Back to Journals » Cancer Management and Research » Volume 11
The prognostic value of preoperative controlling nutritional status score in non-metastatic renal cell carcinoma treated with surgery: a retrospective single-institution study
Authors Song H , Xu B, Luo C , Zhang Z, Ma B, Jin J, Zhang Q
Received 21 March 2019
Accepted for publication 25 July 2019
Published 9 August 2019 Volume 2019:11 Pages 7567—7575
DOI https://doi.org/10.2147/CMAR.S209418
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Haifeng Song,1–3,* Ben Xu,1–3,* Cheng Luo,1–3 Zhenan Zhang,1–3 Binglei Ma,1–3 Jie Jin,1–3 Qian Zhang1–3
1Department of Urology, Peking University First Hospital, Beijing 100034, People’s Republic of China; 2Institute of Urology, Peking University, Beijing 100034, People’s Republic of China; 3National Research Center for Genitourinary Oncology, Beijing 100034, People’s Republic of China
*These authors contributed equally to this work
Purpose: This study aimed to investigate the significance of the controlling nutritional status (CONUT) score as a predictor for survival outcomes for non-metastatic renal cell carcinoma (RCC) patients.
Methods: We retrospectively reviewed 325 patients who received surgical treatment for renal cell carcinoma between 2010 and 2012 at Peking University First Hospital. Patients were divided into two groups according to the optimal cut-off value of CONUT score. Kaplan–Meier method and log-rank test were used for survival analysis according to different CONUT groups. Cox proportional hazards regression models were performed to assess the prognostic value of clinicopathological parameters for overall survival (OS), cancer-specific survival (CSS) and disease-free survival (DFS) respectively.
Results: The optimal cut-off value of CONUT score was 3. High CONUT score significantly correlated to higher tumor grade (P<0.001), later pathological T stage (P<0.001) and tumor necrosis (P<0.001). Patients with higher CONUT score had worse OS (HR 5.34, 95% CI 2.29–12.46, P<0.001), CSS (HR 5.51, 95% CI 2.12–14.33, P<0.001) and DFS (HR 4.23, 95% CI 2.16–8.29, P<0.001). In multivariable analysis, high CONUT score was an independent risk factor for OS, CSS and DFS (OS: HR=3.36, 95% CI 1.73–6.56, P<0.001; CSS: HR=3.34, 95% CI 1.59–6.98, P=0.001; DFS: HR=1.85, ]95% CI 1.07–3.21, P=0.029)
Conclusion: Preoperative CONUT score was an independent prognostic factor for OS, CSS and DFS in non-metastatic RCC patients treated with surgery.
Keywords: CONUT score, renal cell carcinoma, surgery, survival, biomarker
Introduction
Renal cell carcinoma is one of the most common malignancies in urological cancers which accounts for about 2–3% of the adult malignant solid tumors.1 With regular medical checkup increasing, its incidence continues to rise. Over decades, surgical resection is still the foremost treatment strategy for non-metastatic renal cell carcinoma. However, almost 20–40% of the postoperative patients will experience metastasis or local recurrence.2,3 Therefore, it is necessary to seek biomarkers which can precisely predict the prognosis, because early identification of patients with bad prognosis may help risk stratification and appropriate therapeutic decision making and consequently improve survival outcomes.
Increasing evidence has revealed that immune and inflammatory factors played an important role in cancer development, growth and metastasis. Several biomarkers, such as the platelet to lymphocyte ratio (PLR), the neutrophil to lymphocyte ratio (NLR) and the C-reactive protein (CRP), have been reported to be independent prognostic factors in various malignancies, including renal cell carcinoma.4–6 Nutritional status was also reported to be associated with cancer progression and prognosis in RCC. For example, body mass index (BMI),7 prognostic nutritional index (PNI),8,9 sarcopenia10 and modified Glasgow Prognostic Score (mGPS).11 Controlling Nutritional Status (CONUT) is a newly proposed scoring system which is used to assess patient nutritional and immune status. It is calculated from the serum albumin concentration, total blood cholesterol level and total peripheral lymphocyte count.12 Some studies showed that CONUT score can predict prognosis in many solid tumors, such as esophageal carcinoma,13 gastric cancer,14 colorectal cancer15 or upper tract urothelial carcinoma.16 But there are few studies for renal cell carcinoma. Therefore, we investigate the usefulness of CONUT score for predicting prognosis in non-metastatic renal cell carcinoma patients treated with surgery in this study.
Materials and methods
Patients
We retrospectively reviewed a database of patients who underwent radical/partial nephrectomy for renal cell carcinoma between 2010 and 2012 at Peking University First Hospital. We excluded patients with distant metastasis at surgery, those with other tumors, those with immune system disease, those with insufficient data from medical record and those without follow-up information. At last, 325 patients were included in final evaluation in this study.
Both radical and partial nephrectomy were performed following a standard procedure either in laparoscopic or open approach. The pathological slides were made and reviewed by professional urological pathologists. Pathological T stage was determined according to the 2010 TNM classification and tumor grade according to the Fuhrman grading system. Clinical information was obtained medical records of each patient. Blood samples were collected and measured within 1 week before surgery as regular preoperative laboratory tests. Body mass index (BMI) was calculated using patient height and weight which was measured when patients were admitted to the hospital before surgery.
This study was in compliance with the Helsinki declaration and approved by the Institutional Ethical Review Board of Peking University First Hospital. Written informed consent was waived because of the retrospective nature of this study. During the follow-up, patients or their next of kin were informed in detail about the study and verbal consents were obtained. All data were maintained with confidentiality.
Follow-up and endpoint
Follow-up surveillance after surgery included routine laboratory and image examination every 3 months within the first 3 years, every 6 months for the next 2 years and then annually until the death. Imaging methods included chest X-ray, abdominal ultrasonography, CT or MRI. Death was confirmed according to medical records in our hospital and information provided by relatives of the patients during phone call following-up. The primary outcome of this study is overall survival (OS), and the secondary outcome is cancer-specific survival (CSS) and disease-free survival (DFS). CSS was defined as the time (in months) from date of surgery to a cancer-related death. OS was defined as the time (in months) from the date of surgery to individuals’ death of any cause. DFS was defined as the time (in months) from date of surgery to the radiologically or histologically confirmed recurrence or metastasis.
Definition and cut-off value of CONUT score
The preoperative CONUT score was calculated using serum albumin concentration, peripheral lymphocyte count and the total cholesterol concentration, as described in Table 1. The optimal cut-off value for the CONUT score was investigated using the receiver operating characteristic (ROC) curve (Figure 1). According to the maximum Youden index value, the optimal cut-off value for the CONUT score was 3(AUC: 0.723, 95% CI: 0.670–0.771, P<0.001, sensitivity: 51.28%, specificity: 82.52%). Then, we classified the patients into high CONUT group (CONUT≥3) and low CONUT group (CONUT<3).
 |
Table 1 Assessment of nutrition status by controlling nutritional status (CONUT) scoring system |
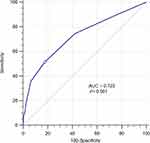 |
Figure 1 ROC curve for CONUT score. Abbreviations: ROC, receiver operating characteristic; CONUT score, controlling nutritional status score. |
Statistical analysis
The clinicopathological characteristics of different groups, including age, gender, hypertension, diabetes mellitus, tumor grade, pathological T stage, histological subtype and surgery type were compared using the chi-square test. The Kaplan–Meier method and log-rank test were used for survival analysis according to different CONUT groups. We performed multivariable analyses using Cox proportional hazards regression models to assess the prognostic value of clinicopathological parameters (age, gender, tumor grade, pathological T stage, histological subtype and CONUT score group) for OS, CSS and DFS, respectively. The hazard ratios (HRs) with 95% confidence intervals (CIs) were presented for each independent factor. The predictive ability of each risk model in the study population was evaluated using the Harrell’s c-index, as previously described.17 Models were refit 200 times using a bootstrap resampling technique to decrease overfit bias. All statistical analyses were performed using Medcalc (Version16.8, Ostend, Belgium), the statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). GraphPad Prism version 7 (GraphPad Software, La Jolla California USA, www.graphpad.com) was used to generate survival curve. A two-sided P<0.05 was considered statistically significant.
Results
Correlations between CONUT score and clinicopathological characteristics
Of the 325 patients included in the study, 231 were male and 94 were female. The median age at surgery of the entire cohort was 57 (IQR 47–66) years. All patients were divided into two groups according to the optimal cut-off value of CONUT score determined by the ROC curve. Seventy patients were included in high CONUT score group while the remaining 255 patients were classified into the low CONUT score group. High CONUT score was significantly correlated with higher tumor grade (P<0.001), later pathological T stage (P<0.001) and presence of tumor necrosis (P<0.001) (Table 2). There were no significant association between CONUT score and age, gender, diabetes, hypertension, tumor histology and surgery type, respectively.
 |
Table 2 Clinicopathological characteristics of the 325 patients according to different controlling nutritional status (CONUT) groups |
Survival analysis for CONUT score in patients with RCC
The median follow-up period for survivors was 64 months (IQR 56.5–69 months). In high CONUT group and low CONUT group, 5-year OS rates were 93.7% and 67.8% (P<0.001) (Figure 2A), 5-year CSS rates were 94.9% and 72.9% (P<0.001) (Figure 2B), 5-year DFS rates were 87.0% and 58.8% (P<0.001) (Figure 2C), respectively. In Kaplan–Meier analysis, as is shown in Table 3, high CONUT score was significantly associated with poor OS (HR 5.34, 95% CI 2.29–12.46, P<0.001), CSS (HR 5.51, 95% CI 2.12–14.33, P<0.001) and DFS (HR 4.23, 95% CI 2.16–8.29, P<0.001). Besides, advanced pathological T stage, high tumor grade and tumor necrosis also had significant correlation to OS, CSS and DFS (all P<0.001).
 |
Table 3 Univariate and multivariate analyses of clinicopathological parameters to predict CSS, DFS and OS in patients with non-metastatic RCC |
Multivariate analysis showed that high CONUT score was an independent risk factor for OS, CSS and DFS (OS: HR=3.36, 95% CI 1.73–6.56, P<0.001; CSS: HR=3.34, 95% CI 1.59–6.98, P=0.001; DFS: HR=1.85, 95% CI 1.07–3.21, P=0.029), independent of advanced pathological T stage and high tumor grade. In addition, tumor necrosis was independently associated with DFS (HR=2.07, 95% CI 1.08–4.02, P=0.030), and diabetes was independently associated with OS (HR=2.24, 95% CI 1.06–4.72 P=0.035) (Table 3). Other factors, such as age, gender, histology and hypertension, were not related to survival outcomes.
Compare CONUT score with other biomarkers in patients with RCC for survival prediction
We used AUCs of ROC curves to estimate the predicting accuracy of CONUT score for the prediction of 5-year OS. The AUC score of PNI and NLR in relation to 5-year OS was 0.701 (95% CI 0.648–0.750) and 0.715 (95% CI 0.662–0.763), respectively. The AUC of CONUT was 0.723 (95% CI 0.670–0.771), which was higher than that of PNI and NLR. However, the difference of AUCs had no statistical significance, which indicates CONUT score may have equivalent accuracy in predicting 5-year OS when compared to NLR and PNI. Similarly, the CONUT score also showed equivalent accuracy in relation to 5-year CSS and DFS prediction. Harrell’s c-index was used to assess the predictive accuracy of the model on multivariate analysis. In the base model which include age, gender, diabetes, hypertension, tumor grade, pathological T stage and histology, the predictive accuracies for OS, CSS and DFS are 80.2%, 81.1% and 84.2%, respectively. When CONUT score was added to the base model, the predictive accuracy was enhanced by 2.5% for OS, 2.5% for CSS and 1.5% for DFS. However, NLR just elevated the predictive accuracy by 1.6% for OS, 0.2% for CSS and 1.2% for DFS, and PNI can elevate the predictive accuracy by 1.9% for OS, 1.4% for CSS and 2.1% for DFS.
Discussion
Our study assessed the association between CONUT score and clinicopathological factors and the prognostic value of CONUT score in non-metastatic renal cancer patients treated with surgery. We found that high CONUT score was significantly correlated with high tumor grade, late pathological T stage and high presence of tumor necrosis. High CONUT score was also significantly associated with worse OS, CSS and DFS in patients with renal cell carcinoma after surgery in univariate analysis; it was an independent prognostic factor for OS, CSS and DFS according to multivariate analysis. The predictive accuracy of CONUT score for survival in postoperative patients with renal cell carcinoma tended to be at least equivalent to other prognostic biomarkers, such as PNI and NLR. The optimal cut-off values of CONUT score were not on all fours in different cancers as reported. In our study, it was 3 according to the ROC curve, which was identical to previous studies.14,18
CONUT was first introduced as an efficient tool for early detection and continuous control of hospital undernutrition. It was calculated from the serum albumin value, the total cholesterol level and the total lymphocyte count, which can be obtained easily from regular blood examination.12 Association between high CONUT score and poor prognosis in cancer patients might relate to the function of these three variables. However, the detailed mechanism underlying remains unclear. Serum albumin is a reliable indicator for host nutritional and systematic inflammatory status, low level of serum albumin was not only considered as malnutritional status but also as systematic inflammatory change. It might be caused by the production of pro-inflammatory cytokines such as interleukin-6 (IL-6) or tumor necrosis factor-alpha (TNF-α), which can modulate the synthesis of albumin by hepatocytes.19,20 It can also be influenced by hepatic function and body fluid volume.21 Researches conducted over the last decade have demonstrated that serum albumin levels, either considered alone or combined with other parameters, can provide prognostic information regarding various cancers which include gastrointestinal cancers, hepatic cancers, lung cancers, breast cancers, ovarian cancers, colorectal cancers, soft tissue sarcoma and some other cancers.22,23
Cholesterol is an essential lipid for maintaining cellular homeostasis. Many epidemiologic observations since the 1980s have shown an inverse association between total serum cholesterol level and cancer incidence and mortality. Lower cholesterol level in plasma was correlated with higher risk in a variety of cancers.24–27 Abnormal lipid metabolism has been proved in close association to the carcinogenesis and tumor development of cancers, but the detailed mechanism is still needed to be clarified. Niendorf et al demonstrated that low-density lipoprotein (LDL) receptor mRNA expression was significantly higher in colorectal tumor tissues than in normal tissues, which induced higher intake of LDL and the plasma cholesterol level decreased consequently. It is indicated that tumor tissues might act as high consumers of cholesterol-rich particles, thus inducing hypocholesterolemia.28 A study from Cengiz et al indicated that hypocholesterolemia was associated with poor tumor prognosis and they hypothesized that tumor activity is a major cause of lowered serum cholesterol values.29
The functional status of immune system significantly affects the prognosis of many human malignancies.30 Lymphocytes play a pivotal role in inhibiting cancer cell proliferation, invasion and migration by initiating a cytotoxic immune response. Studies have proved that tumor-infiltrating lymphocytes (TILs) in tumor microenvironment are associated to prognosis and clinical outcome of immunotherapy in a variety of cancers. It is reported that cancer cells exploit immune checkpoints to inactivate in order to escape from immune surveillance. The presence of TILs in tumor tissues suggests a favorable prognostic role in melanoma, breast cancer, lung cancer and RCC.31,32 As blood lymphocytes represent the level of TILs in a way. Total serum lymphocyte count indicates host immunological status and decreased lymphocyte count predicts worse prognosis in diverse cancers as a result of the insufficient immune response to cancer cells.33,34 Biomarkers based on blood lymphocytes such as PLR and NLR were proved to be useful and easy-available index for immune status and showed prognostic value for diverse solid tumors.35,36
Results of this study remind us the importance of nutritional support in RCC patients. Nutritional intervention should be emphasized during the treatment and follow-up of renal malignancies. Detailed nutritional support strategy needs to be individualized based on nutritional baseline of RCC patients and CONUT score may serve as an index for evaluation of nutritional status. Immunotherapy is emerging as a promising strategy for the treatment of many cancers. Ströhlein et al reported that relative lymphocyte count can predict responding cancer patients to catumaxomab therapy and it is also independently associated with a prolonged survival time.37 So CONUT score may also have potential predictive value for efficacy of immunotherapy in renal cancer patients, while further investigations are needed for this speculation.
Our study has certain limitations. First, it is a small cohort, retrospective study from a single center. Further internal and external validations are needed to clarify the accurate prognostic role of postoperative CONUT score. Second, postoperative CONUT score was not followed-up and taken into account in our study. So, we are not clear whether the change of CONUT score after surgery can influence the survival of RCC patients. Third, due to the lack of relative information, several other nutritional and immune markers, such as mGPS, sarcopenia and PLR were not assessed in this study.
Conclusion
In conclusion, our study suggests that preoperative CONUT score can not only reflect host nutritional and immune status but also is an effective and convenient biomarker for predicting prognosis in patients with non-metastatic RCC treated by surgery. Perioperative nutritional support should be emphasized of RCC patients.
Abbreviations
RCC, renal cell carcinoma; CONUT, controlling nutritional status; OS, overall survival; CSS, cancer-specific survival; DFS, disease-free survival; PLR, platelet to lymphocyte ratio; NLR, the neutrophil to lymphocyte ratio; CRP, C-reactive protein; BMI, body mass index; PNI, prognostic nutritional index; mGPS, modified Glasgow Prognostic Score; HR, hazard ratio; 95% CI, 95% confidence intervals; ROC, receiver operating characteristic; IL-6, interleukin-6; TNF-α, tumor necrosis factor-alpha; LDL, low-density lipoprotein; TILs, tumor-infiltrating lymphocytes.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Kyoda Y, Kobayashi K, Hirobe M, et al. Evaluation of long-term outcome for patients with renal cell carcinoma after surgery: analysis of cancer deaths occurring more than 10 years after initial treatment. Int J Clin Oncol. 2014;19(1):146–151. doi:10.1007/s10147-013-0533-x
3. Gong J, Maia MC, Dizman N, Govindarajan A, Pal SK. Metastasis in renal cell carcinoma: biology and implications for therapy. Asian J Urol. 2016;3(4):286–292. doi:10.1016/j.ajur.2016.08.006
4. Hu H, Yao X, Xie X, et al. Prognostic value of preoperative NLR, dNLR, PLR and CRP in surgical renal cell carcinoma patients. World J Urol. 2017;35(2):261–270. doi:10.1007/s00345-016-1864-9
5. Semeniuk-Wojtas A, Lubas A, Stec R, Syrylo T, Niemczyk S, Szczylik C. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and c-reactive protein as new and simple prognostic factors in patients with metastatic renal cell cancer treated with tyrosine kinase inhibitors: a systemic review and meta-analysis. Clin Genitourin Cancer. 2018;16(3):e685–e693. doi:10.1016/j.clgc.2018.01.010
6. Wang Z, Peng S, Wang A, et al. Platelet-lymphocyte ratio acts as an independent predictor of prognosis in patients with renal cell carcinoma. Clin Chim Acta. 2018;480:166–172. doi:10.1016/j.cca.2018.02.014
7. Byun SS, Hwang EC, Kang SH, et al. Age-dependent prognostic value of body mass index for non-metastatic clear cell renal cell carcinoma: a large multicenter retrospective analysis. J Surg Oncol. 2018;118(1):199–205. doi:10.1002/jso.25104
8. Jeon HG, Choi DK, Sung HH, et al. Preoperative prognostic nutritional index is a significant predictor of survival in renal cell carcinoma patients undergoing nephrectomy. Ann Surg Oncol. 2016;23(1):321–327. doi:10.1245/s10434-015-4614-0
9. Hofbauer SL, Pantuck AJ, de Martino M, et al. The preoperative prognostic nutritional index is an independent predictor of survival in patients with renal cell carcinoma. Urol Oncol. 2015;33(2):
10. Fukushima H, Nakanishi Y, Kataoka M, Tobisu K, Koga F. Prognostic significance of sarcopenia in patients with metastatic renal cell carcinoma. J Urol. 2016;195(1):26–32. doi:10.1016/j.juro.2015.08.071
11. Tsujino T, Komura K, Matsunaga T, et al. Preoperative measurement of the modified glasgow prognostic score predicts patient survival in non-metastatic renal cell carcinoma prior to nephrectomy. Ann Surg Oncol. 2017;24(9):2787–2793. doi:10.1245/s10434-017-5948-6
12. Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20(1):38–45.
13. Yoshida N, Baba Y, Shigaki H, et al. Preoperative nutritional assessment by Controlling Nutritional Status (CONUT) is useful to estimate postoperative morbidity after esophagectomy for esophageal cancer. World J Surg. 2016;40(8):1910–1917. doi:10.1007/s00268-016-3549-3
14. Kuroda D, Sawayama H, Kurashige J, et al. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. 2018;21(2):204–212. doi:10.1007/s10120-017-0744-3
15. Iseki Y, Shibutani M, Maeda K, et al. Impact of the preoperative Controlling Nutritional Status (CONUT) score on the survival after curative surgery for colorectal cancer. PLoS One. 2015;10(7):e0132488. doi:10.1371/journal.pone.0132488
16. Ishihara H, Kondo T, Yoshida K, et al. Preoperative controlling nutritional status (CONUT) score as a novel predictive biomarker of survival in patients with localized urothelial carcinoma of the upper urinary tract treated with radical nephroureterectomy. Urol Oncol. 2017;35(9):
17. Karam JA, Lotan Y, Karakiewicz PI, et al. Use of combined apoptosis biomarkers for prediction of bladder cancer recurrence and mortality after radical cystectomy. Lancet Oncol. 2007;8(2):128–136. doi:10.1016/S1470-2045(07)70002-5
18. Harimoto N, Yoshizumi T, Sakata K, et al. Prognostic significance of Preoperative Controlling Nutritional Status (CONUT) score in patients undergoing hepatic resection for hepatocellular carcinoma. World J Surg. 2017;41(11):2805–2812. doi:10.1007/s00268-017-4097-1
19. Deehan DJ, Heys SD, Simpson W, Herriot R, Broom J, Eremin O. Correlation of serum cytokine and acute phase reactant levels with alterations in weight and serum albumin in patients receiving immunotherapy with recombinant IL-2. Clin Exp Immunol. 1994;95(3):366–372. doi:10.1111/j.1365-2249.1994.tb07005.x
20. Heys SD, Walker LG, Deehan DJ, Eremin OE. Serum albumin: a prognostic indicator in patients with colorectal cancer. J R Coll Surg Edinb. 1998;43(3):163–168.
21. Tanriverdi O. A discussion of serum albumin level in advanced-stage hepatocellular carcinoma: a medical oncologist’s perspective. Med Oncol. 2014;31(11):282. doi:10.1007/s12032-014-0374-0
22. Gupta D, Lis CGJNJ. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9(1):69.
23. Ishizuka M, Nagata H, Takagi K, Kubota K. Influence of inflammation-based prognostic score on mortality of patients undergoing chemotherapy for far advanced or recurrent unresectable colorectal cancer. Ann Surg. 2009;250(2):268–272. doi:10.1097/SLA.0b013e3181b16e24
24. Kark JD, Smith AH, Hames CG. Serum retinol and the inverse relationship between serum cholesterol and cancer. Br Med J. 1982;284(6310):152–154. doi:10.1136/bmj.284.6310.152
25. Kitahara CM, Berrington de Gonzalez A, Freedman ND, et al. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29(12):1592–1598. doi:10.1200/JCO.2010.31.5200
26. Williams RR, Sorlie PD, Feinleib M, McNamara PM, Kannel WB, Dawber TR. Cancer incidence by levels of cholesterol. JAMA. 1981;245(3):247–252.
27. Wu B, Teng L, He D, Yu -D-D, Jiang F. Dose-response relation between serum total cholesterol levels and overall cancer risk: evidence from 12 prospective studies involving 1,926,275 participants. Int J Food Sci Nutr. 2018;70:1–10.
28. Niendorf A, Nägele H, Gerding D, Meyer-Pannwitt U, Gebhardt A. Increased LDL receptor mRNA expression in colon cancer is correlated with a rise in plasma cholesterol levels after curative surgery. Int J Cancer. 1995;61(4):461–464. doi:10.1002/ijc.2910610405
29. Cengiz O, Kocer B, Surmeli S, Santicky MJ, Soran A. Are pretreatment serum albumin and cholesterol levels prognostic tools in patients with colorectal carcinoma? Med Sci Monit. 2006;12(6):CR240–247.
30. Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6(1):149–163. doi:10.2217/fon.09.136
31. Hadrup S, Donia M, Thor Straten P. Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron. 2013;6(2):123–133. doi:10.1007/s12307-012-0127-6
32. Badalamenti G, Fanale D, Incorvaia L, et al. Role of tumor-infiltrating lymphocytes in patients with solid tumors: can a drop dig a stone? Cell Immunol. 2018. doi:10.1016/j.cellimm.2018.01.013
33. Feng JF, Liu JS, Huang Y. Lymphopenia predicts poor prognosis in patients with esophageal squamous cell carcinoma. Medicine (Baltimore). 2014;93(27):e257. doi:10.1097/MD.0000000000000257
34. Saroha S, Uzzo RG, Plimack ER, Ruth K, Al-Saleem T. Lymphopenia is an independent predictor of inferior outcome in clear cell renal carcinoma. J Urol. 2013;189(2):454–460. doi:10.1016/j.juro.2012.09.166
35. Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Jnci-J Natl Cancer I. 2014;106(6):dju124.
36. Zhou X, Du YP, Huang ZB, et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One. 2014;9(6):e101119.
37. Strohlein MA, Lefering R, Bulian DR, Heiss MM. Relative lymphocyte count is a prognostic parameter in cancer patients with catumaxomab immunotherapy. Med Hypotheses. 2014;82(3):295–299. doi:10.1016/j.mehy.2013.12.014
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

