Back to Journals » Breast Cancer: Targets and Therapy » Volume 13
The Proper Ki-67 Cut-Off in Hormone Responsive Breast Cancer: A Monoinstitutional Analysis with Long-Term Follow-Up
Authors Lombardi A , Lazzeroni R , Bersigotti L, Vitale V, Amanti C
Received 6 February 2021
Accepted for publication 2 March 2021
Published 7 April 2021 Volume 2021:13 Pages 213—217
DOI https://doi.org/10.2147/BCTT.S305440
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Augusto Lombardi, Rachele Lazzeroni, Laura Bersigotti, Valeria Vitale, Claudio Amanti
Department of Breast Surgery, Università di Roma La Sapienza – OspedaleSant’Andrea, Rome, Italy
Correspondence: Laura Bersigotti
OspedaleSant’Andrea, via di Grottarossa 1035, Rome, 00189, Italy
Tel +39 3332559243
Fax +39 06 33775649
Email [email protected]
Introduction: Breast cancer is a heterogeneous disease. Our study focuses on a monoinstitutional series of patients affected by Hormone Responsive carcinomas (luminal A and luminal B) and aims to define an optimal Ki-67 cut-off, to correctly stratify these patients into risk classes, using the ImmunoHistoChemical (IHC) surrogates of the Molecular Subtypes, according to the St. Gallen guidelines.
Methods: We analyzed 1685 patients. These patients underwent both radical and conservative surgeries with Sentinel Lymph Node Biopsy eventually followed by Axillary Dissection (AD). Furthermore, all the patients underwent adjuvant therapies according to the guidelines. A retrospective univariate analysis was performed and survival curves (Disease-Related Survival, DRS, and Disease-Free Survival, DFS) were carried out according to the following ki-67 risk classes: Low Risk (Ki-67 ≤ 14%); Intermediate Risk (Ki-67 15% ÷ 20%); High Risk (Ki-67 > 20%).
Results: 14 yy DRS was 98% in LA and 85% in LB with a ki-67 cut-off of 14% (p=0.037) vs 95% (LA) and 83% (LB) with a ki-67 cut-off of 20% (p=0.003). 14yy DFS was 85% in LA and 72% in LB with a ki-67 cut-off of 14% (p=0.017) vs 83% (LA) and 66% (LB) with a ki-67 cut-off of 20% (p< 0.000).
Discussion: Our results confirmed that the 20% Ki-67 cut-off is more reliable in differentiating patients at low or high risk of recurrence and death, and stratifying patients eligible for adjuvant chemotherapy. Thus, despite its poor reproducibility, the identification of the most accurate ki-67 index assumes a pivotal relevance in guiding a tailored strategy among patients with this specific profile of breast cancer, as well as the molecular surrogates, in order to avoid harmful overtreatments.
Keywords: Ki67, molecular subtypes, immunohistochemistry
Introduction
Breast cancer is a heterogeneous disease. An important step forward in the field of breast cancer classification is related to the characterization of molecular subtypes.
About 20 years ago, Perou1 highlighted how each tumor has an extremely precise genetic signature, that influences the neoplastic behavior in terms of growth, aggression, tendency to metastasize, and consequently the prognosis.
Waiting for multigene panels entering in routine clinical practice, the immunohistochemical (IHC) surrogates of the molecular subtypes of breast cancer proposed by the Saint Gallen Consensus Meetings have been widely used to classify and stratify patients into various risk categories.2
The main difficulty concerns with the differentiation of the luminal forms, A and B. In fact, both tumors are estrogen receptor positive (OR +) and HER2 negative. With this aim, the guidelines proposed by the Saint Gallen Consensus Meetings recommend the evaluation of the Ki-67 proliferation index, a nuclear protein, detectable with IHC, that is a marker indicative of cell expansion, which is expressed in all phases of the cell cycle, except G0.3 The Luminal B subtype should exhibit a higher Ki-67 proliferation index than the Luminal A type; however, the Ki-67 cut-off for the differentiation of these two categories has changed over time. In fact, in 2011 the Saint Gallen Consensus Meeting defined as “low proliferation” breast tumors those with an index of Ki-67 <14%,4 a cut-off based on the median value of its distribution. However, during the 2013 Saint Gallen Consensus Meeting, most experts stated that a threshold of ≥20% was indicative of a higher risk class. At the same time, several studies have shown a low reproducibility of the Ki-67 marker, mainly in a subgroup of breast tumors with intermediate proliferation activity, such as between 15 and 30%.5,6
Thus, the aim of this paper is to establish which is the optimal Ki-67 cut-off (14 vs 20%) to stratify and define the proper IHC surrogate of breast cancer molecular subtypes with the ultimate purpose of customizing the therapy for patients as much as possible, in order to avoid harmful overtreatments.7–11
Materials and Methods
This retrospective work is based on a prospective database of 2250 patients affected by primary breast cancer collected from October 2004 to September 2020. They all underwent surgical procedures at the Breast Surgery Unit of the Sant’Andrea Hospital, of Rome. Exclusion criteria include: diagnosis of Carcinoma in situ, neoadjuvant chemotherapy and tumors that do not express estrogen receptors or HER2-enriched.
Therefore, the analysis was conducted among 1685 patients, who underwent both radical and conservative surgery with Sentinel Lymph Node Biopsy and subsequent completion lymph node axillary dissection (AD), when positive. This approach was maintained regardless of the size of the metastasis until 2012 when AD was carried out only in the case of macrometastasis (>2 mm). On the other side, no AD was performed in the case of isolated tumor cells (ITC) on Sentinel Lymph Node.
All the patients underwent adjuvant therapies (Radiation Therapy, Hormone therapy and/or Chemotherapy) according to the guidelines. The instrumental and clinical follow-up was conducted in collaboration with the Radiation Therapy and Oncologic Units of our hospital.
The mean age of the sample was 59 years (± 12.7, range 26–89). The main clinical-pathological characteristics of the sample are reported in the following table (Table 1).
 |
Table 1 Clinical-Pathological Characteristics of the Study Population |
Statistic Analysis and Software
The prospective database was built by Microsoft® Access. The statistical analysis was carried out by IBM-SPSS®. In order to compare categorical and continuous variables, Chi-square test and Student’s t test were used. Disease-Related Survival (DRS) and Disease-Free Survival (DFS) were calculated from the surgical procedure, plotting the curves by the Kaplan–Meier method and the Log-rank test was used for statistical comparisons.
Results
Tumor size, Grading, Lympho-Vascular Invasion and Histological type are highly correlated to the Ki-67 proliferative index, while other variables do not show statistically significant correlation (Table 1).
DRS and DFS on the whole sample were, respectively, 98% and 94% at 5 years, 97% and 79% at 10 years, 88% and 77% at 14 years.
Out of 1114 patients who responded to follow-up, 47 deaths were recorded over a period of 14 years (20 directly due to breast cancer, and 27 related to other causes). The adverse events recorded are shown in the following table (Table 2).
 |
Table 2 Adverse Events Recorded During the Follow-Up |
We stratified our population according to the Ki-67 value, establishing three categories: Low Risk (Ki-67 ≤ 14%); Intermediate Risk (Ki-67 15% ÷ 20%); High Risk (Ki-67 > 20%). The DRS and DFS curves vary according to the risk class (Figure 1), and the risk of death or relapse seems related to the value of Ki-67 (p=0.01 and p=0.002, respectively).
 |
Figure 1 (A) Disease-related survival and (B) disease-free survival by class of risk. |
Then, we evaluated the prognostic correlation of the IHC surrogate molecular subtype (Luminal A vs Luminal B), depending on the cut-off set (Ki-67 14% vs Ki-67 20%) (Table 3, Figure 2).
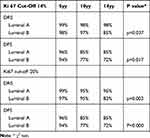 |
Table 3 Disease-Related Survival (DRS) and Disease-Free Survival (DFS) at 5, 10 and 14 Years by Molecular Subtype (IHC Surrogate) According to Ki67 Cut-Off (14% vs 20%) |
Furthermore, a more accurate stratification by risk class, using a 20% cut-off, could be obtained from the analysis on the subpopulation of patients with at least one positive lymph node. In this set, the difference between the DRS and DFS curves with a ki-67 cut-off of 14% showed a not significant p value compared with those stratified by a Ki-67 cut-off of 20% that showed p = 0.006; and p = 0.003, respectively. Such a specific analysis of a subset is essentially focused to verify the prognostic impact of the biomarker in patients with an associated risk factor. This results agree with the last revision of the AJCC Staging System,12 where an information of a purely staging type (in this case the N status) is combined with a biological information (the IHC surrogate of the molecular subtype) to assess a proper therapy and predict a correct prognosis.
Discussion and Conclusions
We can confirm that luminal breast cancer is a pathology with a good prognosis. Further analysis is needed especially focusing on long-term mortality, most of all in luminal B patients.
Despite the usefulness of Ki-67 on the management of breast cancer has been strongly discussed, due to its poor reproducibility, the Saint Gallen Consensus Meeting had suggested, since 2009, to use it for stratifying luminal tumors. Our experimental results confirm that within luminal breast tumors’ setting the 20% Ki-67 cut-off is more reliable in differentiating patients at low or high risk of recurrence and death, identifying patients at higher risk, eligible for adjuvant chemotherapy. This cut-off allows a more correct management of the disease, avoiding an unwarranted overtreatment.
Undoubtedly, we could say that it is time to ascend the phenotypic slope reaching the genotypic top.
The recent results of clinical trials, such as the TAILORx,13 show the actual usefulness of the multigenic panels to assign the most appropriate and effective treatment for these luminal breast cancer patients.
These findings provide significant support to identify a subset of low-risk women who can avoid postsurgical chemotherapy. Just while we are writing, about this issue, the Italian Ministro della Salute (the Public Health Authority) has set up a 20.000.000 € yearly fund to implement into the National Health System the clinical use of the multigene panels in hormone responsive breast cancer patients.
Ethics and Consent Statement
The authors declare that this is a retrospective study and it does not require approval by the Ethics Committee, but rather, only required approval by the Breast Unit Core Team Institutional Review Board (Professor Claudio Amanti, breast surgery, Professor Mattia Falchetto Osti, radiation oncology, and Professor Patrizia Pellegrini, oncology). Professor Amanti, as an author of the paper, recused himself from the review and approval process undertaken by the institutional review board.
Patient consent to review their medical records was not required by the board because all patients included in the study signed, at admission, a standard form about privacy and processing of personal data.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. PMID: 10963602. doi:10.1038/35021093
2. Mittendorf EA, Chavez-MacGregor M, Vila J, et al. Bioscore: a staging system for breast cancer patients that reflects the prognostic significance of underlying tumor biology. Ann Surg Oncol. 2017;24(12):3502–3509. PMID: 28726077. doi:10.1245/s10434-017-6009-x
3. Bustreo S, Osella-Abate S, Cassoni P, et al. Optimal Ki-67 cut-off for luminal breast cancer prognostic evaluation: a large case series study with a long-term follow-up. Breast Cancer Res Treat. 2016;157(2):363–371. doi:10.1007/s10549-016-3817-9
4. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22(8):1736–1747. PMID: 21709140. doi:10.1093/annonc/mdr304
5. Mikami Y, Ueno T, Yoshimura K, et al. Interobserver concordance of Ki67 labeling index in breast cancer: Japan Breast Cancer Research Group Ki67 ring study. Cancer Sci. 2013;104(11):1539–1543. PMID: 23905924. doi:10.1111/cas.12245
6. Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Ann Oncol. 2015;26(8):1533–1546. PMID: 25939896. doi:10.1093/annonc/mdv221
7. Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31(1):13–20. doi:10.1002/ijc.2910310104
8. Lee LH, Yang H, Bigras G. Current breast cancer proliferative markers correlate variably based on decoupled duration of cell cycle phases. Int J Cancer. 1983;31(1):13–20. doi:10.1002/ijc.2910310104
9. Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki-67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103(22):1656–1664. doi:10.1093/jnci/djr393
10. Polley MY, Leung SC, McShane LM, et al. An international Ki-67 reproducibility study. Mod Pathol. 2015;28(6):778–786. doi:10.1038/modpathol.2015.38
11. Polley MY, Leung SC, Gao D, et al. An international study to increase concordance in Ki-67 scoring. Mod Pathol. 2015;28(6):778–786. doi:10.1038/modpathol.2015.38
12. Gabriel NH, James LC, Carl JD, et al.; American Joint Committee on Cancer (AJCC). AJCC Cancer Staging Manual.
13. Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–121. doi:10.1056/NEJMoa1804710
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

