dismutase, catalase and glutathione peroxidase in diabetes: response to insulin and antioxidant therapies. Clin Exp Hypertens. 2004;26(1):43–53.
71. Rahn S, Zimmermann V, Viol F, et al. Diabetes as risk factor for pancreatic cancer: Hyperglycemia promotes epithelial-mesenchymal-transition and stem cell properties in pancreatic ductal epithelial cells. Cancer Lett. 2018;415:129–150. doi:10.1016/j.canlet.2017.12.004
72. Prud’homme GJ, Piccirillo CA. The inhibitory effects of transforming growth factor-beta-1 (TGF-beta1) in autoimmune diseases. Autoimmun. 2000;14:23–42. doi:10.1006/jaut.1999.0339
73. Ciaraldi TP, Sasaoka T. Review on the in vitro interaction of insulinnglargine with the insulin/insulin-like growth factor system: potential implications for metabolic and mitogenic activities. Horm Metab Res. 2011;43(1):1–10. doi:10.1055/s-0030-1267203
74. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi:10.1038/nature01322
75. Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3(4):276–285. doi:10.1038/nrc1046
76. Aljada A, Ghanim H, Mohanty P, Syed T, Bandyopadhyay A, Dandona P. Glucose intake induces an increase in activator protein 1 and early growth response 1 binding activities, in the expression of tissue factor and matrix metalloproteinase in mononuclear cells, and in plasma tissue factor and matrix metalloproteinase concentrations. Am J Clin Nutr. 2004;80(1):51–57.
77. Dhindsa S, Tripathy D, Mohanty P, et al. Differential effects of glucose and alcohol on reactive oxygen species generation and intranuclear nuclear factor-kappaB in mononuclear cells. Metabolism. 2004;53(3):330–334. doi:10.1016/j.metabol.2003.10.013
78. Mohanty P, Ghanim H, Hamouda W, Aljada A, Garg R, Dandona P. Both lipid and protein intakes stimulate increased generation of reactive oxygen species by polymorphonuclear leukocytes and mononuclear cells. Am J Clin Nutr. 2002;75(4):767–772. doi:10.1093/ajcn/75.4.767
79. Housa D, Housova J, Vernerova Z, Haluzik M. Adipocytokines and cancer. Physiol Res. 2006;55(3):233–244.
80. van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2569–2578. doi:10.1158/1055-9965.EPI-09-0372
81. Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–1685. doi:10.2337/dc10-0666
82. Li Y, Ding L, Hassan W, Abdelkader D, Shang J. Adipokines and hepatic insulin resistance. J Diabetes Res. 2013;2013:1–8.
83. O’Sullivan KE, Reynolds JV, O’Hanlon C, O’Sullivan JN, Lysaght J. Could signal transducer and activator of transcription 3 be a therapeutic target in obesity-related gastrointestinal malignancy? J Gastrointest Cancer. 2014;45(1):1–11. doi:10.1007/s12029-013-9555-x
84. Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–771. doi:10.1038/nrc3611
85. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. doi:10.1016/j.immuni.2019.06.025
86. Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–795. doi:10.2337/db09-9028
87. Lago R, Gómez R, Lago F, Gómez-Reino J, Gualillo O. Leptin beyond body weight regulation—current concepts concerning its role in immune function and inflammation. Cell Immunol. 2008;252(1–2):139–145. doi:10.1016/j.cellimm.2007.09.004
88. Smith TJ. Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases? Pharmacol Rev. 2010;62(2):199–236. doi:10.1124/pr.109.002469
89. Wohlfert EA, Nichols FC, Nevius E, Clark RB. Peroxisome proliferator-activated receptor gamma (PPARgamma) and immunoregulation: enhancement of regulatory T cells through PPARgamma-dependent and -independent mechanisms. J Immunol. 2007;178(7):4129–4135. doi:10.4049/jimmunol.178.7.4129
90. Maisonneuve P, Lowenfels AB, Bueno-de-Mesquita HB, et al. Past medical history and pancreatic cancer risk: results from a multicenter case-control study. Ann Epidemiol. 2010;20(2):92–98. doi:10.1016/j.annepidem.2009.11.010
91. Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29(2):254–258. doi:10.2337/diacare.29.02.06.dc05-1558
92. Jo A, Kim Y, Kang S, Kim M, Ko M. The effect of metformin use and mortality among those with pancreatic cancer and type 2 diabetes mellitus: findings from a nationwide population retrospective cohort study. Value Health. 2015;18(7):A439–A439. doi:10.1016/j.jval.2015.09.1072
93. Lee DY, Yu JH, Park S, et al. The influence of diabetes and antidiabetic medications on the risk of pancreatic cancer: a nationwide population-based study in Korea. Sci Rep. 2018;8(1):9719. doi:10.1038/s41598-018-27965-2
94. Pusceddu S, Vernieri C, M D M, et al. Metformin use associates with longer progression-free survival of patients with diabetes and pancreatic neuroendocrine tumors receiving everolimus and/or somatostatin analogues. Gastroenterology. 2018;155(2):479–489.e7. doi:10.1053/j.gastro.2018.04.010
95. Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310(5754):1642–1646. doi:10.1126/science.1120781
96. Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66(21):10269–10273. doi:10.1158/0008-5472.CAN-06-1500
97. Kisfalvi K, Eibl G, Sinnett-Smith J, Rozengurt E. Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res. 2009;69(16):6539–6545. doi:10.1158/0008-5472.CAN-09-0418
98. Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6(1):91–99. doi:10.1016/j.ccr.2004.06.007
99. Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75(75):137–163. doi:10.1146/annurev.biochem.75.103004.142702
100. Mohammed A, Janakiram NB, Brewer M, et al. Antidiabetic drug metformin prevents progression of pancreatic cancer by targeting in part cancer stem cells and mTOR signaling. Transl Oncol. 2013;6(6):649–659. doi:10.1593/tlo.13556
101. Lonardo E, Cioff M, Sancho P, et al. Metformin targets the metabolic achilles heel of human pancreatic cancer stem cells. PLoS One. 2013;8(10):e76518. doi:10.1371/journal.pone.0076518
102. Bonelli L, Aste H, Bovo P, et al. Exocrine pancreatic cancer, cigarette smoking, and diabetes mellitus: a case-control study in northern Italy. Pancreas. 2003;27(2):143–149.
103. Ding XZ, Fehsenfeld DM, Murphy LO, Permert J, Adrian TE. Physiological concentrations of insulin augment pancreatic cancer cell proliferation and glucose utilization by activating MAP kinase, PI3 kinase and enhancing GLUT-1 expression. Pancreas. 2000;21(3):310–320.
104. Azar M, Lyons TJ. Diabetes, insulin treatment, and cancer risk: what is the evidence? F1000 Med Rep. 2010;2(1).
105. Mayer D, Shukla A, Enzmann H. Proliferative effects of insulin analogues on mammary epithelial cells. Arch Physiol Biochem. 2008;114(1):38–44. doi:10.1080/13813450802033909
106. Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev. 2009;25(1):41–49. doi:10.1002/dmrr.v25:1
107. Khadka R, Tian W, Hao X, Koirala R. Risk factor, early diagnosis and overall survival on outcome of association between pancreatic cancer and diabetes mellitus: Changes and advances, a review. Int J Surg. 2018;52:342–346. doi:10.1016/j.ijsu.2018.02.058
108. Chu CK, Mazo AE, Sarmiento JM, et al. Impact of diabetes mellitus on perioperative outcomes after resection for pancreatic adenocarcinoma. J Am Coll Surg. 2010;210(4):463–473. doi:10.1016/j.jamcollsurg.2009.12.029
109. Chu CK, Mazo AE, Goodman M, et al. Preoperative diabetes mellitus and longterm survival after resection of pancreatic adenocarcinoma. Ann Surg Oncol. 2010;17(2):502–513. doi:10.1245/s10434-010-1022-3
110. Cannon RM, LeGrand R, Chagpar RB, et al. Multi-institutional analysis of pancreatic adenocarcinoma demonstrating the effect of diabetes status on survival after resection. HPB (Oxford). 2012;14(4):228–235. doi:10.1111/j.1477-2574.2011.00432.x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.