Back to Journals » Clinical Interventions in Aging » Volume 18
The Prognostic Value of the Age-Adjusted Charlson Comorbidity Index Among the Elderly with Breast Cancer
Authors Wang Z , Zhong Y , Zhou Y, Mao F, Zhang X, Wang C, Sun Q
Received 28 March 2023
Accepted for publication 20 July 2023
Published 26 July 2023 Volume 2023:18 Pages 1163—1174
DOI https://doi.org/10.2147/CIA.S414727
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Zhe Wang, Ying Zhong, Yidong Zhou, Feng Mao, Xiaohui Zhang, Changjun Wang, Qiang Sun
Department of Breast Surgery, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, 100730, People’s Republic of China
Correspondence: Qiang Sun, Email [email protected]
Purpose: This study aimed to assess the effect of comorbidities on prognosis using the Age-adjusted Charlson Comorbidity Index (ACCI) among the elderly with breast cancer (BC).
Methods: This study included 745 patients divided into two groups following the ACCI score (≤ 3 vs > 3). Multivariate logistic regression analysis was conducted for all kinds of outcomes, including BC-specific death (BCSD) and non-breast cancer-specific death (NBCSD). The Kaplan–Meier curves were plotted, and survival analysis was conducted for disease-free survival (DFS), overall survival (OS), BC-specific survival (BCSS), and non-BCSS (NBCSS).
Results: A significantly higher NBCSD was found in the high-score (ACCI > 3) group than in the low-score (ACCI < 3) group (p = 0.032). The multivariate logistic regression analysis revealed ACCI score as an independent affecting factor for all-cause death (hazard ratio [HR] = 0.42, 95% confidence interval [CI]: 0.22– 0.83, p = 0.012) and NBCSD (HR = 0.41, 95% CI: 0.20– 0.87, p = 0.020). The Kaplan–Meier curves revealed statistical differences only in NBCSS between the two groups (p = 0.039). Subgroup analysis revealed a worse prognosis in the high-score group for OS and NBCSS among hormone receptor-positive participants and those who without undergoing axillary dissection or receiving chemotherapy (all p < 0.05). Multivariate Cox regression analysis revealed ACCI as an independent prognostic predictor for OS (HR = 2.18, 95% CI: 1.22– 3.92, p = 0.009) and NBCSS (HR = 2.04, 95% CI: 1.02– 4.08, p = 0.044).
Conclusion: ACCI was indeed an effective indicator of the effects of comorbidities on survival among elderly patients with BC. However, the co-effect from age and comorbidities was not significant enough on cancer-specific prognosis, although it exerted a significant effect on treatments received.
Keywords: comorbidity, elderly, breast cancer, Age-adjusted Charlson Comorbidity Index, prognosis
Introduction
Breast cancer (BC) incidence and mortality rates remain increasing in both developing and developed countries.1 The number of females aged ≥70 years who are affected by BC worldwide is dramatically increasing.2 BC is rapidly increasing among the elderly in China based on the largest population and increasing aging. The epidemiological profile data regarding BC in China reported the second onset age peak of BC morbidity after 70 years old, and the proportion of patients aged >65 is expected to exceed one-fifth in 2020 and reach 27.0% by 2030.3 However, some controversies remain to be resolved due to the lack of high-quality evidence. Tumor biological behavior, treatment tolerance, and comorbid status among the elderly prominently differ from their young counterparts.4 Studies focusing on the global population confirmed more favorable biological behavior of tumors among elderly patients compared with younger patients.5,6 Age and comorbidity are both important decisive factors. Previous studies revealed that the presence of comorbid conditions and age at diagnosis were significantly associated with overall survival (OS) and all-cause mortality.7 Moreover, a large population cohort study including >60,000 patients from Denmark confirmed an increased risk of BC-specific death with increasing severity of comorbid conditions.8 However, no study focused on the correlation between comorbidity and prognosis among elderly patients with BC in China.
Several methods for evaluating comorbidities (eg, the Charlson Comorbidity Index [CCI]) have been introduced in the comprehensive geriatric assessment (CGA) by the National Comprehensive Cancer Network Clinical Practice Guidelines,9 although the updated recommendations from the International Society of Geriatric Oncology and European Society of Breast Cancer Specialists emphasized that robust evidence remains lacking on the effectiveness of CGA.10 The CCI was proposed by Charlson et al in 1987, and the Age-adjusted CCI (ACCI) was put forward based on their study results in 1994.11,12 To date, ACCI has shown predictive value in assessing the relationship between comorbidity and survival in various cancers.13–18 ACCI was proven as a better tool than the CCI and Elixhauser comorbidity indices even in patients with lung cancer.19 No studies focused on the co-effect of comorbidity and age among elderly patients with BC by ACCI, although comorbidities were particularly common in the elderly patients. This study aimed to use the ACCI for assessing the effect of comorbidities on prognosis among elderly patients with BC by survival analysis from multiple perspectives and further determine ACCI as a decisive factor for the prognosis of the target population through regression analysis.
Materials and Methods
Patients Selection
The consensus on breast carcinoma treatment in elderly patients in China recommended that patients aged ≥70 years with BC were regarded as elderly BC cases.20 This study included female patients who were diagnosed as BC and underwent breast conserving surgery (BCS) or mastectomy at our hospital from January 1, 2000, to December 31, 2015. Patients who had bilateral simultaneous or heterochronous (age at first onset <70) BC and who had distant metastasis at diagnosis were all excluded from the study to facilitate the detection of postoperative local recurrence or distant metastasis. Finally, this study included 745 patients following the inclusion and exclusion criteria (Figure 1). Tumor, lymph node, and metastasis (TNM) stage was determined according to the American Joint Committee on Cancer 8th edition. Written informed consent was obtained from all patients before commencing any of the medical procedures when they were admitted into our department. All patients were regularly followed up by our dedicated staff. Follow-ups were conducted once every six months for three years postoperatively and annually thereafter. The main methods included telephone queries, outpatient clinic visits, and WeChat interviews. Follow-up was terminated on January 31, 2020, with a median duration of 63 months. The specific cause of death was recorded and classified as BC-specific death (BCSD) and non-BCSD (NBCSD). Disease-free survival (DFS), OS, BC-specific survival (BCSS), and non-BCSS (NBCSS) were separately analyzed according to the cause of death.
 |
Figure 1 The flowchart. |
Comorbidities and ACCI Score
All participants’ comorbid statuses were recorded in detail upon admission, including self-reported medical histories and previous medical records. Then, their comorbid statuses were measured according to ACCI score criteria. Specifically, the ACCI score was calculated by adding one point to the CCI score for every decade starting from 70 years old (70–79 score: +1, 80–89 score: +2, and ≥90 score: +3). This study did not include BC in the ACCI calculation.
Statistical Analyses
All patients were divided into two groups according to ACCI scores: the low-score group (≤3 points) and the high-score group (>3 points). A score of >3 is associated with mortality risk in other malignancies, as previously reported.21 Thus, this study selected an ACCI score of 3 as the cut-off value. Associations between ACCI and patient characteristics were analyzed by Chi-Square tests. Multivariate logistic regression analysis was used to estimate the hazard ratio (HR) with a corresponding 95% confidence interval (CI) range for relationships between ACCI and different ending events. Survival analysis was plotted by the Kaplan–Meier curve with Log rank test results. Cox proportional hazard models were used to detect whether ACCI was an independent predicting factor for survival outcomes. All statistical analyses were performed by Statistical Package for the Social Sciences Statistics version 23 (IBM Corp., Armonk, NY, USA). All p-values were two-sided and p-values of <0.05 were considered statistically significant.
Results
Demographic Characteristics
All participants’ data (n = 745) were shown in Table S1. In our cohort, the patients’ ages range from 70 to 93, 52% between 70 and 74 years, 29.1% between 75 and 79 years, 14.0% between 80 and 84 years, 3.4% between 85 and 89 years, 1.1% over 90 years. About 75.2% patients developed one or more kinds of comorbidity. For ACCI Score, 41.6% score 1, 29.4% score 2, 19.2% score 3, 9.8% score over 3. Regarding surgical methods, patients underwent BCS with axillary dissection at the rate of 2.6% while without axillary dissection at 48.5%, and patients underwent mastectomy with axillary dissection at 46.8% while without axillary dissection at 2.1%. As for adjuvant therapy, 66.0% patients received endocrine therapy, 23.9% received chemotherapy and 5.4% received radiotherapy. For outcomes, 17.6% patients had recurrence or metastasis with 6.6% recurrence and 13.0% metastasis, 17.3% patients were in all cause death with 5.8% BCSD and 11.5% NBCSD.
ACCI Score and Pathological Features
The 745 patients were divided into two groups according to ACCI score: ACCI ≤ 3 group (n = 672) and ACCI > 3 group (n = 73). The differences in pathological factors between two groups were analysed by the chi-squared test (Data are shown in Table 1). The proportion of hormone receptor positive patients was obviously higher in the ACCI > 3 group (88.1% vs 76.2%, p = 0.037). The ACCI score was only correlated with hormone receptor status, and there was no statistically significant association obtained with other factors such as histologic type, grade, lymph node status, TNM stage, disease degree, and molecular subtype. Therefore, the pathological features of BC are not related to other comorbidities of the BC patients.
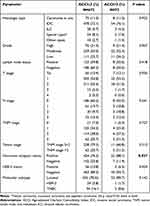 |
Table 1 Pathological Features According to ACCI Score |
ACCI Score and Treatment Methods or Outcomes
The differences in treatment methods or outcome incidence between two groups were also analysed by the chi-squared test (Table 2). In the ACCI > 3 group, a much higher proportion of patients underwent BCS than mastectomy (71.2% vs 48.8%, p < 0.001) and underwent axillary treatment than not underwent axillary dissection (48.1% vs 74.0%, p < 0.001). Regarding adjuvant therapy, the proportion of ACCI ≤ 3 group who received postoperative chemotherapy was significantly higher than ACCI > 3 group (31.0% vs 15.3%, p = 0.012). There is no significant difference between two groups for radiotherapy (7.0% vs 3.4%, p = 0.413) and endocrine therapy (78.1% vs 82.0%, p = 0.484). There was also no obvious difference in local recurrence or distant metastasis, all-cause death, or BCSD incidence (all p > 0.05), but the incidence of NBCSD was significantly higher in the ACCI > 3 group than the ACCI ≤ 3 group (19.4% vs 10.7%, p = 0.032).
 |
Table 2 Treatment and Outcomes According to ACCI Score |
ACCI Score and Specific Comorbid Diseases
Independent statistical analyses in Table 3 were performed for comorbid diseases. The ACCI > 3 group had a significantly higher incidence rate in overall (98.6% vs 72.6%, p < 0.001) and almost every single type of disease such as hypertension, arrhythmia, coronary heart disease, diabetes mellitus, asthma or chronic obstructive pulmonary disease, cerebrovascular disease, peripheral vascular disease, other endocrine diseases, connective tissue disease, chronic kidney disease, chronic liver disease, and other malignant solid tumors (p < 0.05). We also investigated the connection between single comorbid disease and outcomes. There was no significant difference in the distribution of local recurrence or distant metastasis for patients with every specific disease (all p > 0.05). However, patients with arrhythmia (27.3% vs 16.4%, p = 0.026), heart failure (75.0% vs 17.1%, p = 0.018), and chronic kidney disease (34.6% vs 16.7%, p = 0.030) had a higher risk of all-cause death in different degrees. And patients with arrhythmia had a higher risk of NBCSD (19.7% vs 10.8%, p = 0.030). No other single disease was significantly associated with BCSD (p > 0.05) except for coronary heart disease (0.8% vs 6.8%, p = 0.010) (Table S2).
 |
Table 3 Incidence of Specific Comorbidities According to ACCI Score |
Univariate and Multivariate Logistic Regression Analysis
The univariate analysis results indicated that ACCI was only significantly related to incidence of NBCSD (HR = 0.56, 95% CI: 0.33–0.94, p = 0.032). However, in the multivariate logistic regression analysis, ACCI score was an independent affecting factor for all-cause death (HR = 0.42, 95% CI: 0.22–0.83, p = 0.012), NBCSD (HR = 0.41, 95% CI: 0.20–0.87, p = 0.020) that patients with low score had better prognosis, but not for local recurrence, distant metastasis (HR = 0.63, 95% CI: 0.32–1.25, p = 0.188) or BCSD (HR = 0.60, 95% CI: 0.19–1.92, p = 0.390) (Table 4).
 |
Table 4 Univariate Analysis and Multivariate Logistic Regression Analysis |
Subgroup Analysis
All the significantly different mentioned parameters between the two groups in Table 1 and 2 were included in the subgroup analysis for further investigation (Table 5 and 6). Stratified by hormone receptor status, a significantly higher incidence of distant metastasis and all-cause death appeared in the high-score group among HR positive patients (21.2% vs 10.4%, p = 0.021; 25.0% vs 12.8%, p = 0.017). Additionally, all-cause death and NBCSD proportion were significantly higher in the high-score group among patients not receiving chemotherapy (26.0% vs 10.9%, p = 0.003; 20.0% vs 8.0%, p = 0.016) and similar phenomenon also occurred in cases without undergoing axillary dissection (31.5% vs 16.5%, p = 0.009; 25.9% vs 11.8%, p = 0.005). Patients undergoing BCS in the high-score group had a higher risk of all-cause death (30.8% vs 15.6%, p = 0.008) and NBCSD compared with those in the low-score group (25.0% vs 11.3%, p = 0.007).
 |
Table 5 Subgroup Analysis Between ACCI Scores and Recurrence or Metastasis |
 |
Table 6 Subgroup Analysis Between ACCI Scores and Death Events |
Survival Analysis
The Kaplan–Meier curve results revealed that statistical difference was observed in NBCSS between two groups that the ACCI > 3 group has shorter survival interval (p = 0.039). There was no significant difference in OS and DFS between the two groups, although the ACCI > 3 group was shorter in survival interval (p > 0.05) (Figure 2). Stratified by factors such as hormone receptor status, surgical methods and without receiving chemotherapy, OS was dramatically different between two groups in hormone receptor positive patients (p = 0.038); OS and NBCSS were also significantly different between two groups in patients without receiving chemotherapy (p = 0.013, p = 0.029) or without undergoing axillary dissection (p = 0.015, p = 0.033) (Figure 3). In univariate analysis, it showed that the difference was prominent merely in NBCSS that ACCI ≤ 3 patients lived longer (HR = 1.80, 95% CI: 1.01–3.20, p = 0.045). After being adjusted for the disease degree, grade, hormone receptor status, molecular subtypes, without receiving chemotherapy, breast surgical methods and axillary dissection methods, multivariate Cox regression analysis demonstrated that ACCI score could be an independent predictor for OS (HR = 2.18, 95% CI: 1.22–3.92, p = 0.009) and NBCSS (HR = 2.04, 95% CI: 1.02–4.08, p = 0.044). No significant difference was found for BCSS neither in univariate analysis nor in multivariate Cox regression analysis (Table 7).
 |
Table 7 Univariate and Multivariate Cox Regression Analysis for Survival Outcomes |
Discussion
Previous studies specially for the elderly women with BC in the Netherlands suggested that competing mortality risks from comorbidity should not be neglected in daily clinical practice as well as in clinical researches.22 Certain comorbid ailments had been proved to impair the prognosis of BC patients such as the type 2 diabetes mellitus.23 Some researches even observed an increasing risk of BCSD among BC patients with type 2 diabetes mellitus.24 In our study, participants with arrhythmia, heart failure, and chronic kidney disease have a significantly increasing risk of all-cause death. Besides, patients with arrhythmia had a higher risk of NBCSD. However, there was no single one disease that significantly increased the risk of BCSD. ACCI score was an independent predictor for all-cause death, NBCSD, OS and NBCSS that patients with low score (ACCI ≤ 3) tended to less death event significantly. ACCI score has no correlation with BCSD and BCSS.
It is believed that comorbidity and age at diagnosis could influence whether the standard treatments could be given, thereby resulting in different outcomes.25 This phenomenon was observed both in surgical methods and postoperative adjuvant chemotherapy in this cohort. Patients who merely undergoing wide local excision were approximately 1.5 times more in the high-score group than the low-score group (71.2% vs 46.0%). Evidence was lacking to support the benefit of more aggressive treatment or so-called standard treatment schedules according to guidelines for younger counterparts.26 However, the subgroup analysis revealed no significantly different effect of age and comorbid status on operation choices on the BCSD between the two groups. Apart from surgical methods, a literature review reported a dramatically decreasing compliance with chemotherapy and inferior long-term survival outcomes among patients with comorbidity compared to those without.27 Our cohort demonstrated no significantly higher incidence of local relapse, distant metastasis, and BCSD in the high-score group regardless of chemotherapy. A significantly higher incidence of all-cause death and NBCSD was detected in the high-score group in cases without receiving chemotherapy. Therefore, it can conclude that the presence of comorbidity has no correlation with BCSD, the reason may be patients received more or less aggressive treatments for the comorbidity in our clinical center.
There is still a paradoxical thought that, on the one hand, the risk of dying from BC may increase if patients do not receive the standard treatment, on the other hand, they may not benefit to live long enough and may even suffer more pain if they receive the standard treatment. Particularly, more attention has been paid to reducing the incidence of postoperative complications and improving the quality of life among elderly people with shorter life expectancy. Glas et al revealed that elderly patients with comorbidities had a higher risk of postoperative complications but the risk of BC-specific mortality was not higher, which indicated that high relative mortality was most likely due to geriatric parameters, such as age and comorbidity, but not postoperative complications.28 Wu et al confirmed that comorbidities had dramatically negative impacts on postoperative symptoms and the quality of life among patients with BC.29 However, relevant data collection and statistical analysis on the above aspects were not available in our study. Further study may focus on the balance between the necessity for standard treatment and other demands in postoperative life considering the comorbid status.
This study was the first to explore the impact of comorbidity on elderly patients with BC in China, and the first to use ACCI as an independent variable to detect the impact of comorbidity worldwide. However, our study had some limitations. The comorbid conditions may be underestimated due to the retrospective nature of the study. Additionally, CCI was originally put forward based on the BC population and involved 19 kinds of comorbidities several decades ago. However, the current disease spectrum is extremely different from what existed 30 years ago (for example, common cardiovascular system conditions, such as hypertension, hyperlipidemia, and arrhythmia, were not included in the list). Thus, comorbid conditions were probably underestimated in disease types and severity. Improved tool models should be enacted, including optimal disease spectrum, and tested in large specific populations in the future. The median follow-up duration was 63 months, and prospective large-scale population study and long-term follow-up are needed to make the results more convincing given the relatively better prognosis of BC and increased life expectancy.
Conclusion
In conclusion, the ACCI score is an independent predictor for all-cause death and NBCSD. Moreover, it is a significant predictor for OS and NBCSS in survival analysis. However, the co-effect of age and comorbidities was not significant on cancer-specific prognosis, although it exerted a significant effect on treatments received. ACCI may assist doctors in clinical decision-making progress as an effective indicator for the impact of comorbidities on survival among elderly BC patients in line with their comorbid status at diagnosis, especially for hormone receptor-positive patients and those not receiving axillary dissection or postoperative chemotherapy.
Abbreviations
ACCI, Age-adjusted Charlson Comorbidity Index; BC, breast cancer; BCSD, breast cancer-specific death; BCSS, breast cancer-specific survival; BCS, breast conserving surgery; CCI, Charlson Comorbidity Index; DFS, disease-free survival; NBCSD, non-breast cancer-specific death; NBCSS, non-breast cancer-specific survival; OS, overall survival.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The institutional review board (IRB) of Peking Union Medical College Hospital reviewed the study and deemed it exempt from the requirement for approval. The need for informed consent was waived by IRB because of the retrospective nature of the study and the anonymous analysis of the data.
Consent for Participate and Publication
Written informed consent for participation and publication was obtained from every participant when they were admitted into our department.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors have no financial and competing interests to disclose.
References
1. Fitzmaurice C, Abate D, Abate D, et al.; Global Burden of Disease Cancer C. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–1768. doi:10.1001/jamaoncol.2019.2996.
2. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–271. doi:10.3322/caac.21235
3. Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol. 2014;15(7):e279–289. doi:10.1016/S1470-2045(13)70567-9
4. Minicozzi P, Van Eycken L, Molinie F, et al. Comorbidities, age and period of diagnosis influence treatment and outcomes in early breast cancer. Int J Cancer. 2019;144(9):2118–2127. doi:10.1002/ijc.31974
5. Ma CD, Zhou Q, Nie XQ, et al. Breast cancer in Chinese elderly women: pathological and clinical characteristics and factors influencing treatment patterns. Crit Rev Oncol Hematol. 2009;71(3):258–265. doi:10.1016/j.critrevonc.2008.11.005
6. Engels CC, Kiderlen M, Bastiaannet E, et al. The clinical prognostic value of molecular intrinsic tumor subtypes in older breast cancer patients: a FOCUS study analysis. Mol Oncol. 2016;10(4):594–600. doi:10.1016/j.molonc.2015.11.002
7. Patnaik JL, Byers T, Diguiseppi C, et al. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst. 2011;103(14):1101–1111. doi:10.1093/jnci/djr188
8. Land LH, Dalton SO, Jensen MB, et al. Impact of comorbidity on mortality: a cohort study of 62,591 Danish women diagnosed with early breast cancer, 1990–2008. Breast Cancer Res Treat. 2012;131(3):1013–1020. doi:10.1007/s10549-011-1819-1
9. Network NCC: National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology: older adult oncology; 2018.
10. Biganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol. 2012;13(4):e148–160. doi:10.1016/S1470-2045(11)70383-7
11. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
12. Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi:10.1016/0895-4356(94)90129-5
13. Park JW, Koh DH, Jang WS, et al. Age-adjusted Charlson Comorbidity Index as a prognostic factor for radical prostatectomy outcomes of very high-risk prostate cancer patients. PLoS One. 2018;13(6):e0199365. doi:10.1371/journal.pone.0199365
14. Robbins JR, Gayar OH, Zaki M, et al. Impact of age-adjusted Charlson comorbidity score on outcomes for patients with early-stage endometrial cancer. Gynecol Oncol. 2013;131(3):593–597. doi:10.1016/j.ygyno.2013.10.007
15. Suidan RS, Leitao MM Jr, Zivanovic O, et al. Predictive value of the age-adjusted Charlson comorbidity index on perioperative complications and survival in patients undergoing primary debulking surgery for advanced epithelial ovarian cancer. Gynecol Oncol. 2015;138(2):246–251. doi:10.1016/j.ygyno.2015.05.034
16. Kang HW, Kim SM, Kim WT, et al. The age-adjusted Charlson comorbidity index as a predictor of overall survival of surgically treated non-metastatic clear cell renal cell carcinoma. J Cancer Res Clin Oncol. 2020;146(1):187–196. doi:10.1007/s00432-019-03042-7
17. Mulcahy CF, Mohamed ASR, Kanwar A, et al.; Multidisciplinary Larynx Cancer Working G. Age-adjusted comorbidity and survival in locally advanced laryngeal cancer. Head Neck. 2018;40(9):2060–2069. doi:10.1002/hed.25200.
18. Maezawa Y, Aoyama T, Kano K, et al. Impact of the Age-adjusted Charlson comorbidity index on the short- and long-term outcomes of patients undergoing curative gastrectomy for gastric cancer. J Cancer. 2019;10(22):5527–5535. doi:10.7150/jca.35465
19. Yang CC, Fong Y, Lin LC, et al. The age-adjusted Charlson comorbidity index is a better predictor of survival in operated lung cancer patients than the Charlson and Elixhauser comorbidity indices. Eur J Cardiothorac Surg. 2018;53(1):235–240. doi:10.1093/ejcts/ezx215
20. elderly Ccgodatobcit. Consensus on the treatment of breast carcinoma in elderly Chinese patients. Med J Peking Union Med Coll Hosp. 2018;9(4):307–312.
21. Chang CM, Yin WY, Wei CK, et al. Adjusted age-adjusted Charlson comorbidity index score as a risk measure of perioperative mortality before cancer surgery. PLoS One. 2016;11(2):e0148076. doi:10.1371/journal.pone.0148076
22. Kiderlen M, van de Velde CJH, Liefers GJ, et al. Targeted therapy in older women with breast cancer - What’s the target? Eur J Surg Oncol. 2017;43(5):944–948. doi:10.1016/j.ejso.2017.01.014
23. Kaplan MA, Pekkolay Z, Kucukoner M, et al. Type 2 diabetes mellitus and prognosis in early stage breast cancer women. Med Oncol. 2012;29(3):1576–1580. doi:10.1007/s12032-011-0109-4
24. Cleveland RJ, North KE, Stevens J, et al. The association of diabetes with breast cancer incidence and mortality in the long island breast cancer study project. Cancer Causes Control. 2012;23(7):1193–1203. doi:10.1007/s10552-012-9989-7
25. Schonberg MA, Marcantonio ER, Li D, et al. Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. J Clin Oncol. 2010;28(12):2038–2045. doi:10.1200/JCO.2009.25.9796
26. Liang S, Hallet J, Simpson JS, et al. Omission of axillary staging in elderly patients with early stage breast cancer impacts regional control but not survival: a systematic review and meta-analysis. J Geriatr Oncol. 2017;8(2):140–147. doi:10.1016/j.jgo.2016.12.003
27. Lee L, Cheung WY, Atkinson E, et al. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review. J Clin Oncol. 2011;29(1):106–117. doi:10.1200/JCO.2010.31.3049
28. de Glas NA, Kiderlen M, Bastiaannet E, et al. Postoperative complications and survival of elderly breast cancer patients: a FOCUS study analysis. Breast Cancer Res Treat. 2013;138(2):561–569. doi:10.1007/s10549-013-2462-9
29. Wu HS, Davis JE, Chen L. Impact of comorbidity on symptoms and quality of life among patients being treated for breast cancer. Cancer Nurs. 2019;42(5):381–387. doi:10.1097/NCC.0000000000000623
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


