Back to Journals » International Journal of Nanomedicine » Volume 18
The Refined Application and Evolution of Nanotechnology in Enhancing Radiosensitivity During Radiotherapy: Transitioning from Gold Nanoparticles to Multifunctional Nanomaterials
Received 20 August 2023
Accepted for publication 21 October 2023
Published 1 November 2023 Volume 2023:18 Pages 6233—6256
DOI https://doi.org/10.2147/IJN.S436268
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Anqi Zhang,1 Lei Gao2
1Oncology Department, Huabei Petroleum Administration Bureau General Hospital, Renqiu, Hebei, People’s Republic of China; 2Medical Imaging Department, Huabei Petroleum Administration Bureau General Hospital, Renqiu, Hebei, People’s Republic of China
Correspondence: Lei Gao, Medical Imaging Department, Huabei Petroleum Administration Bureau General Hospital, Renqiu, Hebei Province, 062550, People’s Republic of China, Tel +86 152 9739 4123, Fax +86-0317-2723381#, Email [email protected]
Abstract: Radiotherapy is a pivotal method for treating malignant tumors, and enhancing the therapeutic gain ratio of radiotherapy through physical techniques is the direction of modern precision radiotherapy. Due to the inherent physical properties of high-energy radiation, enhancing the therapeutic gain ratio of radiotherapy through radiophysical techniques inevitably encounters challenges. The combination of hyperthermia and radiotherapy can enhance the radiosensitivity of tumor cells, reduce their radioresistance, and holds significant clinical utility in radiotherapy. Multifunctional nanomaterials with excellent biocompatibility and safety have garnered widespread attention in tumor hyperthermia research, demonstrating promising potential. Utilizing nanotechnology as a sensitizing carrier in conjunction with radiotherapy, and high atomic number nanomaterials can also serve independently as radiosensitizing carriers. This synergy between tumor hyperthermia and radiotherapy may overcome many challenges currently limiting tumor radiotherapy, offering new opportunities for its further advancement. In recent years, the continuous progress in the synthesis and design of novel nanomaterials will propel the future development of medical imaging and cancer treatment. This article summarizes the radiosensitizing mechanisms and effects based on gold nanotechnology and provides an overview of the advancements of other nanoparticles (such as bismuth-based nanomaterials, magnetic nanomaterials, selenium nanomaterials, etc.) in the process of radiation therapy.
Keywords: nanotechnology, gold nanoparticles, tumor radiotherapy, hyperthermia, radiosensitization
Introduction
Malignant tumors hold the second-highest mortality rate globally, surpassed only by cardiovascular diseases. Radiotherapy, chemotherapy, and surgical intervention stand as the three most prevalent treatment modalities.1,2 Radiotherapy, a critical approach in treating malignant tumors, is incorporated into the frontline treatment plans for approximately 60% of newly diagnosed patients.3–5 Modern precision radiotherapy aims to enhance the therapeutic gain ratio through advanced physics technology, thereby maximizing the radiation dose delivered to the target lesion while minimizing unnecessary exposure to surrounding healthy tissues and organs. This has led to the widespread application of sophisticated techniques such as Intensity-Modulated Radiation Therapy (IMRT), Image-Guided Radiation Therapy (IGRT), and Stereotactic Radiosurgery (SRS).6–8 However, due to the inherent physical properties of high-energy radiation, enhancing the therapeutic gain ratio through radiophysical technology inevitably encounters limitations. Factors affecting the efficacy of tumor radiotherapy extend beyond radiophysical technology to include intracellular oxygen content and reoxygenation, glutathione levels, radiation damage repair capacity, radiosensitivity across different cell cycles, expression of radioresistant genes in tumors, immune evasion by tumors, and radiation-induced side effects.9–11
Combining hyperthermia with radiotherapy enhances the radiosensitivity of tumor cells and diminishes their radioresistance, holding significant clinical value in tumor treatment. With the rapid advancements in nanoscience and biomedicine in recent years, numerous studies indicate that, due to their unique physicochemical and biological properties, nanomaterials offer vast prospects for enhancing tumor radiosensitivity. This has paved new avenues for the combined application of tumor hyperthermia and radiotherapy, introducing innovative approaches.12,13 Multifunctional nanomaterials with commendable biocompatibility and safety have garnered extensive attention in tumor hyperthermia applications, demonstrating promising potential.14,15 Integrating nanothermal technology as a sensitizing agent in radiotherapy not only synergistically enhances the effects of hyperthermia and radiotherapy but also allows nanomaterials to serve independently as radiosensitizing agents. This dual approach may overcome numerous challenges currently constraining tumor radiotherapy, thereby presenting new opportunities for its further advancement.16–18 This article provides a comprehensive review of the research progress in tumor hyperthermia and radiosensitization based on nanotechnology, aiming to inform subsequent research and clinical translation of this promising technique.
Overview of Nanotechnology-Enhanced Thermoradiotherapy Radiosensitization
Tumor hyperthermia is a treatment modality that employs physical factors (such as radiofrequency, microwaves, ultrasound, and lasers) to elevate the temperature of tumor tissues and/or the entire body, utilizing the cytotoxic effects of elevated temperatures and their secondary effects to treat tumors.19,20 It serves as a comprehensive cancer treatment approach, supplementing surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy. Tumor hyperthermia is regarded as a green, non-toxic, safe, and widely accepted radiosensitizing technique. Tumor hyperthermia combined with radiotherapy primarily functions by directly inhibiting tumor cells and enhancing the radiosensitizing effects induced by heat, thereby augmenting the efficacy of radiotherapy and chemotherapy.21,22 The mechanisms underlying hyperthermia-induced radiosensitization are complex, with established mechanisms including cell cycle complementation, hypoxia alleviation, and DNA repair inhibition.23 In terms of cell cycle complementation, radiotherapy is most effective during the late G2 and M phases of tumor cell mitosis. During the S phase of mitosis, when glutathione synthesis increases, cells are relatively radioresistant. In contrast, hyperthermia is sensitive to tumor cells in the S phase, and the combined application of both treatments can produce a synergistic effect.24,25 To alleviate tumor hypoxia, hyperthermia can enhance tumor tissue perfusion, increase vascular permeability, and augment tumor metabolism and oxygenation, thereby reversing tumor tissue hypoxia and enhancing radiosensitivity. DNA strand breaks are key factors in radiation-induced tumor cell death. While double-strand breaks are often irreparable and lethal to tumor cells, single-strand breaks can be repaired by DNA polymerases, affecting the cytotoxic impact of radiation. Hyperthermia can reduce DNA polymerase activity, thereby inhibiting DNA strand repair and enhancing tumor radiosensitivity by suppressing the repair of sublethal and potentially lethal damage.26,27
Traditional tumor hyperthermia involves the use of external electromagnetic energy sources, such as infrared radiation, radiofrequency, microwaves, and ultrasound, to heat the region containing the tumor tissue. This non-selective heating technique, which affects both tumor and normal tissues, can result in damage to healthy tissues, a factor that has limited the further development and application of hyperthermia in the context of modern precision medicine.28 The rapid advancement of nanotechnology offers a multitude of potential treatment strategies for cancer. Utilizing the unique tissue permeability and retention (EPR) effect of nanomaterials, combined with technologies such as electromagnetic navigation, enables the specific targeting of nanomaterials to tumor cells. Selecting nanomaterials with photothermal and electromagnetic heating properties, and applying external electromagnetic waves, can overcome the limitations of traditional hyperthermia techniques, achieving precise intracellular tumor hyperthermia.29,30 Moreover, certain high atomic number nanomaterials, due to their unique photoelectric attenuation characteristics, can be targeted to tumor tissues. Under the photoelectric and Compton effects of high-energy X-rays, these materials can generate high levels of energy absorption, modify the tumor microenvironment, enhance radiosensitivity, and ultimately achieve a synergistic effect between hyperthermia and radiotherapy. These materials are often employed as radiation sensitizers.31–33
Radiosensitizing Effects of Gold Nanoparticles
Gold, a transition metal and an inert element, is one of the most malleable metals known.34 Its excellent biocompatibility, multivalent surface properties, and ease of modification with targeted molecular probes make it a focal point in the field of nanomedicine.35,36 Gold nanoparticles (AuNPs) utilize surface plasmon resonance technology (SPR) for photothermal conversion, serving as photothermal transducers for photothermal therapy. Surface plasmon resonance enhances, to a certain extent, the light absorption and scattering effects of gold nanoparticles, thereby increasing the absorbed dose in tumor tissues. In cancer treatment, gold nanoparticles also function as drug carriers, imaging probes, and radiation sensitizers.37–40 Under X-ray irradiation, gold nanoparticles enhance local photoelectric absorption effects, accelerate DNA cleavage, and alter the cell cycle and intracellular environment, thus emerging as novel sensitizing agents in current radiation therapy for tumors,41 as depicted in Figure 1.
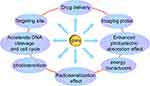 |
Figure 1 The multifaceted capabilities of gold nanoparticles. |
Radiosensitizers are defined as chemical or biological compounds that can increase the effective dose of radiation therapy in cancer cells,42 serving as substances that enhance the radiosensitivity of targeted lesions. Due to its high atomic number, gold can produce a certain radiosensitizing effect, and the synergy between the two can multiply the therapeutic effect.43,44 Tabatabaie et al conducted experimental research on human melanoma and prostate cancer cell lines using gold nanoparticles (GNPs) and 6 MV X-rays (0–8Gy), and quantified the mitochondrial stress response. The results showed that the addition of GNPs significantly increased mitochondrial stress, peaking at 4Gy. Radiation-induced mitochondrial damage was quantified through increased ROS activity. This enhanced mitochondrial stress can lead to more effective killing of GNP-treated cells, further enhancing the applicability of functionally guided nanoparticles.45 Huynh et al, using gold nanoparticles (GNPs) as radiation sensitizers, found that the addition of gold nanoparticles reduced the total radiation dose and radiation toxicity. Experiments demonstrated that GNP radiosensitization can overcome hypoxia-induced radiation resistance and treatment-induced accelerated repopulation of cancer cells in HNC, improving radiation therapy outcomes and offering an optimistic prognosis and approach for the treatment of head and neck cancers.46 The radiosensitizing potential of GNPs has been supported and confirmed by various experimental data (Table 1)
 |
Table 1 Studies on Radiosensitization of GNPs |
The Intricate Mechanism of Gold Nanoparticles in Enhancing Radiosensitivity During Radiotherapy
The use of radiosensitizers can increase the local dose and overcome the heterogeneity of responses within hypoxic and rapidly proliferating regions of tumors. This enhances the contrast between tumor and normal tissues, yielding additional therapeutic benefits. Initially, the radiosensitizing effect of gold nanoparticles (GNPs) was believed to be solely due to the physical dose enhancement caused by the strong photoelectric absorption of gold (Au). However, as research has progressed, it has become apparent that biological systems undergo a series of processes under infrared radiation. These processes can be categorized into three stages: the physical phase, the chemical phase, and the biological phase. These three processes complement each other and collectively influence the interaction between GNPs and radiation, thereby exerting a radiosensitizing effect, as illustrated in Figures 2–4.
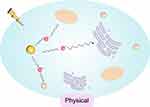 |
Figure 2 Physical Mechanisms. |
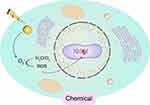 |
Figure 3 Biological Mechanisms. |
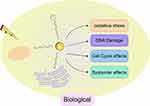 |
Figure 4 Chemical Mechanisms. |
Physical Mechanisms
In the physical phase, which occurs within the first nanoseconds of exposure, ionizing radiation (IR) interacts with biomolecules, causing ionization or excitation and generating free radicals (ROS). Among the various cellular components, DNA is the primary target that determines the radiobiological effects.71,72 If sufficient energy is available, ejected electrons will further collide with subsequent atoms, producing a cascade of ionization events.
In the context of kV-level radiation, photons primarily interact with matter through either the Compton effect or the photoelectric effect. In the Compton effect, incident photons are scattered when they collide with weakly bound electrons; during this process, part of the energy is transferred from the photon to the electron, which is then ejected from the atom. In the photoelectric effect, incident photons transfer all their energy to an electron, liberating it from its atomic binding and creating a photoelectron. This results in the ejection of an inner-shell electron, leaving an atom in an excited state with a vacancy in its electron orbitals. The atom returns to its ground state by emitting characteristic X-rays or Auger electrons. When outer-shell electrons fall to fill the vacancy, lower-energy photons (fluorescence) and cascading secondary electrons (Auger electrons) are released.Soft tissues are primarily composed of elements such as C, H, O, and N, which have low electron densities and photoelectric cross-sections. As a result, they absorb relatively little energy when interacting with kV-level radiation.
The primary principle of using GNPs (Gold Nanoparticles) as radiosensitizers is as follows: compared to soft tissues, the presence of gold (Au) allows for an enhanced physical dose due to its distinct energy absorption characteristics.73 Gold has a high atomic number (Z=79), and its electron density is significantly higher than that of soft tissues. When photon energy ranges between 10 and 500 KeV, the photoelectric effect becomes the dominant physical process, as described by the formula σph∝(Z /E),3 where the probability of the photoelectric effect occurring is inversely proportional to the cube of the incident photon energy and directly proportional to the cube of the target atomic number. This process results in the generation of photoelectrons, characteristic X-rays of gold atoms, and Auger electrons. A large number of inner-shell electron transitions (Auger transitions) can effectively produce a high density of local ionizations. These low-energy electrons distribute energy in a high-density manner along a path of approximately 10 nm, doubling the energy deposition at the surface of the gold nanoparticles and potentially producing radiobiological effects similar to those of high LET (Linear Energy Transfer) radiation.
In the clinical context of 1 to 6 MeV X-rays, where the Compton effect is predominant, gold atoms are more than 10 times as likely to undergo Compton scattering as soft tissues (79/7.4). The scattered photons and Compton electrons produced can undergo inelastic scattering with neighboring gold nanoparticles, leading to photoelectric effects and Auger cascades, although the photoelectric effect is lower than that with kV-level radiation.74,75 When interacting with kV-level radiation, the cross-section for the photoelectric effect occurring when radiation strikes the surface of gold nanoparticles is also significantly higher than that for soft tissues. This results in a large number of secondary and Auger electrons, generating additional secondary electrons and energy deposition.76 This process increases the photoelectric absorption in tumor tissues, damages the DNA of cancer cells, and accelerates tumor cell death. This constitutes the physical basis of the radiosensitizing effect of GNPs.77
For example, Leung et al, using Monte Carlo computational methods, demonstrated that the enhancement of radiotherapy with gold nanoparticles is achieved by amplifying photoelectric absorption, thereby increasing the production of photoelectrons and Auger/Coster-Kronig electrons by two orders of magnitude. This amplification effect is dependent on the photon spectrum.78 Carter et al calculated the microdosimetric distribution of X-ray irradiation from the surface of gold nanoparticles to a distance of 3 nm from the surface. They found that, due to the surge of low-energy Auger electrons at the particle surface, the energy deposition within the nanoscale range of the nanoparticle surface is significantly stronger than the average energy deposition in the solution.79
Numerous scholars believe that high atomic number materials, when positioned within the cell nucleus, can induce more efficient DNA damage and radiobiological effects, while cytoplasmic events are also significant mechanisms leading to cell death.80 Gold nanoparticles, located in the cytoplasm, are exposed to radiation that generates a large number of secondary electrons and free radicals. These attack critical organelles within the cytoplasm, resulting in lethal damage to these organelles and subsequent cell death.81,82 Furthermore, some researchers propose that after gold nanoparticles enter the tumor vasculature, they adhere to the endothelial cells of the tumor blood vessels. When exposed to X-ray radiation, these nanoparticles generate low-energy, short-range photoelectrons, increasing the damage to the vascular endothelium and the dose within the tumor microenvironment. This is also considered one of the mechanisms through which gold nanoparticles enhance the internal sensitizing effect of radiation.83
Biological Mechanisms
Research indicates that the radiosensitizing effect of GNPs is higher than the effect caused solely by the deposition of GNPs. This suggests that the mechanism involved in the radiosensitization process of GNPs is not limited to the enhancement of physical doses caused by the Compton effect and the photoelectric effect. It is speculated that biological mechanisms also play a significant role.77,84 Through experimental research analysis, the following three key biological pathways related to radiosensitization are widely recognized: 1) Induction of oxidative stress response; 2) Impact on cell cycle progression; 3) Inhibition of DNA repair. In addition, other related biological mechanisms such as autophagy, endoplasmic reticulum (ER) stress, and effects on tumor blood vessels have been proposed.
Gold Nanoparticles Induce Oxidative Stress
One of the primary mechanisms by which radiation induces cell death is through the radiation-induced decomposition of water molecules, leading to the production of free radicals and ROS, which then interact with various cellular components. ROS includes superoxide anion radicals (O2-), hydrogen peroxide (H2O2), and hydroxyl radicals (·OH). Studies have found that ROS can oxidize the mitochondrial membrane, disrupt its potential, and leak more superoxide anions into the cell solute. These can then be transformed into H2O2 molecules, which further diffuse across the cell membrane and damage DNA. ROS can also indirectly cause cell apoptosis or necrosis through the oxidation of lipids, proteins, and DNA, as well as mitochondrial dysfunction. This might be one of the potential biological mechanisms of the radiosensitizing effect of GNPs.
The precise mechanism by which gold nanoparticles instigate oxidative stress remains enigmatic. Nevertheless, a plethora of empirical studies suggests that its genesis is intertwined with an escalation in intracellular ROS, culminating in mitochondrial impairment, DNA double-strand ruptures, and consequent cellular apoptosis or necrosis. Research by Chithrani et al unveiled that post-irradiation of HeLa cells with 220kVp X-rays in conjunction with GNPs, the expression levels of γ-H2AX and 53BP1 markedly surpassed those solely irradiated. Elevated expression of radiation-induced γ-H2AX and 53BP1 serves as a salient hallmark of intracellular DNA double-strand breaks. Furthermore, in cell clusters irradiated with internalized gold nanoparticles, a moderate augmentation in the number of lesions per nucleus was discerned.49 Butterworth et al, in their exploration of the cytotoxic and radiation-enhancing effects of 1.9 nm gold particles in oncological treatments, discerned that GNPs precipitated an upsurge in DSBs in irradiated cells, coupled with pronounced cell-specific toxicity, apoptosis, and oxidative stress.48 Geng et al’s empirical study on thiolated glucose-bound gold nanoparticles enhancing the radiocytotoxic targeting of ovarian cancer revealed that 14nm thiolated glucose-bound gold nanoparticles (Glu-GNP) can be harnessed as sensitizers to amplify the radiotherapy of ovarian cancer. The interplay between X-rays and 14nm Glu-GNPs significantly induced a surge in intracellular ROS levels, leading to heightened oxidative stress and increased cellular apoptosis.50 Khalil et al, employing plasmid DNA in aerated aqueous solutions as a probe, ascertained the type of ROS engendered post ultra-soft X-ray (USX) absorption that partakes in biomolecular damage, in the presence or absence of gold nanoparticles (GNP) and specific scavengers. Their findings underscored the radiosensitizing prowess of citrate-coated GNPs and elucidated the dependency between the gold core’s size and the radiosensitizing efficacy – the smaller the core, the more pronounced the effect. Although H2O2 exhibits relative inertness towards DNA, the majority of this molecule, synthesized by the amalgamation of two ·OH radicals post ionizing radiation exposure, becomes exceedingly reactive in the presence of gold nanoparticles due to its catalytic decomposition into hydroxyl radicals. H2O2 is postulated as a reservoir of ·OH radicals released by gold nanoparticles, leading to the inference that H2O2 is pivotal in radiosensitization.85 Some studies accentuate that the most conspicuous biological effects are not when the cell nucleus is directly targeted, but rather when the cytoplasm is. Mitochondria, customarily compromised under oxidative duress, serve as quintessential participants in modulating cellular death trajectories. Antecedent research indicates that mitochondria are the linchpin cytoplasmic mediators of radiation damage and GNP susceptibility.86 Ghita et al, employing soft X-ray carbon (K shell, 278 eV) microbeams on MDA-MB-231 breast cancer and AG01522 fibroblasts for nuclear and cytoplasmic irradiation with or without GNP to delineate DNA damage and repair dynamics, corroborated that GNP radiosensitization is orchestrated by mitochondrial functionality, and consequently, cytoplasmic damage can also instigate significant DNA impairment.87 Tsai et al, irradiating A431 cells with Cs-137γ rays, discerned that post GNP-intake cell irradiation, ROS was excessively expressed, followed by observations of ROS-induced cytoskeletal disintegration and mitochondrial dysfunction. Evaluating the cell survival fraction against the radiation dose curve, it was evident that GNPs can catalyze ROS production, thereby debilitating tumor cells, and significantly augmenting the tumor-killing efficacy of Cs-137 radiation.88 Thus, it’s been substantiated as a promising radiosensitizer, especially for the therapeutic intervention of certain radioresistant tumor cells. Through this avenue, the tumoricidal efficacy of radiotherapy can be enhanced.
GNPs can modulate the expression of pivotal proteins in the apoptosis process, thereby regulating cell death. The Bcl-2 family plays a significant role in orchestrating apoptosis, with Bax and Bcl-2 being integral members. Bax promotes apoptosis, while Bcl-2 inhibits it. Zhang et al discovered that, compared to solely external irradiation, the combination of GNPs and external irradiation generated more ROS within cells. This ROS depletion of superoxide dismutase and glutathione, intrinsic cellular antioxidants, leads to DNA damage. Concurrently, it induces an upregulation of Bax and Caspase-3 expression in HepG2 cells and a downregulation of Bcl-2, culminating in cell apoptosis. p53 is a pivotal regulator of Bcl-2 family protein expression, suggesting that GNPs might exert their radiosensitizing effects by modulating the p53/Bcl-2 pathway. This study further underscores the paramount role of ROS in the radiosensitizing effects of GNPs.89 Jabir and associates, in their research on the cytotoxic effects of linalool-GNP (LG) and linalool-GNP-CALNN peptide conjugates (LGC) on ovarian cancer cells and the potential mechanisms inducing apoptosis, found that LG and LGC exhibited significant antiproliferative effects on SKOV-3 cells. Cytotoxicity assays revealed that LG and LGC displayed selective toxicity in cancer cells, marking them as promising compounds. They induce cell apoptosis by activating caspase-8, p53 protein, and various proteins involved in the apoptosis of ovarian cancer (SKOV-3) cells.
Influencing the Cell Cycle Process
The cell cycle is one of the primary determinants of a cell’s sensitivity to radiation. By halting or slowing the progression of cells through the cell cycle, cells maintain genomic integrity either by repairing radiation-induced damage or activating cell death when repair is unsuccessful. The sensitivity of tumor cells to radiation is closely related to their position in the cell cycle. The G2/M phase is the most sensitive, the G1 phase is moderately sensitive, the G0 phase has some resistance, and the S phase is the least sensitive.90,91 GNPs have been shown to cause cell cycle disruption, an effect dependent on various factors. Roa et al explored the mechanism by which glucose-modified gold nanoparticles enhance the radiosensitivity of human prostate cancer cells. Compared to X-rays alone, 2Gy irradiation combined with Glu-GNPs exhibited a 1.5–2.0 fold increase in growth inhibition. Analyzing cell cycle changes, Glu-GNPs induced acceleration in the G0/G1 phase and accumulation of cells in the G2/M phase. Glu-GNPs promoted an increase in the expression of cell cycle proteins cyclin B1 and cyclin E in DU-145 cells, while inhibiting the expression of p53 and Cyclin A, ultimately causing cells to rapidly pass through the G1/S phase and stall in the G2/M phase. In essence, Glu-GNPs trigger the activation of CDK kinases, leading to an accelerated cell cycle in the G0/G1 phase and accumulation in the G2/M phase, with this activation accompanied by a significant increase in sensitivity to ionizing radiation.92 On one hand, Glu-GNPs suppress the expression of p53, leading to overexpression of Cyclin E, thereby accelerating the G1/S phase. The suppression of p53 also results in an increase in Cyclin B1 expression, causing a G2/M phase block. On the other hand, Glu-GNPs inhibit the expression of Cyclin A, which can also cause a G2/M phase block. Wang et al, in their study on the radiosensitizing effects of glucose-terminated GNPs (Glu-GNPs) of different sizes (16nm and 49nm) on MDA-MB-231 cells, found that MDA-MB-231 cells absorbed more 49-nm Glu-GNPs than 16-nm Glu-GNPs. Glu-GNPs might enhance radiation effects by regulating cell cycle distribution, causing MDA-MB-231 cells to stall in the G2/M phase and inducing cell accumulation.60 Jeon et al, in their study using anti-HER2 antibody-conjugated gold nanoparticles (GNP-HER2) to investigate the induction and mechanism of selective apoptosis in G361 melanoma cells, observed an increase in cells with nuclear condensation indicative of apoptotic phenomena and sub-G1 phase cells, as well as the translocation of apoptosis-inducing factors and cytochrome c from mitochondria to the nucleus and cytoplasm. They also observed a downregulation of cell cycle proteins A, D1, E, cdk 2, cdk 4, and cdc 2 and an upregulation of p21, thereby confirming that treatment with GNP-HER 2 can induce cell cycle progression.93
Influencing Cellular Autophagy
GNPs can also impact cellular autophagy. Autophagy is a vital pathway for cellular material metabolism and the renewal of certain organelles.94 Cellular autophagy plays a significant role in various physiological and pathological processes, including cellular homeostasis, aging, immunity, tumor development, and neurodegenerative diseases.95 A basal level of autophagy is essential for maintaining intracellular stability. Ma et al found that GNPs enter cells through receptor-mediated endocytosis and accumulate in lysosomes, alkalinizing the pH within the lysosomes, weakening their degradative capacity, and also causing obstacles in the fusion of autophagosomes with lysosomes. Cells treated with GNPs showed an increase in autophagosomes, an elevated expression of the autophagy marker protein LC3II, and a blockade in the degradation of the autophagy substrate protein p62, leading to increased p62 expression. An increase in LC3II IIexpression indicates the initiation of autophagy, while p62 protein, mainly degraded through autophagy, increases, suggesting weakened autophagy. If both LC3II and P62 levels rise, it indicates normal autophagy initiation but a downstream blockage, meaning autophagosomes and lysosomes cannot fuse. This suggests that GNPs increase autophagosomes by blocking autophagic flux rather than inducing autophagy, thereby affecting cellular homeostasis.96 Joshi et al, in their exploration of the potential application of 11-mercaptoundecanoic acid-modified gold nanoparticles (∼7nm) conjugated with chloroquine in cancer treatment, found that the primary pathway for cell death was mediated by autophagy. They also demonstrated the anticancer activity of the chloroquine-gold nanoparticle conjugate (GNP-Chl) in MCF-7 breast cancer cells.97 Ding et al, in their study on the cytotoxic effects of GNPs in hypoxic renal tubular epithelial cells, found that GNP treatment can cause autophagy under normoxic conditions. Under hypoxic conditions, GNP exposure leads to the production of reactive oxygen species, loss of mitochondrial membrane potential (ΔΨM), and an increase in both apoptosis and autophagic cell death, suggesting the potential toxicity of GNPs in hypoxic HK-2 cells.98 Luo et al discovered that the cervical cancer cell line overexpresses JAK2, while quercetin-coupled GNPs can inhibit JAK2 expression, thereby inducing apoptosis and autophagy and inhibiting cervical cancer cell proliferation. This effect is achieved through the STATs-regulated Bcl-2/Caspase-3 signaling pathway and the PI3K/Akt-related GSK and mTOR signaling pathways.99
Inhibition of DNA Repair
Radiation therapy can induce various DNA damages, including single-strand breaks (SSB), double-strand breaks (DSB), DNA-protein crosslinks, and DNA base modifications. The repair of these damages is crucial for cell survival. Failure to repair DNA DSBs affects genomic stability and can lead to cell death in multiple ways. The phosphorylated histone variant γ-H2AX and p53 binding protein 1 (53BP1) are considered the earliest sensitive markers. The dynamic monitoring of γ-H2AX and 53BP1 post GNP radiation damage to DNA repair has been validated in numerous experiments.100–102 Thus, the inhibition of DNA repair is believed to be another vital biological mechanism of GNP radiosensitization. Chithrani et al observed an increase in the number of γ-H2AX and 53BP1 foci 4h and 24h after irradiating HeLa cells incubated with 50nm citrate-GNPs at 220 kVp and 6 MV energies. Based on the increased residual breaks in the presence of nanoparticles, it’s speculated that this is due to the inhibition or delay of DNA repair, considered another mechanism of radiosensitization.49 Additionally, GNPs have been found to induce DSBs in hepatocellular carcinoma cells post-radiation exposure. Residual damage was also observed when cells were irradiated in the presence of nano-gold, indicating an impact on cellular repair mechanisms.89 Currently, there is no consensus in the literature regarding the specific role of GNPs in the DNA damage repair process. Further studies are needed on the different properties of GNPs, irradiation conditions, and the biological responses of various cell lines to DNA damage and repair to elucidate the potential mechanisms by which GNPs affect DNA damage responses.
Other Biological Mechanisms
In addition, GNPs can exert a range of effects by acting on tumor blood vessels. Amato et al simulated and evaluated the dose-enhancing effect of GNPs during treatment with X-rays operated at 150kV (E= 55keV and Emax= 150 keV) using the Geant4 Monte Carlo code. They also studied the role of GNPs in anti-tumor processes from the perspectives of anti-angiogenesis and cytotoxicity. The study found that the dose enhancement factor (DEF) increases with the depth of GNPs, and the relative difference between the DEF at the surface (depth= 0cm) and the deepest target location (depth=5cm) increases with GNP concentration. The radial average DEF distribution around the vessels is closely related to the radial distribution of GNP concentration. From the experimental results, it is inferred that the diffusion mechanism (range and radial distribution) is significant for anti-angiogenesis (controlled by the dose to the capillary endothelium) and cytotoxicity to live tumor cells (achieved by giving a minimum lethal dose to all tumor cells, especially those at the tumor margin). Therefore, the potential role of GNP diffusion in anti-angiogenesis and cytotoxic dose enhancement is further explored.83 Joh et al studied the anti-tumor effects of RT combined with PEGylated gold nanoparticles (PEGylated-GNPs) in experiments on brain tumors like glioblastoma multiforme in cell culture experiments and animal models. The results showed that GNPs significantly increase ionizing radiation-induced cell DNA damage in human GBM-derived cell lines, leading to a decrease in clonogenic survival. The combination of GNPs and RT also resulted in a significant increase in DNA damage to the brain vasculature and the survival time of mice with in-situ GBM tumors. Subsequent in vitro experiments confirmed that the combination of GNPs and RT led to a significant increase in DNA damage in brain-derived endothelial cells. Previous treatment of mice with brain tumors resulted in increased extravasation and tumor deposition of GNPs, suggesting that the radiation-induced blood-brain barrier (BBB) can be used to improve GNP tumor tissue targeting, further optimizing GNP radiosensitization of brain tumors. These encouraging results together indicate that GNPs can be effectively integrated into the RT treatment process of brain tumors, with potential benefits produced by increased radiosensitization of tumor cells to the tumor-associated vascular system.53 Lin et al studied the induction of different vascular injuries and radiosensitization effects of gold nanoparticles (GNP) under proton, megavolt (MV) photon, and kilovolt (kV) photon irradiation conditions. The results showed that the addition of GNPs could potentially cause high dose peaks and enhance radiotherapy by inducing vascular system damage. If GNPs actively accumulate at the walls of the tumor vascular system, vascular system damage is significantly increased. Due to the photoelectric effect, kilovolt photon irradiation causes stronger radiosensitization; while MV photons and protons can cause a high local dose increase (>15 Gy) to the vascular area, indicating that the combination with GNPs can potentially help disrupt the function of vessels in the tumor.103 The antagonist peptide of EGFA/VEGFB, called VGB3, can recognize and neutralize VEGFR 1 and VEGFR 2 on endothelial cells and tumor cells, thereby inhibiting angiogenesis and tumor growth. Zanjanchi et al studied the conjugation of VGB3 to GNPs to enhance its efficacy and extend the interval between treatments. They found that GNP-VGB3 more effectively induces cell cycle arrest, excessive ROS production, and apoptosis, and inhibits the proliferation and migration of endothelial cells and tumor cells than unconjugated VGB3 or GNPs. The results proved that conjugation with GNPs not only improved the efficacy of VGB3 but also enhanced anti-angiogenic and anti-tumor activity, extended the interval between treatments without increasing side effects, indicating that GNP-VGB3 provides a new direction for clinical anti-tumor treatment.104
Chemical Mechanisms
Compared to the physical and biological pathways of radiation enhancement with GNPs, the chemical enhancement mechanism has not been extensively studied. Current research indicates that even at low concentrations of Au, there is an impact on radiosensitization, highlighting the importance of chemical contributions to radiation enhancement. The “chemical mechanism” of GNP radiosensitization mainly involves participating in free radical reactions that repair damage or by weakening DNA bonds, making DNA more susceptible to radiation-induced damage.
Contrary to the widely accepted view that gold nanoparticles are chemically inert, an increasing number of studies report that the surface of gold nanoparticles is electronically active and can catalyze chemical reactions.105 Research has shown that small particles with a large surface area (<5nm) have demonstrated more active catalytic activity by mediating electron transfer from surface-bound donor groups to O2 to produce superoxide radicals through GNP.106 The unique catalytic properties of AuNP are attributed to the small size and high curvature of the nanoparticles, and the change in the electronic configuration of surface atoms allows the generation of free radicals at the reactive surface of AuNPs.107 Current experiments have confirmed the catalytic activity of the AuNP surface, and gold nanoparticles mainly interact with molecular oxygen on the surface, promoting surface-mediated electron transfer to produce ROS.108,109 For example, Ito et al showed that 15 nm citrate gold nanoparticles can enhance the cytotoxic effect of 5’-aminolevulinic acid (5’ALA) by enhancing the production of reactive oxygen species (ROS).110 The combination of 5’-ALA with gold nanoparticles achieves catalysis of superoxide and hydroxyl radicals in two steps: 5’-ALA interacts with the surface of gold nanoparticles and then binds with molecular oxygen to produce ROS. This proves that the catalytic action of GNP mainly occurs through surface interaction with molecular oxygen, facilitating surface-mediated electron transfer to produce ROS. The increase in ROS is closely related to the photon and Auger electron emission of GNP and the secondary radiolysis of water, leading to indirect damage to DNA, proteins, and lipid membranes through oxidative defense, thereby inducing cell apoptosis/death. Further research on the relationship between GNP particle size and ROS production found that smaller AuNPs with a larger surface area produce higher levels of ROS, confirming the catalytic activity of the AuNP surface.111 In summary, based on the above research data, it can be concluded that GNP achieves chemical enhancement of radiation by catalyzing free radical reactions and increasing ROS production, ultimately enhancing and repairing radiation-induced cell damage.
Factors Influencing Radiosensitization by Gold Nanoparticles
There are several factors that influence the radiosensitization effect of gold nanoparticles (GNPs), such as cell type, type of radiation, radiation dose, particle size, shape, surface functionalization, concentration, biological distribution, localization, and the anticancer drugs used in combination. This indicates that optimizing different parameters of GNPs could potentially achieve high radiation efficiency.
The size of the gold nanoparticles affects their distribution and accumulation in tumor tissues. Smaller particle sizes can enhance permeability and absorption in tumor tissues.112 However, particles that are too small might be cleared rapidly, reducing the sensitizing effect. Typically, particle sizes between 10–100 nanometers are considered optimal. Recent in vivo studies have shown that small GNPs (less than 6nm) are cleared through the kidneys within minutes. For monolayer cells, the smaller the GNP, the more GNPs are found in each cell. Due to their ultra-small nanostructure, 2nm and 6nm nanoparticles showed high levels of accumulation in tumor tissues in mice. Surprisingly, both 2nm and 6nm GNPs were distributed in both the cytoplasm and nucleus of cancer cells both in vitro and in vivo, while 15nm GNPs were only found in the cytoplasm, indicating that smaller GNPs have higher penetration capabilities and achieve high levels of tumor accumulation.113 Gold nanoparticles have been proven to enhance RT-induced DNA damage and cytotoxicity in MCF7 breast cancer cells. Janic et al studied the effects of AuNP on RT cytotoxicity, survival, and immune modulation of the tumor microenvironment (TME) in a human triple-negative breast cancer (TNBC) xenograft mouse model, as well as the importance of nanoparticle size in these effects. The results showed that mice treated with either 4nm or 14nm AuNPs exhibited significant tumor growth delay. Compared to 4nm AuNPs, 14nm AuNPs significantly enhanced RT, indicating size-dependent RT enhancement by AuNPs. Both sizes of AuNPs enhanced RT-induced immunogenic cell death (ICD), but significant macrophage infiltration was coupled in mice pretreated with 14nm AuNPs.114
The shape of gold nanoparticles also influences their biological distribution and cellular uptake.112 Ma et al explored the radiosensitizing effects of gold nanostructures in cancer radiation therapy. The researchers synthesized Au nanostructures of different shapes but similar average sizes (~50nm), including spherical gold nanoparticles (GNP), gold nanostars (GNS), and gold nanorods (GNR), and functionalized them with poly(ethylene glycol) (PEG) molecules. Although all these Au nanostructures were coated with the same PEG molecules, they exhibited significant differences in cellular uptake behavior. In experiments with human oral epidermoid carcinoma (KB) cells, after incubating the three nanostructures with KB cancer cells for 24 hours, spherical gold nanoparticles showed the highest cellular response and uptake, compared to gold nanostars and gold nanorods (based on the same gold mass). The sensitization ratios (Sers) calculated for GNP, GNSs, and GNRs treatments were 1.62, 1.37, and 1.21, respectively, indicating that spherical gold nanoparticles exhibited superior anticancer efficiency and radiation enhancement effects under X-ray irradiation.115 In a study by Pakravan et al, where they investigated the influence of GNP geometric structures (star-shaped, hollow, rod-shaped, cage-like, spherical, Fe-Au, and Si-Au core-shell) as photothermal sensors on cellular uptake and photothermal therapy (PTT) efficacy, it was found that although all these GNPs could absorb near-infrared light and convert it into thermal energy, gold nanostars exhibited the lowest cellular toxicity, highest cellular uptake, and highest heat generation compared to other structures.116 This demonstrates that the geometric shape of GNPs affects cellular uptake, heat generation, and the pathways of cellular destruction through apoptosis. Different shapes of GNPs can enhance the radiosensitizing effects for various types of cancers.
The surface modification of gold nanoparticles can alter their biocompatibility, stability, and targeting capabilities. Surface modification and functionalization of gold nanoparticles can enhance cellular uptake and improve radiosensitizing effects. For instance, polyethylene glycol (PEG) is the most widely used surface engineering strategy to optimize the properties of nanoparticles, including their pharmacokinetic characteristics, active targeting, and mucosal permeability. It enhances biocompatibility, while modifications with antibodies or peptides can enhance targeting.117,118 The steric hindrance produced by surface PEGylation also prevents nanoparticle aggregation, increasing their colloidal stability. Enferadi et al studied the uptake, toxicity, and radiosensitivity of GNP-PEG-cRGDfKs in ALTS1C1 cells exposed to protons, kilovolt photons, and megavolt photons. The in vitro uptake and toxicity of GNPs in AML12 liver cells and RAW 264.7 macrophage cell lines were evaluated in hepatocytes and Kupffer cells. The results showed that under radiation with protons, kilovolt photons, and megavolt photons, the observed sensitization enhancement ratios and dose enhancement factors were 1.21–1.66 and 1.14–1.33, respectively. This indicates that ultra-small GNP-PEG-cRGD can be considered as a radiosensitizer, and further efforts can increase GNP uptake in tumors while reducing uptake in off-target organs.119
In conclusion, the factors influencing the radiosensitizing effects of gold nanoparticles include particle size, shape, surface modification, concentration, LET (Linear Energy Transfer), redox environment, cell type, radiation dose and fractionation, combined drug treatment, and temporal factors, among others. A deeper understanding and research into these factors will help optimize the application of gold nanoparticles in radiation therapy, enhancing therapeutic outcomes and minimizing side effects.
The Nuanced Radiosensitizing Roles of Alternative Nanoparticles in Radiotherapy
Bismuth-Based Nanomaterials
In recent years, an increasing variety of bismuth (Bi)-based nanomaterials have been developed for research in the biomedical field.120 Bismuth is an element of the V main group and the sixth period of the periodic table, with an atomic number of 83. It is the heaviest stable element and mainly exists in the form of +3 valence in compounds. Due to the strong X-ray absorption capability and photothermal conversion ability of Bi-based nanomaterials, they have shown promising prospects in the field of tumor therapy.
Song et al prepared a PEG-functionalized hollow Bi₂Se₃ nanoparticle loaded with perfluorocarbon (PFC) as an oxygen carrier through a cation exchange method.121 Cellular and animal experiments demonstrated that under the action of 808nm near-infrared light, the nanoparticles produce heat and release oxygen, aiming to counteract the radiation resistance induced by tumor hypoxia. In the PEG-Bi2Se3 @PFC@ O2 system, Bi2Se3 itself can act as a radiosensitizer to enhance the efficiency of RT, while PFC can serve as an oxygen carrier to moderately improve tumor oxygenation. More notably, due to the strong near-infrared absorption of Bi2Se3, near-infrared laser irradiation can generate a pronounced photothermal effect, triggering a burst release of oxygen, thereby significantly promoting tumor oxygenation and further overcoming radiation resistance associated with hypoxia. Furthermore, due to its high atomic number, bismuth-based nanomaterials possess a strong radiation absorption capability for X-rays, concentrating radiation energy locally within the tumor. Coupled with the photothermal conversion characteristics of bismuth-based materials, they hold promising potential in tumor hyperthermia and radiation therapy.
Recognizing the potential of bismuth nanoparticles (BNPs) as radiosensitizers in tumor radiotherapy, Chen et al developed bismuth nanorods coated with mesoporous silica and camouflaged with platelet membrane (PM), termed BMN-R@PM.122 Compared to BMSNR, the PM camouflage enhanced the tumor-targeting ability of the bismuth-based nanomaterial, facilitating more precise tumor radiotherapy sensitization. The study revealed that after treatment with 808nm near-infrared radiation, BMN-R@PMs altered the cell cycle distribution of mouse 4T1 cancer cells, decreasing the proportion of cells in the S phase and increasing those in the G2/M phase, enhancing the radiosensitivity of the 4T1 cancer cells. The research demonstrated that BMN-R@PMs effectively eradicated cancer cells through the combined action of photothermal therapy and in vivo radiotherapy, significantly improving the survival rate of mice bearing 4T1 tumors. The synergistic therapeutic effect was superior to treatments using either photothermal therapy or radiotherapy alone. The BMN-R@PM multifunctional bismuth-containing nanoparticle platform is an integrated platform with tumor targeting, photothermal therapy, immune evasion, and radiosensitization capabilities, representing an excellent radiosensitizing nanoreagent platform.
Zeng et al synthesized multifunctional bismuth sulfide (Bi₂S₃) nanoparticles and constructed a core-shell Bi₂S₃@Ce₆-CeO₂ nanocomposite for research in near-infrared-triggered photothermal therapy.123 As a direct narrow-bandgap n-type semiconductor, Bi₂S₃ nanomaterials exhibit significant near-infrared-triggered photothermal effects. The study introduced the photosensitizer Ce₆ with good photodynamic properties and CeO₂ with O₂ release characteristics, designing the core-shell structure of Bi₂S₃@Ce₆-CeO₂ nanocomposites (Bi₂S₃@Ce₆-CeO₂NCs). Bi₂S₃@Ce₆-CeO₂NCs demonstrated significant synergistic photothermal and photodynamic therapeutic effects both in vitro and in vivo, proving their potential application in radiosensitization of tumor thermoradiotherapy.
To create a highly biocompatible nanoparticle platform capable of synergistic therapy and real-time imaging, researchers synthesized a novel Au@Bi2S3 core-shell nanosphere (NB) with Au nanorods as the core (Au@Bi2S3 NBs).124 The combination of Au nanorods with a Bi2S3 thin film endowed the Au@Bi2S3 nanorods with ultra-high photothermal conversion efficiency, excellent photoacoustic imaging, and high CT performance. In vitro and in vivo studies showed that Au@Bi2S3-PVP nanoparticles possess a range of characteristics required for tumor treatment, including extremely low toxicity, excellent biocompatibility, high drug-loading capacity, precise tumor targeting, and effective accumulation. Subsequently, the poly(N-vinylpyrrolidone)-modified Au@Bi2S3 NBs (Au@Bi2S3-PVP NBs) successfully loaded the anticancer drug doxorubicin (DOX), achieving a satisfactory pH-sensitive release profile, revealing the immense potential of Au@Bi2S3-PVP NBs as drug carriers to deliver DOX to cancer cells. Au@Bi2S3-PVP NBs, serving as contrast-enhancing probes and therapeutic agents, provided outstanding NIR-triggered multimodal PT/PA/CT imaging-guided PTT and effectively inhibited the growth of HepG 2 liver cancer cells through synergistic chemotherapy/PT treatment. Hence, Au@Bi2S3 NBs may emerge as promising nanotheranostics for PT/PA/CT imaging.
Magnetic Nanomaterials
Over the past decade, research has significantly advanced the therapeutic potential of magnetic nanoparticles (MNPs) as nanomedicines for cancer.125 Due to the nanoscale size effect, magnetic nanoparticles exhibit physicochemical properties that are distinctly different from those of macroscopic magnetic materials. Emerging magnetic nanoparticles offer various advantages, such as broader working temperatures, wider size ranges, lower toxicity, simpler preparation methods, and reduced production costs.126 MNPs possess a range of unique and superior physicochemical properties, holding immense potential in medical applications and the biomedical field.127,128
Magnetic nanomaterials, under the unique electron paramagnetic resonance (EPR) characteristic inherent to nanomaterials, can be targeted more to tumor sites through nanoparticle modifications. Additionally, with the assistance of an exogenous magnetic field, the targeting of nanomaterials to tumor cells can be enhanced. Under the influence of an exogenous alternating magnetic field, electromagnetic energy is converted into thermal energy through magnetic hysteresis and relaxation effects, leading to intracellular hyperthermia. Combined with precise radiotherapy, this approach not only utilizes hyperthermia to radiosensitize tumor tissues but also avoids thermal damage to normal tissues, achieving true precision thermoradiotherapy.
Superparamagnetic iron oxide nanoparticles (SPIONs) are a novel tool suitable for numerous applications, including magnetic targeting, drug delivery, gene delivery, hyperthermia, cell tracking, or multifunctionality. Marekova et al investigated SPIONs targeted to tumor cell proteins or the tumor vascular system as magnetic resonance imaging contrast agents for tumors.129 In mouse models, SPIONs have delivered drugs to GB tumors. Beyond imaging or drug delivery targeting tumor cells, SPIONs have also proven effective for targeted hyperthermia. Moving forward, researchers have conducted human trials on various modes of SPION use, providing crucial scientific foundations for further preclinical and clinical experiments.
Lyu et al synthesized a Fe₃O₄@MnO₂ core-shell magnetic particle and combined it with glucose oxidase (GOX) for radiosensitization studies.130 Glucose is oxidized by GOX, producing an excess of H₂O₂ in the acidic extracellular microenvironment. The MnO₂ shell reacts with H₂O₂ to generate O₂, overcoming tumor hypoxia. Simultaneously, intracellular glutathione (GSH), which limits the effect of radiotherapy, can also be oxidized by the MnO₂ shell. The Fe₃O₄ core possesses superior magnetic hyperthermia properties and excellent magnetic targeting capabilities. This study indicates that Fe₃O₄·MnO₂ is a highly biocompatible thermoradiotherapy sensitizing material with magnetic targeting effects.
Meidanchi et al successfully synthesized magnesium-doped spinel copper ferrite superparamagnetic nanoparticles, Mg(1-x)CuxFe₂O₄ SPMNPs (where x ranges from 0.2 to 0.8), via a hydrothermal method and conducted cytological studies on these nanoparticles as nanoradiosensitizers in MCF-7 human breast cancer cells.131 Mg(1-x)CuxFe₂O₄ SPMNPs were exposed to human breast cancer cells MCF-7 at different concentrations of 0.1, 1.0, 10.0, and 100.0μg/mL, and the cytotoxic effects and cell viability of the breast cancer cells were tested before and after radiotherapy. The results showed that Mg(1-x)CuxFe₂O₄ SPMNPs, with x values at concentrations of 0.1, 1.0, and 10.0μg/mL, exhibited no significant cytotoxicity. By increasing the Cu content and concentration, the cell destruction capability of MCF-7 human breast cancer cells post X-ray irradiation was enhanced. The superparamagnetic properties of Mg(1-x)CuxFe₂O₄ SPMNPs are targeted and cleared only through an external magnetic field. Under their superparamagnetic characteristics, their excellent magnetic hyperthermia performance can also serve as an effective targeted magnetic hyperthermia carrier. The study suggests that Mg(1-x)CuxFe₂O₄ SPMNPs with x=0.2 (10μg/mL) and x=0.6 (1μg/mL) can be further researched and applied as nanothermoradiotherapy sensitizers.
Selenium Nanomaterials
Selenium is an essential trace element, integral to numerous vital processes, ensuring the proper function of the immune system and playing a pivotal role in human health. Deficiencies in selenium correlate with a myriad of physiological ailments, including an elevated risk of cancer onset. In recent years, the discovery of antitumor drugs based on the essential trace element selenium (Se) has heralded promising prospects for cancer therapy. Notably, selenium nano-drugs (Se nanoparticles; SeNPs) exhibit superior bioavailability, antioxidative activity, and diminished toxicity compared to inorganic selenium (Inorg-Se) and organic selenium (Org-Se). Over the past decade, SeNPs have garnered extensive attention due to their potential applications in pharmacology. SeNPs can also serve as drug delivery vectors, adeptly modulating protein and DNA biosynthesis and protein kinase C activity, thereby inhibiting cancer cell proliferation. Furthermore, SeNPs effectively activate antigen-presenting cells, stimulate cellular immunity, modulate both innate and adaptive immunity, and bolster cancer immunotherapy, as depicted in Figure 5. Dana et al endeavored to investigate the inhibitory effects of chitosan-coated selenium nanoparticles (Cs-SeNPs) on the proliferation, migration, and invasiveness of GBM cells. They developed chitosan-coated SeNPs (Cs-SeNPs) to further stabilize SeNPs and assess their impact on glioma cells, concurrently treating GBM cells with a combination of Cs-SeNPs and the chemotherapy drug 5-fluorouracil (5-FU) to observe cell growth in a 3D tumor spheroid model. They also evaluated the influence of Cs-SeNPs on the sensitivity of glioma cells to the chemotherapy drug 5-fluorouracil (5-FU), offering a novel alternative therapeutic strategy for GBM. The results revealed that Cs-SeNPs effectively suppressed GBM cell proliferation, migration, and invasion, and the use of Cs-SeNPs significantly heightened the sensitivity of GBM cells to 5-FU.132
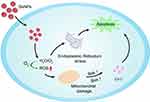 |
Figure 5 The intricate mechanisms of selenium nanoparticles. |
Sonkusre et al delved into exploring less toxic forms of selenium to supplement its potential high anticancer activity. The findings revealed that biogenic selenium nanoparticles derived from lichen bacillus induced necrotic apoptosis and necrosis in LNCaP-FGC cells at a minimal concentration of 2 μg Se/mL, without compromising RBC integrity. Subsequent administration of these endotoxin-free selenium nanoparticles at tenfold concentration (50 mg Se/kg body weight) to C3 H/HeJ mice significantly reduced toxicity. The study suggests that biogenic SeNPs, while inducing cell apoptosis and necrosis, might serve as a safer form of selenium supplement, exhibiting effective anticancer activity.133
The advantages of SeNPs render them superior to organic nanoparticles or inorganic metal nanoparticles in drug/gene delivery. Hence, SeNPs have progressively evolved into one of the most promising carriers for chemotherapeutic drugs or genes.134 To delve deeper into the issue of drug carriers lacking tumor-targeting capabilities, Wang et al, in their study, employed RGDfC peptide-modified selenium nanoparticles (SeNPs) to prepare biocompatible siRNA carriers (R-SeNPs). They loaded MEF 2D-siRNA onto R-SeNPs, fabricating functionalized selenium nanoparticles R-Se@ MEF 2D-siRNA, introduced into SKOV 3 cells for ovarian cancer treatment. The chemical properties of R-SeNPs were characterized, and the anticancer efficacy and related mechanisms of R-Se@ MEF 2D-siRNA were further explored. In vitro tests revealed that R-Se@ MEF 2D-siRNA significantly inhibited the proliferation of SKOV 3 cells and further induced their apoptosis. Moreover, the excessive production of reactive oxygen species (ROS) during the study indicated that mitochondrial dysfunction and ROS generation play a pivotal role in R-Se@ MEF 2D-siRNA-induced SKOV 3 cell apoptosis. In vivo experiments demonstrated that R-Se@ MEF 2D-siRNA primarily exhibited robust antitumor activity by inhibiting tumor cell proliferation and inducing apoptosis in tumor-bearing nude mice, with minimal side effects, offering a novel strategy for clinical treatment of ovarian cancer.135
The use of125 I particles in tumor radiotherapy offers advantages such as low dosage and continuous irradiation, presenting superior long-term efficacy and fewer side effects compared to traditional X-ray radiotherapy. However, its clinical application still faces certain limitations. Thus, exploring sensitizers that can enhance the sensitivity of cancer cells to 125I particles is of paramount importance. Selenium nanoparticles (SeNPs) have demonstrated significant potential in cancer chemotherapy and as drug carriers. In this study, Chan et al discovered that, based on the Auger electron effect and Compton effect of Se atoms, tumor-targeted SeNPs combined with 125I particles synergistically inhibited cancer cell growth and colony formation by inducing cell apoptosis and cell cycle arrest. Further research revealed that the combined treatment effectively activated excessive intracellular ROS production, modulated P53-mediated DNA damage, apoptosis signaling pathways, and the phosphorylation of MAPKs, while simultaneously inhibiting cancer cell self-repair. Consequently, the combination of SeNPs with 125I particles holds promise as a safe and effective clinical application strategy for next-generation tumor radiochemotherapy. Although significant progress has been made in the research of SeNPs in recent years, there remain challenges for future applications, such as enhancing the storage stability of SeNPs, understanding the synergistic interaction mechanisms between SeNPs and other chemotherapy drugs, and addressing potential long-term cytotoxicity during application.
Conclusion and Outlook
The theoretical studies, cellular experiments, and animal tests based on nano-targeted thermoradiotherapy have shown promising application prospects.136,137 Under the technology of nano-thermoradiotherapy sensitization, targeted photothermal therapy has been clinically tested. By using nano-targeted photothermal therapy as a sensitizing carrier and combining it with radiotherapy, thermoradiotherapy sensitization has been achieved.138,139 With the known mechanisms of thermoradiotherapy sensitization and the enhanced radiation energy absorption of high atomic number nanoparticles, nano-targeted thermoradiotherapy sensitization technology is now ready for clinical application.
Gold nanomaterials, endowed with unique optical, electronic, and chemical properties, hold vast potential for applications in the realm of biomedicine. Notably, their high atomic number facilitates the generation of a plethora of secondary electrons under radiation, thereby amplifying the effects of radiotherapy. Radiotherapy stands as one of the primary modalities for cancer treatment; however, certain tumors exhibit resistance to it. Gold nanomaterials can serve as radiosensitizers, enhancing the efficacy of radiotherapy. When these nanomaterials are delivered to tumor tissues and subjected to radiation, they bolster the cytotoxic impact of the radiation on tumor cells. Beyond gold nanomaterials, a spectrum of multifunctional nanomaterials, such as bismuth-based, selenium, and magnetite nanomaterials, are under exploration for radiosensitization. Bismuth-based nanomaterials, owing to their high atomic number element characteristics, effectively absorb X-rays, positioning them as potent radiosensitizers to augment radiotherapeutic outcomes. Magnetic nanomaterials serve as contrast agents for magnetic resonance imaging (MRI), while magnetite nanomaterials can be employed as contrast agents for ultrasonography, ensuring precise localization and concurrently acting as radiosensitizers to enhance therapeutic outcomes. Selenium, leveraging its antioxidative properties, shields normal cells from radiation-induced damage. These nanomaterials not only amplify radiotherapeutic effects but also find utility in drug delivery, magnetic resonance imaging, and a myriad of other biomedical applications, showcasing superior biocompatibility and reduced toxicity.
With advancements in nanotechnology, it is anticipated that an array of nanomaterials will be devised for radiosensitization and other biomedical applications. Amidst the rapid evolution of modern nanotechnology in tumor radiotherapy, the construction of integrated nano-agents for combined thermoradiotherapy has emerged, enabling precise intracellular radiosensitization and enhancing the therapeutic gain of radiotherapy. However, the transition of most inorganic nanomaterials from research to clinical application still grapples with potential long-term toxicity due to prolonged retention in the human body. Furthermore, research into the biodistribution, toxicity, and excretion of nanomaterials is poised to become more comprehensive, ensuring safer and more effective clinical applications. Future endeavors may potentially surmount the myriad challenges currently impeding tumor radiotherapy, paving the way for novel opportunities in advancing radiotherapeutic techniques.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mullard A. Addressing cancer’s grand challenges. Nat Rev Drug Discov. 2020;19(12):825–826. doi:10.1038/d41573-020-00202-0
2. Dolgin E. Cancer’s new normal. Nature Cancer. 2021;2(12):1248–1250. doi:10.1038/s43018-021-00304-7
3. Minniti G, Goldsmith C, Brada M. Radiotherapy. Handb Clin Neurol. 2012;104:215–228.
4. Schaue D, McBride WH. Opportunities and challenges of radiotherapy for treating cancer. Nat Rev Clin Oncol. 2015;12(9):527–540. doi:10.1038/nrclinonc.2015.120
5. Chen LC, Lin HY, Hung SK, Chiou WY, Lee MS. Role of modern radiotherapy in managing patients with hepatocellular carcinoma. World J Gastroenterol. 2021;27(20):2434–2457. doi:10.3748/wjg.v27.i20.2434
6. Steers JM, Fraass BA. IMRT QA and gamma comparisons: the impact of detector geometry, spatial sampling, and delivery technique on gamma comparison sensitivity. Med Phys. 2021;48(9):5367–5381. doi:10.1002/mp.14997
7. Kong V, Hansen VN, Hafeez S. Image-guided adaptive radiotherapy for bladder cancer. Clin Oncol. 2021;33(6):350–368. doi:10.1016/j.clon.2021.03.023
8. Shampain KL, Hackett CE, Towfighi S, et al. SBRT for HCC: overview of technique and treatment response assessment. Abdom Radiol. 2021;46(8):3615–3624. doi:10.1007/s00261-021-03107-7
9. Arneth B. Tumor Microenvironment. Medicina. 2019;56(1). doi:10.3390/medicina56010015
10. Buckley AM, Lynam-Lennon N, O’Neill H, O’Sullivan J. Targeting hallmarks of cancer to enhance radiosensitivity in gastrointestinal cancers. Nat Rev Gastroenterol Hepatol. 2020;17(5):298–313. doi:10.1038/s41575-019-0247-2
11. Ashrafizadeh M, Farhood B, Eleojo Musa A, Taeb S, Najafi M. Damage-associated molecular patterns in tumor radiotherapy. Int Immunopharmacol. 2020;86:106761. doi:10.1016/j.intimp.2020.106761
12. Zhang C, Yan L, Gu Z, Zhao Y. Strategies based on metal-based nanoparticles for hypoxic-tumor radiotherapy. Chem Sci. 2019;10(29):6932–6943.
13. Siddique S, Chow JCL. Recent advances in functionalized nanoparticles in cancer theranostics. Nanomaterials. 2022;12:16.
14. Wang Y, Zou L, Qiang Z, Jiang J, Zhu Z, Ren J. Enhancing targeted cancer treatment by combining hyperthermia and radiotherapy using Mn-Zn ferrite magnetic nanoparticles. ACS Biomat Sci Engin. 2020;6(6):3550–3562. doi:10.1021/acsbiomaterials.0c00287
15. Fan W, Tang W, Lau J, et al. Breaking the Depth Dependence by Nanotechnology-Enhanced X-Ray-Excited Deep Cancer Theranostics. Advan Mat. 2019;31(12):e1806381. doi:10.1002/adma.201806381
16. DuRoss AN, Neufeld MJ, Rana S, Thomas CR, Sun C. Integrating nanomedicine into clinical radiotherapy regimens. Adv Drug Deliv Rev. 2019;144:35–56. doi:10.1016/j.addr.2019.07.002
17. Mirzaghavami PS, Khoei S, Khoee S, Shirvalilou S, Mahdavi SR, Pirhajati Mahabadi V. Radio-sensitivity enhancement in HT29 cells through magnetic hyperthermia in combination with targeted nano-carrier of 5-Flourouracil. Mater Sci Eng C Mater Biol Appl. 2021;124:112043. doi:10.1016/j.msec.2021.112043
18. Xie J, Gong L, Zhu S, Yong Y, Gu Z, Zhao Y. Emerging strategies of nanomaterial-mediated tumor radiosensitization. Advan Mat. 2019;31(3):e1802244. doi:10.1002/adma.201802244
19. Dunne M, Regenold M, Allen C. Hyperthermia can alter tumor physiology and improve chemo- and radio-therapy efficacy. Adv Drug Deliv Rev. 2020;163–164:98–124. doi:10.1016/j.addr.2020.07.007
20. Gavazzi S, van Lier A, Zachiu C, et al. Advanced patient-specific hyperthermia treatment planning. Int J Hyperthermia. 2020;37(1):992–1007. doi:10.1080/02656736.2020.1806361
21. Minaei SE, Khoei S, Khoee S, Mahdavi SR. Sensitization of glioblastoma cancer cells to radiotherapy and magnetic hyperthermia by targeted temozolomide-loaded magnetite tri-block copolymer nanoparticles as a nanotheranostic agent. Life Sci. 2022;306:120729. doi:10.1016/j.lfs.2022.120729
22. Zhu L, Altman MB, Laszlo A, et al. Ultrasound Hyperthermia Technology for Radiosensitization. Ultrasound Med Biol. 2019;45(5):1025–1043. doi:10.1016/j.ultrasmedbio.2018.12.007
23. Datta NR, Ordóñez SG, Gaipl US, et al. Local hyperthermia combined with radiotherapy and-/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev. 2015;41(9):742–753. doi:10.1016/j.ctrv.2015.05.009
24. Elming PB, Sørensen BS, Oei AL, et al. Hyperthermia: the optimal treatment to overcome radiation resistant hypoxia. Cancers. 2019;11:1. doi:10.3390/cancers11010060
25. Eppink B, Krawczyk PM, Stap J, Kanaar R. Hyperthermia-induced DNA repair deficiency suggests novel therapeutic anti-cancer strategies. Inter J Hyperth. 2012;28(6):509–517. doi:10.3109/02656736.2012.695427
26. Chen Y, Yang J, Fu S, Wu J. Gold nanoparticles as radiosensitizers in cancer radiotherapy. Int J Nanomedicine. 2020;15:9407–9430. doi:10.2147/IJN.S272902
27. Wang H, Mu X, He H, Zhang XD. Cancer Radiosensitizers. Trends Pharmacol Sci. 2018;39(1):24–48. doi:10.1016/j.tips.2017.11.003
28. Xu W, Lin Q, Yin Y, et al. A review on cancer therapy based on the photothermal effect of gold nanorod. Curr Pharm Des. 2019;25(46):4836–4847. doi:10.2174/1381612825666191216150052
29. Siddique S, Chow JCL. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials. 2020;10:9.
30. Jabeen M, Chow JCL. Gold Nanoparticle DNA Damage by Photon Beam in a Magnetic Field: a Monte Carlo Study. Nanomaterials. 2021;11:7.
31. Moloudi K, Samadian H, Jaymand M, Khodamoradi E, Hoseini-Ghahfarokhi M, Fathi F. Iron oxide/gold nanoparticles-decorated reduced graphene oxide nanohybrid as the thermo-radiotherapy agent. IET Nanobiotechnol. 2020;14(5):428–432. doi:10.1049/iet-nbt.2020.0106
32. Sood A, Dev A, Sardoiwala MN, et al. Alpha-ketoglutarate decorated iron oxide-gold core-shell nanoparticles for active mitochondrial targeting and radiosensitization enhancement in hepatocellular carcinoma. Mater Sci Eng C Mater Biol Appl. 2021;129:112394. doi:10.1016/j.msec.2021.112394
33. Kadkhoda J, Tarighatnia A, Barar J, Aghanejad A, Davaran S. Recent advances and trends in nanoparticles based photothermal and photodynamic therapy. Photodiagnosis Photodyn Ther. 2022;37:102697. doi:10.1016/j.pdpdt.2021.102697
34. Singh P, Pandit S, Mokkapati V, Garg A, Ravikumar V, Mijakovic I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int J Mol Sci. 2018;19:7. doi:10.3390/ijms19071979
35. Nejabat M, Samie A, Ramezani M, Alibolandi M, Abnous K, Taghdisi SM. An overview on gold nanorods as versatile nanoparticles in cancer therapy. J Control Release. 2023;354:221–242. doi:10.1016/j.jconrel.2023.01.009
36. Yang S, Han G, Chen Q, et al. Au-Pt nanoparticle formulation as a radiosensitizer for radiotherapy with dual effects. Int J Nanomedicine. 2021;16:239–248. doi:10.2147/IJN.S287523
37. Chen Y, Feng X. Gold nanoparticles for skin drug delivery. Int J Pharm. 2022;625:122122. doi:10.1016/j.ijpharm.2022.122122
38. Niikura K, Iyo N, Matsuo Y, Mitomo H, Ijiro K. Sub-100 nm gold nanoparticle vesicles as a drug delivery carrier enabling rapid drug release upon light irradiation. ACS Appl Mater Interfaces. 2013;5(9):3900–3907. doi:10.1021/am400590m
39. Gholipourmalekabadi M, Mobaraki M, Ghaffari M, et al. Targeted drug delivery based on gold nanoparticle derivatives. Curr Pharm Des. 2017;23(20):2918–2929. doi:10.2174/1381612823666170419105413
40. Siddique S, Chow JCL. Gold nanoparticles for drug delivery and cancer therapy. Appl Sci. 2020;10:11. doi:10.3390/app10113824
41. Moore JA, Chow JCL. Recent progress and applications of gold nanotechnology in medical biophysics using artificial intelligence and mathematical modeling. Nano Express. 2021;2(2):022001. doi:10.1088/2632-959X/abddd3
42. Shrestha S, Cooper LN, Andreev OA, Reshetnyak YK, Antosh MP. Gold nanoparticles for radiation enhancement in vivo. Jacobs J Radiat Oncol. 2016;3:1.
43. Bromma K, Chithrani DB. Advances in gold nanoparticle-based combined cancer therapy. Nanomaterials. 2020;10:9.
44. Alhussan A, Bozdoğan EPD, Chithrani DB. Combining gold nanoparticles with other radiosensitizing agents for unlocking the full potential of cancer radiotherapy. Pharmaceutics. 2021;13:4. doi:10.3390/pharmaceutics13040442
45. Tabatabaie F, Franich R, Feltis B, Geso M. Oxidative damage to mitochondria enhanced by ionising radiation and gold nanoparticles in cancer cells. Int J Mol Sci. 2022;23:13. doi:10.3390/ijms23136887
46. Huynh M, Kempson I, Bezak E, Phillips W. Predictive modeling of hypoxic head and neck cancers during fractionated radiotherapy with gold nanoparticle radiosensitization. Med Phys. 2021;48(6):3120–3133. doi:10.1002/mp.14872
47. Chang MY, Shiau AL, Chen YH, Chang CJ, Chen HH, Wu CL. Increased apoptotic potential and dose-enhancing effect of gold nanoparticles in combination with single-dose clinical electron beams on tumor-bearing mice. Cancer Sci. 2008;99(7):1479–1484. doi:10.1111/j.1349-7006.2008.00827.x
48. Butterworth KT, Coulter JA, Jain S, et al. Evaluation of cytotoxicity and radiation enhancement using 1.9 nm gold particles: potential application for cancer therapy. Nanotechnology. 2010;21(29):295101. doi:10.1088/0957-4484/21/29/295101
49. Chithrani DB, Jelveh S, Jalali F, et al. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiat Res. 2010;173(6):719–728. doi:10.1667/RR1984.1
50. Geng F, Song K, Xing JZ, et al. Thio-glucose bound gold nanoparticles enhance radio-cytotoxic targeting of ovarian cancer. Nanotechnology. 2011;22(28):285101. doi:10.1088/0957-4484/22/28/285101
51. Jain S, Coulter JA, Hounsell AR, et al. Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int J Radiat Oncol Biol Phys. 2011;79(2):531–539. doi:10.1016/j.ijrobp.2010.08.044
52. Coulter JA, Jain S, Butterworth KT, et al. Cell type-dependent uptake, localization, and cytotoxicity of 1.9 nm gold nanoparticles. Int J Nanomedicine. 2012;7:2673–2685. doi:10.2147/IJN.S31751
53. Joh DY, Sun L, Stangl M, et al. Selective targeting of brain tumors with gold nanoparticle-induced radiosensitization. PLoS One. 2013;8(4):e62425. doi:10.1371/journal.pone.0062425
54. Chattopadhyay N, Cai Z, Kwon YL, Lechtman E, Pignol JP, Reilly RM. Molecularly targeted gold nanoparticles enhance the radiation response of breast cancer cells and tumor xenografts to X-radiation. Breast Cancer Res Treat. 2013;137(1):81–91. doi:10.1007/s10549-012-2338-4
55. Cui L, Tse K, Zahedi P, et al. Hypoxia and cellular localization influence the radiosensitizing effect of gold nanoparticles (AuNPs) in breast cancer cells. Radiat Res. 2014;182(5):475–488. doi:10.1667/RR13642.1
56. Jain S, Coulter JA, Butterworth KT, et al. Gold nanoparticle cellular uptake, toxicity and radiosensitisation in hypoxic conditions. Radiother Oncol. 2014;110(2):342–347. doi:10.1016/j.radonc.2013.12.013
57. Khoshgard K, Hashemi B, Arbabi A, Rasaee MJ, Soleimani M. Radiosensitization effect of folate-conjugated gold nanoparticles on HeLa cancer cells under orthovoltage superficial radiotherapy techniques. Phys Med Biol. 2014;59(9):2249–2263. doi:10.1088/0031-9155/59/9/2249
58. Taggart LE, McMahon SJ, Currell FJ, Prise KM, Butterworth KT. The role of mitochondrial function in gold nanoparticle mediated radiosensitisation. Cancer Nanotechnol. 2014;5(1):5. doi:10.1186/s12645-014-0005-7
59. Liu Y, Liu X, Jin X, et al. The dependence of radiation enhancement effect on the concentration of gold nanoparticles exposed to low- and high-LET radiations. Phys Med. 2015;31(3):210–218. doi:10.1016/j.ejmp.2015.01.006
60. Wang C, Jiang Y, Li X, Hu L. Thioglucose-bound gold nanoparticles increase the radiosensitivity of a triple-negative breast cancer cell line (MDA-MB-231). Breast Cancer. 2015;22(4):413–420. doi:10.1007/s12282-013-0496-9
61. Wolfe T, Chatterjee D, Lee J, et al. Targeted gold nanoparticles enhance sensitization of prostate tumors to megavoltage radiation therapy in vivo. Nanomedicine. 2015;11(5):1277–1283. doi:10.1016/j.nano.2014.12.016
62. Li S, Penninckx S, Karmani L, et al. LET-dependent radiosensitization effects of gold nanoparticles for proton irradiation. Nanotechnology. 2016;27(45):455101. doi:10.1088/0957-4484/27/45/455101
63. Dou Y, Guo Y, Li X, et al. Size-Tuning ionization to optimize gold nanoparticles for simultaneous enhanced CT imaging and radiotherapy. ACS nano. 2016;10(2):2536–2548. doi:10.1021/acsnano.5b07473
64. Soleymanifard S, Rostami A, Aledavood SA, Matin MM, Sazgarnia A. Increased radiotoxicity in two cancerous cell lines irradiated by low and high energy photons in the presence of thio-glucose bound gold nanoparticles. Int J Radiat Biol. 2017;93(4):407–415. doi:10.1080/09553002.2017.1268282
65. Liu S, Piao J, Liu Y, et al. Radiosensitizing effects of different size bovine serum albumin-templated gold nanoparticles on H22 hepatoma-bearing mice. Nanomedicine. 2018;13(11):1371–1383. doi:10.2217/nnm-2018-0059
66. Molinari A, Iovenitti G, Mancini A, et al. AuNP Pyrazolo[3,4-d]pyrimidine nanosystem in combination with radiotherapy against glioblastoma. ACS Med Chem Lett. 2020;11(5):664–670. doi:10.1021/acsmedchemlett.9b00538
67. Luo D, Johnson A, Wang X, et al. Targeted radiosensitizers for MR-guided radiation therapy of prostate cancer. Nano Lett. 2020;20(10):7159–7167. doi:10.1021/acs.nanolett.0c02487
68. Cunningham C, de Kock M, Engelbrecht M, Miles X, Slabbert J, Vandevoorde C. Radiosensitization effect of gold nanoparticles in proton therapy. Front Public Health. 2021;9:699822. doi:10.3389/fpubh.2021.699822
69. Marques A, Belchior A, Silva F, et al. Dose rate effects on the selective radiosensitization of prostate cells by GRPR-targeted gold nanoparticles. Int J Mol Sci. 2022;23:9. doi:10.3390/ijms23095279
70. Kim JY, Lee WS, Seo SJ, Jung CW, Kim EH. Effects of gold nanoparticles on normal hepatocytes in radiation therapy. Transl Cancer Res. 2022;11(8):2572–2581. doi:10.21037/tcr-21-1855
71. Chow JCL, Santiago CA. DNA damage of iron-gold nanoparticle heterojunction irradiated by kV photon beams: a monte carlo study. Appl Sci. 2023;13:15. doi:10.3390/app13158942
72. Santiago CA, Chow JCL. Variations in gold nanoparticle size on DNA damage: a monte carlo study based on a multiple-particle model using electron beams. Appl Sci. 2023;13:8. doi:10.3390/app13084916
73. Dimitriou NM, Tsekenis G, Balanikas EC, et al. Gold nanoparticles, radiations and the immune system: current insights into the physical mechanisms and the biological interactions of this new alliance towards cancer therapy. Pharmacol Ther. 2017;178:1–17. doi:10.1016/j.pharmthera.2017.03.006
74. Douglass M, Bezak E, Penfold S. Monte Carlo investigation of the increased radiation deposition due to gold nanoparticles using kilovoltage and megavoltage photons in a 3D randomized cell model. Med Phys. 2013;40(7):071710. doi:10.1118/1.4808150
75. Cui L, Her S, Borst GR, Bristow RG, Jaffray DA, Allen C. Radiosensitization by gold nanoparticles: will they ever make it to the clinic? Radiother Oncol. 2017;124(3):344–356. doi:10.1016/j.radonc.2017.07.007
76. McMahon SJ, Hyland WB, Muir MF, et al. Biological consequences of nanoscale energy deposition near irradiated heavy atom nanoparticles. Sci Rep. 2011;1:18. doi:10.1038/srep00018
77. Butterworth KT, McMahon SJ, Currell FJ, Prise KM. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale. 2012;4(16):4830–4838. doi:10.1039/c2nr31227a
78. Leung MK, Chow JC, Chithrani BD, Lee MJ, Oms B, Jaffray DA. Irradiation of gold nanoparticles by x-rays: monte Carlo simulation of dose enhancements and the spatial properties of the secondary electrons production. Med Phys. 2011;38(2):624–631. doi:10.1118/1.3539623
79. Carter JD, Cheng NN, Qu Y, Suarez GD, Guo T. Nanoscale energy deposition by X-ray absorbing nanostructures. J Phys Chem B. 2007;111(40):11622–11625. doi:10.1021/jp075253u
80. Prise KM, Schettino G, Folkard M, Held KD. New insights on cell death from radiation exposure. Lancet Oncol. 2005;6(7):520–528. doi:10.1016/S1470-2045(05)70246-1
81. Sun H, Jia J, Jiang C, Zhai S. Gold nanoparticle-induced cell death and potential applications in nanomedicine. Int J Mol Sci. 2018;19(3). doi:10.3390/ijms19030754
82. Gupta N, Malviya R. Understanding and advancement in gold nanoparticle targeted photothermal therapy of cancer. Bioch Bio Acta Rev Cancer. 2021;1875(2):188532. doi:10.1016/j.bbcan.2021.188532
83. Amato E, Italiano A, Leotta S, Pergolizzi S, Torrisi L. Monte Carlo study of the dose enhancement effect of gold nanoparticles during X-ray therapies and evaluation of the anti-angiogenic effect on tumour capillary vessels. J Xray Sci Technol. 2013;21(2):237–247. doi:10.3233/XST-130374
84. Berbeco RI, Korideck H, Ngwa W, et al. DNA damage enhancement from gold nanoparticles for clinical MV photon beams. Radiat Res. 2012;178(6):604–608. doi:10.1667/RR3001.1
85. Khalil TT, Bazzi R, Roux S, Fromm M. The contribution of hydrogen peroxide to the radiosensitizing effect of gold nanoparticles. Colloids Surf B Biointerfaces. 2019;175:606–613. doi:10.1016/j.colsurfb.2018.12.041
86. Tartier L, Gilchrist S, Burdak-Rothkamm S, Folkard M, Prise KM. Cytoplasmic irradiation induces mitochondrial-dependent 53BP1 protein relocalization in irradiated and bystander cells. Cancer Res. 2007;67(12):5872–5879. doi:10.1158/0008-5472.CAN-07-0188
87. Ghita M, McMahon SJ, Taggart LE, Butterworth KT, Schettino G, Prise KM. A mechanistic study of gold nanoparticle radiosensitisation using targeted microbeam irradiation. Sci Rep. 2017;7(1):44752. doi:10.1038/srep44752
88. Tsai SW, Lo CY, Yu SY, et al. Gold nanoparticles enhancing generation of ROS for Cs-137 radiotherapy. Nanoscale Res Lett. 2022;17(1):123. doi:10.1186/s11671-022-03761-w
89. Zheng Q, Yang H, Wei J, Tong JL, Shu YQ. The role and mechanisms of nanoparticles to enhance radiosensitivity in hepatocellular cell. Biomed Pharmacoth. 2013;67(7):569–575. doi:10.1016/j.biopha.2013.04.003
90. Mahmoudi M, Azadmanesh K, Shokrgozar MA, Journeay WS, Laurent S. Effect of nanoparticles on the cell life cycle. Chem Rev. 2011;111(5):3407–3432. doi:10.1021/cr1003166
91. Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59(4):928–942. doi:10.1016/j.ijrobp.2004.03.005
92. Roa W, Zhang X, Guo L, et al. Gold nanoparticle sensitize radiotherapy of prostate cancer cells by regulation of the cell cycle. Nanotechnology. 2009;20(37):375101. doi:10.1088/0957-4484/20/37/375101
93. Jeon HJ, Choi BBR, Park KH, Hwang DS, Kim UK, Kim GC. Induction of melanoma cell-selective apoptosis using anti-HER2 antibody-conjugated gold nanoparticles. Yonsei Med J. 2019;60(6):509–516. doi:10.3349/ymj.2019.60.6.509
94. Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20(3):460–473. doi:10.1089/ars.2013.5371
95. Levy JMM, Towers CG, Thorburn A, Siddique S. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17(9):528–542. doi:10.1038/nrc.2017.53
96. Ma X, Wu Y, Jin S, et al. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS nano. 2011;5(11):8629–8639. doi:10.1021/nn202155y
97. Joshi P, Chakraborti S, Ramirez-Vick JE, et al. The anticancer activity of chloroquine-gold nanoparticles against MCF-7 breast cancer cells. Colloids Surf B Biointerfaces. 2012;95:195–200. doi:10.1016/j.colsurfb.2012.02.039
98. Ding F, Li Y, Liu J, et al. Overendocytosis of gold nanoparticles increases autophagy and apoptosis in hypoxic human renal proximal tubular cells. Int J Nanomedicine. 2014;9:4317–4330. doi:10.2147/IJN.S68685
99. Luo CL, Liu YQ, Wang P, et al. The effect of quercetin nanoparticle on cervical cancer progression by inducing apoptosis, autophagy and anti-proliferation via JAK2 suppression. Biomed Pharmacoth. 2016;82:595–605. doi:10.1016/j.biopha.2016.05.029
100. Bhogal N, Jalali F, Bristow RG. Microscopic imaging of DNA repair foci in irradiated normal tissues. Int J Radiat Biol. 2009;85(9):732–746. doi:10.1080/09553000902785791
101. Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8(3):180–192. doi:10.1038/nrc2344
102. Banáth JP, Olive PL. Expression of phosphorylated histone H2AX as a surrogate of cell killing by drugs that create DNA double-strand breaks. Cancer Res. 2003;63(15):4347–4350.
103. Lin Y, Paganetti H, McMahon SJ, Schuemann J. Gold nanoparticle induced vasculature damage in radiotherapy: comparing protons, megavoltage photons, and kilovoltage photons. Med Phys. 2015;42(10):5890–5902. doi:10.1118/1.4929975
104. Zanjanchi P, Asghari SM, Mohabatkar H, Shourian M, Shafiee Ardestani M. Conjugation of VEGFR1/R2-targeting peptide with gold nanoparticles to enhance antiangiogenic and antitumoral activity. J Nanobiotechnology. 2022;20(1):7. doi:10.1186/s12951-021-01198-4
105. Ionita P, Conte M, Gilbert BC, Chechik V. Gold nanoparticle-initiated free radical oxidations and halogen abstractions. Org Biomol Chem. 2007;5(21):3504–3509. doi:10.1039/b711573c
106. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–627. doi:10.1126/science.1114397
107. Her S, Jaffray DA, Allen C. Gold nanoparticles for applications in cancer radiotherapy: mechanisms and recent advancements. Adv Drug Deliv Rev. 2017;109:84–101. doi:10.1016/j.addr.2015.12.012
108. Wahab R, Dwivedi S, Khan F, et al. Statistical analysis of gold nanoparticle-induced oxidative stress and apoptosis in myoblast (C2C12) cells. Colloids Surf B Biointerfaces. 2014;123:664–672. doi:10.1016/j.colsurfb.2014.10.012
109. Tang Y, Shen Y, Huang L, et al. In vitro cytotoxicity of gold nanorods in A549 cells. Environ Toxicol Pharmacol. 2015;39(2):871–878. doi:10.1016/j.etap.2015.02.003
110. Ito S, Miyoshi N, Degraff WG, Nagashima K, Kirschenbaum LJ, Riesz P. Enhancement of 5-Aminolevulinic acid-induced oxidative stress on two cancer cell lines by gold nanoparticles. Free Radic Res. 2009;43(12):1214–1224. doi:10.3109/10715760903271249
111. Cheng NN, Starkewolf Z, Davidson RA, et al. Chemical enhancement by nanomaterials under X-ray irradiation. J Am Chem Soc. 2012;134(4):1950–1953. doi:10.1021/ja210239k
112. Caldorera-Moore M, Guimard N, Shi L, Roy K. Designer nanoparticles: incorporating size, shape and triggered release into nanoscale drug carriers. Expert Opin Drug Deliv. 2010;7(4):479–495. doi:10.1517/17425240903579971
113. Huang K, Ma H, Liu J, et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS nano. 2012;6(5):4483–4493. doi:10.1021/nn301282m
114. Janic B, Brown SL, Neff R, et al. Therapeutic enhancement of radiation and immunomodulation by gold nanoparticles in triple negative breast cancer. Cancer Biol Ther. 2021;22(2):124–135. doi:10.1080/15384047.2020.1861923
115. Ma N, Wu FG, Zhang X, et al. Shape-dependent radiosensitization effect of gold nanostructures in cancer radiotherapy: comparison of gold nanoparticles, nanospikes, and nanorods. ACS Appl Mater Interfaces. 2017;9(15):13037–13048. doi:10.1021/acsami.7b01112
116. Pakravan A, Salehi R, Mahkam M. Comparison study on the effect of gold nanoparticles shape in the forms of star, hallow, cage, rods, and Si-Au and Fe-Au core-shell on photothermal cancer treatment. Photodiagnosis Photodyn Ther. 2021;33:102144. doi:10.1016/j.pdpdt.2020.102144
117. Shi L, Zhang J, Zhao M, et al. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale. 2021;13(24):10748–10764. doi:10.1039/D1NR02065J
118. Uzonwanne VO, Navabi A, Obayemi JD, et al. Triptorelin-functionalized PEG-coated biosynthesized gold nanoparticles: effects of receptor-ligand interactions on adhesion to triple negative breast cancer cells. Biomat Advan. 2022;136:212801. doi:10.1016/j.bioadv.2022.212801
119. Enferadi M, Fu SY, Hong JH, et al. Radiosensitization of ultrasmall GNP-PEG-cRGDfK in ALTS1C1 exposed to therapeutic protons and kilovoltage and megavoltage photons. Int J Radiat Biol. 2018;94(2):124–136. doi:10.1080/09553002.2018.1407462
120. Shahbazi MA, Faghfouri L, Ferreira MPA, et al. The versatile biomedical applications of bismuth-based nanoparticles and composites: therapeutic, diagnostic, biosensing, and regenerative properties. Chem Soc Rev. 2020;49(4):1253–1321.
121. Song G, Liang C, Yi X, et al. Perfluorocarbon-loaded hollow Bi2Se3 nanoparticles for timely supply of oxygen under near-infrared light to enhance the radiotherapy of cancer. Advan Mat. 2016;28(14):2716–2723. doi:10.1002/adma.201504617
122. Chen Y, Zhao G, Wang S, et al. Platelet-membrane-camouflaged bismuth sulfide nanorods for synergistic radio-photothermal therapy against cancer. Biomat Sci. 2019;7(8):3450–3459. doi:10.1039/C9BM00599D
123. Zeng L, Zhao H, Zhu Y, et al. A one-pot synthesis of multifunctional Bi(2)S(3) nanoparticles and the construction of core-shell Bi(2)S(3)@Ce6-CeO(2) nanocomposites for NIR-triggered phototherapy. J Mate Chem. 2020;8(18):4093–4105.
124. Ouyang R, Cao P, Jia P, et al. Bistratal Au@Bi(2)S(3) nanobones for excellent NIR-triggered/multimodal imaging-guided synergistic therapy for liver cancer. Bioact Mat. 2021;6(2):386–403. doi:10.1016/j.bioactmat.2020.08.023
125. Rabaan AA, Bukhamsin R, AlSaihati H, et al. Recent Trends and Developments in Multifunctional Nanoparticles for Cancer Theranostics. Molecules. 2022;27:24.
126. Srivastava P, Sharma PK, Muheem A, Warsi MH. Magnetic nanoparticles: a review on stratagems of fabrication an d its biomedical applications. Recent Pat Drug Deliv Formul. 2017;11(2):101–113. doi:10.2174/1872211311666170328150747
127. Fatima H, Charinpanitkul T, Kim KS. Fundamentals to apply magnetic nanoparticles for hyperthermia therapy. Nanomaterials. 2021;11(5). doi:10.3390/nano11051203
128. Chow JCL. 9 - Magnetic nanoparticles as contrast agents in magnetic resonance imaging and radiosensitizers in radiotherapy. In: Hussain CM, Patankar KK, editors. Fundamentals and Industrial Applications of Magnetic Nanoparticles. Woodhead Publishing; 2022:291–316.
129. Marekova D, Turnovcova K, Sursal TH, Gandhi CD, Jendelova P, Jhanwar-Uniyal M. Potential for treatment of glioblastoma: new aspects of superparamagnetic iron oxide nanoparticles. Anticancer Res. 2020;40(11):5989–5994. doi:10.21873/anticanres.14619
130. Lyu M, Zhu D, Kong X, et al. Glutathione-depleting nanoenzyme and glucose oxidase combination for hypoxia modulation and radiotherapy enhancement. Adv Healthcare Mater. 2020;9(11):e1901819. doi:10.1002/adhm.201901819
131. Meidanchi A. Mg((1-x))Cu(x)Fe(2)O(4) superparamagnetic nanoparticles as nano-radiosensitizer agents in radiotherapy of MCF-7 human breast cancer cells. Nanotechnology. 2020;31(32):325706. doi:10.1088/1361-6528/ab8cf2
132. Dana P, Pimpha N, Chaipuang A, et al. Inhibiting metastasis and improving chemosensitivity via chitosan-coated selenium nanoparticles for brain cancer therapy. Nanomaterials. 2022;12:15.
133. Sonkusre P. Specificity of biogenic selenium nanoparticles for prostate cancer therapy with reduced risk of toxicity: an in vitro and in vivo study. Front Oncol. 2019;9:1541. doi:10.3389/fonc.2019.01541
134. Xia Y, Tang G, Chen Y, et al. Tumor-targeted delivery of siRNA to silence Sox2 gene expression enhances therapeutic response in hepatocellular carcinoma. Bioact Mat. 2021;6(5):1330–1340. doi:10.1016/j.bioactmat.2020.10.019
135. Wang C, Xia Y, Huo S, et al. Silencing of MEF2D by siRNA loaded selenium nanoparticles for ovarian cancer therapy. Int J Nanomedicine. 2020;15:9759–9770. doi:10.2147/IJN.S270441
136. Tabei M, Zeinizade E, Beik J, et al. Insights into nano-photo-thermal therapy of cancer: the kinetics of cell death and effect on cell cycle. Anticancer Agents Med Chem. 2020;20(5):612–621. doi:10.2174/1871520620666200129111332
137. Youssef Z, Yesmurzayeva N, Larue L, et al. New targeted gold nanorods for the treatment of glioblastoma by photodynamic therapy. J Clin Med. 2019;8(12):2205. doi:10.3390/jcm8122205
138. Dan Q, Hu D, Ge Y, et al. Ultrasmall theranostic nanozymes to modulate tumor hypoxia for augmenting photodynamic therapy and radiotherapy. Biomat Sci. 2020;8(3):973–987. doi:10.1039/C9BM01742A
139. Rastinehad AR, Anastos H, Wajswol E, et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc Natl Acad Sci U S A. 2019;116(37):18590–18596. doi:10.1073/pnas.1906929116
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
