Back to Journals » Drug Design, Development and Therapy » Volume 16
The Preventive and Therapeutic Effects of Ajwa Date Fruit Extract Against Acute Diclofenac Toxicity-Induced Colopathy: An Experimental Study
Authors Hassan SMA , Aboonq MS , Albadawi EA, Aljehani Y, Abdel-Latif HM, Mariah RA, Shafik NM, Soliman TM, Abdel-Gawad AR , Omran FM, Abdellah WA, Shehata A , Shahada H, Baghdadi HH, Aly HY, Saad A, Nabo MMH, Almilaibary A, Eltahir HM, El Sayed SM, Abu-Elnaga MAM, Elbastawisy YM
Received 13 October 2021
Accepted for publication 13 May 2022
Published 6 August 2022 Volume 2022:16 Pages 2601—2616
DOI https://doi.org/10.2147/DDDT.S344247
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Georgios Panos
Shaimaa Mohamed Abdelfattah Hassan,1 Moutasem Salih Aboonq,2 Emad A Albadawi,3 Yasmeen Aljehani,4,5 Hytham Mahmoud Abdel-Latif,6 Reham A Mariah,7 Noha M Shafik,7 Tamer M Soliman,8 Abdelhady Ragab Abdel-Gawad,8 Faten M Omran,6 Wafaa A Abdellah,6 Ahmed Shehata,9,10 Husam Shahada,11 Hussam H Baghdadi,12 Hanan Yousef Aly,13 Alfarazdeg Saad,12 Manal Mohamed Helmy Nabo,14 Abdullah Almilaibary,15 Heba M Eltahir,16 Salah Mohamed El Sayed,12,17 Mustafa AM Abu-Elnaga,18,19 Yasser M Elbastawisy3,20
1Histology and Cell Biology Department, Faculty of Medicine, Menoufia University, Menoufia, Egypt; 2Department of Medical Physiology, Taibah Faculty of Medicine, Taibah University, Al-Madinah Al-Munawwarah, Saudi Arabia; 3Anatomy and Embryology Department, Taibah College of Medicine, Taibah University, Al-Madinah, Saudi Arabia; 4Academic Affairs, Training and Research (CAO), King Salman Bin Abdelaziz Medical City, Al-Madinah Al-Munawwarah, Saudi Arabia; 5Family Medicine, Research and Studies Department and Health Affairs in Al-Madinah Region, Al-Madinah Al-Munawwarah, Saudi Arabia; 6Department of Medical Pharmacology, Sohag Faculty of Medicine, Sohag University, Sohag, Egypt; 7Department of Medical Biochemistry, Tanta Faculty of Medicine, Tanta University, Tanta, Egypt; 8Department of Clinical Pathology, Sohag Faculty of Medicine, Sohag University, Sohag, Egypt; 9Department of Pharmacology and Toxicology, College of Pharmacy, Taibah University, Al-Madinah Al-Munawwarah, Saudi Arabia; 10Department of Pharmacology and Toxicology, Faculty of Pharmacy, Beni-Suef University, Beni-Suef, Egypt; 11Department of Pharmaceutical Care, Uhud Hospital, Al-Madinah, Saudi Arabia; 12Department of Clinical Biochemistry, Taibah Faculty of Medicine, Taibah University, Al-Madinah Al-Munawwarah, Saudi Arabia; 13Department of Neuropsychiatry, Sohag Faculty of Medicine, Sohag University, Sohag, Egypt; 14Department of Pediatrics, Al-Rayyan Medical College, Al-Madinah, Saudi Arabia; 15Family and Community Medicine Department, Faculty of Medicine, Albaha University, Albaha, Saudi Arabia; 16Department of pharmacology and toxicology, Biochemistry Division, Taibah University, Medina, Saudi Arabia; 17Department of Medical Biochemistry, Sohag Faculty of Medicine, Sohag University, Sohag, Egypt; 18Department of Anatomy, College of Medicine, Al-Rayyan Medical Colleges, Al-Madinah, Saudi Arabia; 19Department of Anatomy, Faculty of Medicine, Al-Azhar University, Damietta, Egypt; 20Anatomy & Embryology Department, Faculty of Medicine, Mansoura University, Mansoura, Egypt
Correspondence: Alfarazdeg Saad, Department of Clinical Biochemistry and Molecular Medicine, Taibah Faculty of Medicine, Taibah University, Al-Madinah Al-Munawwarah, Saudi Arabia, Tel +249-91-219-0492, Email [email protected]
Background: Studies regarding treatment of acute toxicity with diclofenac (ATD) are quite few. Diclofenac is commonly prescribed in neurology, psychiatry, and general medicine practice. This study investigated possible colon-protective effects exerted by Ajwa date fruit extract (ADFE), a prophetic medicine remedy native to Al-Madinah, Saudi Arabia against ATD. Phytochemicals in ADFE as gallic acid and quercetin have reported protective effects against ATD.
Methods: Total phenols and flavonoids in ADFE were estimated as equivalents to gallic acid and quercetin. Four experimental groups were allocated each of six rats: control group, ATD group received a single dose of 150 mg diclofenac intraperitoneally, toxicity prevention group received a single dose of ADFE orally followed 4 hours later by diclofenac injection, and toxicity treatment group received a similar diclofenac dose followed 4 hours later by a single dose of ADFE. Four days later, animals were sacrificed. Histological and biochemical examinations were done.
Results: ADFE has a total phenolic content of 331.7 gallic acid equivalent/gram extract and a total flavonoid content of 70.23 quercetin equivalent/gram. ATD significantly increased oxidative stress markers as serum malondialdehyde (MDA) and hydrogen peroxide (H2O2). Serum MDA and H2O2 were significantly scavenged by ADFE. ATD significantly (p< 0.001) decreased antioxidant power as serum total antioxidant capacity and catalase activity. That was reversed by ADFE in both prevention and treatment groups. Histologically, ATD caused complete destruction of colonic crypts architecture, patchy loss of the crypts, loss of the surface epithelium, absent goblet cells and submucosal exudate, heavy infiltration of the lamina propria and submucosa with inflammatory cells, mainly lymphocytes and eosinophils. There were mucosal haemorrhages and submucosal dilated congested blood vessels. All that was prevented and treated using ADFE.
Conclusion: ADFE is rich in quercetin and gallic acid equivalents that exert potent antitoxic effects. ADFE is strongly recommended for preventive and therapeutic colon effects against ATD.
Keywords: diclofenac, acute toxicity, Ajwa date extract, colon, histology, polyphenols and flavonoids
Introduction
Reports regarding treatment of life-threatening acute toxicity with diclofenac (ATD) are quite few. Being an over-the-counter (OTC) drug, acute diclofenac overdosage is a commonly missed health problem in general medicine practice, neurology and psychiatry1–3 that causes colopathy ranging from colitis to colonic perforation. Incidence of gastrointestinal side effects of diclofenac is 37%, which is significantly higher than the rates for ibuprofen and for acetaminophen that are 7.2% and 7.6%, respectively.2,3 Diclofenac toxicity causes direct cellular toxicity to the intestinal cells,4,5 diaphragm-like strictures, ulcerations of the colon, bloody stools, and anaemia.6 Diclofenac toxicity caused massive small intestinal ulcerations, perforated duodenal ulcers, and multiple ileal perforations that may cause pneumoperitoneum and peritonitis. ATD caused death of experimental animals7,8 possibly due to colonic strictures, inflammatory bowel diseases and/or gut perforation. Colonic perforation is a differential diagnosis in patients having acute abdominal symptoms following toxicity with NSAIDs.9
Ajwa date fruit (Phoenix dactylifera L.) is native to Al-Madinah, Saudi Arabia (Figure 1A–E). Ajwa date fruit is famous in prophetic medicine (for its antitoxic effects). Prophetic medicine is the medical knowledge gained from teachings of Prophet Muhammad peace be upon him. Medicinal purposes of Ajwa dates are diverse owing to its phytochemical and nutritive components, eg, polyphenols as gallic acid and flavonoids as quercetin.10 Gallic acid mitigated diclofenac-induced liver toxicity by modulating oxidative stress and suppressing IL-1β gene expression in male rats.11 Similarly, quercetin exerted protective effects against diclofenac-induced liver toxicity through mitigation of inflammatory response and oxidative stress.12 Harmfull effects of ATD to the liver and lungs were significantly alleviated with Ajwa date fruits.13 Ajwa date fruit significantly reduced hospital admissions and infections and improved survival in pediatric oncology practice.14 Ajwa date fruits maximally inhibited bacterial biofilm and protected the calf thymus DNA against damage caused by hydroxyl radicals produced by Fenton reagent.15 Ajwa date fruit extract (ADFE) exhibited potent antitoxic effects that abrogated carbon tetrachloride-induced hepatotoxicity.16 The phenolic contents of ADFE improved lipid profile and antioxidant defense system in rats fed a cholesterol-rich diet.17 In an interesting recent report, ADFE reverted malignantly induced hepatocellular carcinoma cells (exposed to the hepatocarcinogen diethylnitrosamine) to a normal hepatocyte phenotype.18
 |
Figure 1 Continued. |
Ajwa palm trees are grown in gardens in Aliah region at southern areas of Al-Madinah, Saudi Arabia and Ajwa date fruit is recommended in prophetic medicine for antitoxin prevention and treatment giving protection for a daytime duration (about 12 hours).19,20
This study aimed at investigating the preventive and tissue-protective effects exerted by ADFE to the colon against ATD. This study also aims at introducing ADFE as a natural source for promising future antitoxins and therapeutics.
Materials and Methods
Study Design and Ethical Aspects
A prior ethical committee approval was gained from the College of Medicine, Taibah University, Saudi Arabia (no. IORG0008716-IRB00010413). Study ID was 039–1440.
Preparation of Ajwa Date Fruit Extract (ADFE)
Ajwa date fruits (Phoenix dactylifera L.) cultivated in Al-Madinah (Figure 1A–E) were harvested in summer season from a local farm in Al-Madinah Al-Munawwarah, Saudi Arabia. ADFE preparation was carried out at the pharmacognosy department at Taibah College of Pharmacy. Ajwa dates were air-dried to keep the active ingredients, sliced into small portions and then minced. Three kilograms of Ajwa dates were lyophilized and then dried via freezing and were macerated twice in 3 liters of ethyl alcohol (ethanol) and water in a ratio of 80:20 and then incubated at room temperature for 72 hours. ADFE was filtered using Whatman filter paper Grade 4: having circles of 27 mm to 400 mm in a Büchner funnel.
Ethanolic extract of Ajwa date fruit was concentrated under reduced pressure at 50 °C using rotary vacuum evaporator. The solvent-free plant extracts (1800 grams) were kept in a refrigerator at 4 °C for further tests and applications.
Determination of Total Phenolic Compounds
Folin-Ciocalteau reagent was utilized for the evaluation of total phenolic content of Ajwa extract as described earlier.21,22 Briefly, 0.5 mL of the extract solution (10 mg/mL) was mixed with 0.5 mL of 10% Folin-Ciocalteau and incubated for 5 minutes, then 2.5 mL of 7% NaNO2 (w/v) was added to the extract-reagent mixture and incubated for 1 hour in the dark room. Finally, absorbance of the resulting blue color was measured at 765 nm using a UV/Vis spectrophotometer. For calibration, serial dilutions of gallic acid (0.078, 0.156, 0.312, 0.625 and 1.25 mg/mL) were incubated similarly with Folin-Ciocalteau and 7% NaNO2 solution. The experiments were done in triplicate and the amount of total phenolic content was calculated as gallic acid equivalents (GAE) per gram of the extract based on the calibration curve equation (y = 1.0036x + 0.0821, R2=0.9862).
Determination of Total Flavonoids Content
Aluminum chloride colorimetric method was used to evaluate the flavonoid content of the extract as described elsewhere.23 In brief, 1 mL of the extract solution (10 mg/mL) was filled up to 5 mL with double distilled water, then 0.3 mL of NaNO2 (5% w/v, in water) was added, mixed thoroughly and incubated for 5 minutes before adding 0.3 mL Aluminum chloride solution. After another incubation of 5 minutes, 2 mL of 1M NaOH was added and the volume was filled up to 10 mL with double distilled water. The absorbance of the reaction product was measured at 416 nm using a UV/Vis spectrophotometer. A calibration curve was established by conducting the same procedures for quercetin using a series of concentrations (20, 40, 60, 80, and 100 µg/mL) and blotting the observed absorbance against these concentrations. The total amount of flavonoid content was calculated as quercetin equivalents (QE) per gram of extract based on the calibration curve equation (y = 0.0013x + 0.0117, R2=0.9997).
Experimental Studies
Our study investigated possible colon-damaging effects after a single intraperitoneal injection of a toxic dose of diclofenac (150 mg/kg). Study timeline was indicated (Figure 2). White albino Sprague-Dawley rats weighing 350–400 grams were purchased from the central animal facility of King Abdul-Aziz University, Jeddah, Saudi Arabia. The animals were maintained for three weeks prior to experimentation in the animal facility unit at Taibah University in completely aseptic and pathogen-free conditions. The animals were kept inside polypropylene cages at a temperature of 22 ± 3 °C with a relative humidity of 65% to 86%. The rats received a standard laboratory pellet chow diet (oral feeding) in addition to pathogen-free water ad libitum throughout the duration of the experiment. Appropriate care and hygienic measures were taken to ensure a healthy location and atmosphere for the rats all the time. They were housed under standard conditions of temperature and lighting (12-hour light/dark cycles). The animals’ weights were periodically evaluated. All rats received care in accordance with the rules and regulations of the medical research ethics committee of Taibah College of Pharmacy, Taibah University, Al-Madinah Al-Munawwarah, Saudi Arabia.
Selection of an acute dose of diclofenac toxicity in this study (150 mg/kg once) on the colon was based on two previous reports: the former by Aljuhani et al who used a single injection of diclofenac at a dose of 200 mg/kg13 and Elshopakey et al used a dose of 100 mg/kg.24 We used the average of the two doses.
Twenty-four rats, including 12 male rats and 12 female rats, were enrolled in this study. Average age was six months and average body weight was about 375 grams per rat. They were assigned into four experimental groups. A total of six rats, including three males and three females per group, were allocated.
1. Control group was group I that received sterile water injection.
2. Positive control was group II that received a single dose of 150 mg diclofenac injected intraperitoneally.
3. Toxicity treatment group was group III that received a single dose of 150 mg diclofenac intraperitoneally followed four hours later by a single dose of ADFE orally using oral gavage.
4. Toxicity prevention group was group IV that received a single dose of ADFE 2 grams/kg orally using oral gavage followed four hours later by a diclofenac at a dose of 150 mg diclofenac intraperitoneally.
Four days later, the animals were sacrificed by decapitation and blood samples were collected. Blood samples were drawn (after decapitation) and cardiac puncture. Blood was collected in sterile plain tubes and centrifuged (4000 rpm/minute) and the serum was kept in −30 C0 for future biochemical assays. A histological examination of the colon was carried out in the four experimental groups.
Assay of Serum Malondialdehyde (MDA)
The levels of serum MDA (μM) were assayed using the MDA ELISA Kit (E-EL-0060, Elabscience, TX, USA). Briefly, the standards and samples were pipetted into microplates coated with a monoclonal antibody and incubated. Positive and negative controls were included in each assay. Biotin was added to all wells, then streptavidin-HRP was added. After incubation, 4 washes were performed to remove unbound reagents. After adding chromogen solutions, stop solution was added, and the resulting optical density was measured at 450 nm using a plate reader (Biotek Synergy multimode microplate reader (VT, USA)). The detection range of the kits was between 31.25 and 2000 ng/mL. The concentrations of MDA were calculated from the standard curve for each assay.
Serum Hydrogen Peroxide Assay
Serum hydrogen peroxide assay was done using hydrogen peroxide assay kits (Elabscience, TX, USA), following the manufacturer’s instructions. Briefly, 60 mmol/L H2O2 of standard solution was used. Blank tubes (containing distilled water), standard tubes (containing 60 mmol/L H2O2 standard solution) and sample tubes (containing serum) were prepared. Buffer solution and then ammonium molybdate solution were added according to the manufacturer’s instructions. Absorbance readings were taken at 405 nm wavelength using a Biotek Synergy multimode microplate reader (VT, USA). A standard curve was drawn and H2O2 concentrations were determined.
Serum Catalase Assay
Serum catalase activity (antioxidant) assay was done using catalase assay kits (Elabscience, TX, USA) according to the manufacturer’s instructions and was based on the principle that catalase decomposes H2O2 and that can be quickly stopped by ammonium molybdate. The residual H2O2 reacts with ammonium molybdate to generate a yellowish complex. Briefly, blank tubes (containing distilled water), and test sample tubes (containing serum) were prepared using buffer solution, reagent and chromogenic agents supplied in the kits. All tubes were allowed to stand at room temperature for ten minutes followed by taking 200 μL of reaction solution to the microplate with a micropipette. Absorbance readings were taken at 405 nm wavelength using the Biotek Synergy multimode microplate reader. A standard curve was drawn and catalase activity in the samples was assayed.
Total Antioxidant Capacity Assay
Total antioxidant capacity was assayed using total antioxidant capacity assay kits (elabscience, TX, USA) according to manufacturer’s instructions. Briefly, fresh blood was collected and stood at 25°C for 30 min to clot. Then, centrifugation was done at 2000 g for 15 min at 4°C. In test tubes, 100 μL of buffer solution was added to 1.5 mL eppendorf tubes followed by adding 10 μL of serum. Then, 200 μL of chromogenic agent working solution and 50 μL of ferric salt working solution was added to the samples tubes and control tubes. That was mixed fully to react at 37°C for 30 min. Ferric salt diluent, stop solution and clarificant were sequentially added. After mixing fully and standing for 10 min at room temperature, 300 μL of the reaction liquid was added to the 96 well microplates and the OD value of each well was measured at 520 nm using Biotek Synergy multimode microplate reader.
Tissue Sample Preparation and Light Microscopic Study
Four days after starting this acute toxicity study experiment, all rats in the study were sacrificed using ether anaesthesia followed by decapitation. Decapitation was used to gain blood samples. Colon was extracted from rats in all the experimental groups. Ether anaesthesia did not affect the blood or tissues in all animals in the different experimental groups. In addition, the animals were sacrificed immediately after administration of ether anaesthesia. Rat’s abdomen was dissected, and samples of the proximal colon were obtained and washed with saline solution to remove faeces.
Colon specimens from each rat were cut into pieces, fixed in 10% phosphate buffered formaldehyde, and embedded in paraffin wax. Then, 5-μm-thick sections were cut using a microtome and fixed into glass slides for haematoxylin and eosin (H&E) staining. The slides were examined under a high-resolution microscope (Nikon, Tokyo, Japan). Histological colonic specimens received further preparation processes, such as staining using haematoxylin and eosin for general morphology evaluation.
Specimens were fixed in 10% buffered formaldehyde for two days at 4°C and processed for paraffin sections. Sections (5 µm thick) were cut and stained with haematoxylin and eosin (H&E), as was previously reported.25
Morphometric Study
The haematoxylin and eosin slides were examined under a standard microscope using an X40 lens, and five fields of each slide were photographed using a Nikon E400 digital microphotography system (N150, Nikon, Tokyo, Japan). The number of goblet cells and lymphocytes were calculated using the Digimizer software program, version 4.6.1 (MedCalc Software Ltd, Acacialaan, Belgium).26
Quantitative Assessment and Statistics
All data (number of goblet cells and lymphocytes) was statistically analysed using SPSS software (version 16.00; SPSS Inc., Chicago, Illinois, USA). Data was expressed as mean± SD and analysed by using one-way ANOVA analysis of variance followed by Bonferroni’s multiple comparison post-hoc tests for comparison between all groups. * indicates p <0.05, ** indicates p <0.01 and ***indicates p <0.001. Testing for normality distribution of data was performed using numerical methods in SPSS to determine skewness and kurtosis using SPSS as previously indicated.27
Results
Total Phenolic and Total Flavonoid Contents in Ajwa Date Fruit Extract
The total phenolic content of the extract was quantified using Folin-Ciocalteau reagent, where the content was calculated by the aid of gallic acid calibration curve. Based on these calculations, the extract had a total phenolic content of 331.7 GAE/g extract (Figure 3A).
The total flavonoid content of the extract was quantified using aluminum chloride method, where the content was calculated by the aid of quercetin calibration curve. Based on these calculations, the extract had a total flavonoid content of 70.23 QE/g extract (Figure 3B).
Acute Diclofenac Toxicity-Induced Oxidative Stress That Was Counteracted by ADFE
Acute diclofenac toxicity significantly induced increased oxidative stress. That was evidenced by significantly increased serum MDA following acute diclofenac toxicity (p<0.01). Serum MDA was significantly lower (p<0.01) in both toxicity treatment and toxicity prevention groups (Figure 4A). Acute diclofenac toxicity-induced oxidative stress was also evidenced by increased serum H2O2 (74.63± 26.65 mmol/L) versus baseline values (40.28± 5.08 mmol/L). The H2O2 quantity was significantly scavenged by prior treatment with ADFE (prevention group) to below baseline values (33.54± 7.91 mmol/L). Giving ADFE after an acute toxic dose of diclofenac (treatment group) significantly decreased serum H2O2 to near baseline values (43.93± 5.57 mmol/L) (Figure 4B). Oxidative stress data was approximately normally distributed for diclofenac-induced H2O2 generation with a skewness of −0.098 (SE= 0.918) and a kurtosis of −1.908 (SE=2.0). Similarly, data on diclofenac-induced MDA generation was approximately normally distributed with a skewness of −0.738 (SE=0.845) and a kurtosis of −1.178 (SE=1.741).
ADFE Significantly Prevented and Treated Diclofenac Toxicity-Induced Decrease in Serum Catalase and TAC
Acute diclofenac toxicity significantly (p<0.001) decreased antioxidant power as indicated by decreased serum catalase activity to 5.91± 0.73 U/mL versus 25.21± 2.04 U/mL at baseline values. A prior intake of ADFE in prevention group significantly protected against such decrease and increased serum catalase levels to about 27.66± 2.18 U/mL at baseline values. Giving ADFE after an acute toxic dose of diclofenac in the toxicity treatment group significantly increased serum catalase to a higher value reaching 15.39± 1.4 U/mL (Figure 4C). Serum TAC significantly decreased (p<0.001) upon acute diclofenac toxicity. Serum TAC significantly increased (p<0.001) to near normal values upon the intake of ADFE after acute toxic dose of diclofenac in the toxicity treatment group and also when giving ADFE prior to giving a toxic dose of diclofenac (Figure 4D). Data of ADFE-induced increase in serum catalase was approximately normally distributed with a skewness of −0.098 (SE=0.918) and a kurtosis of 0.753 (SE=0.687). Similarly, data on ADFE-induced increase in TAC was approximately normally distributed with a skewness of −0.073 (SE=0.913) and a kurtosis of −1.477 (SE=2.00).
Light Microscopic Results (Haematoxylin and Eosin Staining)
Normal Colon Histology in the Control Group
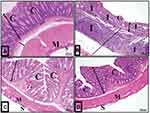
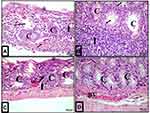
Examination of colon sections of the control group (group I) revealed normal histological architecture of its mucosa demonstrating numerous, regularly arranged, evenly spaced and tightly packed crypts. The crypts occupied the whole thickness of the mucosa resting on the muscularis mucosa. The surface epithelium and crypt lining were formed mainly of simple columnar absorptive cells and goblet cells. The number of simple columnar absorptive cells decreased when going deeper into the crypt, while goblet cells became more numerous. Connective tissue lamina propria housed normally resident immune cells, mainly lymphocytes (Figures 5A and 6A).
Acute Diclofenac Toxicity Effects on the Colon
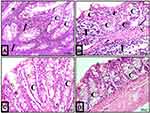
Examination of colon sections of group II (acute diclofenac toxicity group) showed many features of mucosal affection, namely distorted mucosa, absence of foldings, loss of columnar surface cells and goblet cells, and malformed crypts with even patchy loss of colonic crypts (Figures 5B, 6B, 7A–7C and 5D). The lumen of some crypts showed cellular debris (Figure 5B). There was a heavy inflammatory infiltration in the mucosa and in the widened submucosa together with the presence of polymorph nuclear leukocytes and lymphocytes (Figures 5B, 6B and 7A) and eosinophil in the lamina propria (Figures 6B and 7C). Also, areas of mucosal haemorrhage (Figure 7C), submucosal exudate (Figures 6B and 7A) and dilated congested blood vessels in submucosa were found (Figure 7D).
Therapeutic Effects of Ajwa Date Fruit Against Acute Diclofenac Toxicity
Examination of the colon sections of the toxicity treatment group (that received an acute toxic dose of diclofenac and then treated few hours later with ADFE) showed apparently normal histological architecture. Colon mucosa exhibited preserved crypts with intact continuous surface epithelium and numerous goblet cells (Figures 5D and 6D).
Preventive Effects of Ajwa Date Fruit Against Acute Diclofenac Toxicity
Examination of colon sections of the toxicity prevention group of rats (that were treated with ADFE and exposed few hours later to an acute toxic dose of diclofenac) showed marked improvement, with restored normal colon histological architecture. Colon mucosa exhibited preserved crypts that were lined with columnar epithelium and numerous goblet cells (Figures 5C and 6C).
Morphometric Results
There was a significant decrease in goblet cells count in the acute diclofenac toxicity group compared to the control group. Also, toxicity treatment group showed a significant decrease in goblet cell count compared to the control group and a significant increase compared to acute diclofenac toxicity group. Toxicity prevention group showed insignificant differences in goblet cell counts compared with the control group, but showed statistically significant differences in comparison with groups II (diclofenac toxicity) and toxicity prevention group (Figure 8).
 |
Figure 8 Goblet cells count (Mean ± SD) in different study groups. *means p< 0.05 and ***means p< 0.001. |
There was a highly significant increase in the number of lymphocytes in group II compared to the control group (group I) (p<0.001). Group III (toxicity treatment group) showed a significant increase in the number of lymphocytes compared to the control group (group I) (p < 0.05), but group IV (toxicity prevention group) showed insignificant differences compared to the control group (Figure 9).
 |
Figure 9 Lymphocytes count (Mean ± SD) in different study groups. *means p< 0.05 and ***means p< 0.001. Abbreviation: ATD, acute toxicity with diclofenac. |
Discussion
Toxicity with diclofenac is a vital health issue as NSAIDs are commonly used drugs in a wide range of medical specialties, eg, neuropsychiatry,28 orthopaedics,29 rheumatology,30 internal medicine, and others. NSAIDs commonly induce acute and chronic toxicity to the upper gastrointestinal mucosa, for example, causing inflammation and ulcerations in the colon. Diclofenac is an OTC treatment1 that may cause acute and chronic toxicity upon treating painful conditions, particularly when taken at home away from medical supervision. Our data (Figures 5–7) agreed with the report that diclofenac toxicity may present with abdominal pain, weight loss and diarrhoea. Proctitis, colonic ulceration and associated complications, such as colon perforation, bleeding, stricture formation and even fistula formation may also occur.31 Lack of effective pharmacological remedies to prevent and treat diclofenac toxicity encouraged the authors to investigate a possible role of ADFE for potential preventive and therapeutic benefits to ATD.
Ajwa date fruit is a promising medicinal plant that is recommended in prophetic medicine for prevention and treatment of toxicity in general.19 Ajwa date fruit is native to Al-Madinah, Saudi Arabia, where Ajwa palm trees are a major part of the natural scenery there (Figure 1). Gallic acid is an active ingredient in Ajwa date fruit. Hamad et al found p-coumaric acid, gallic acid and ferulic acid derivatives were the most dominant phenolic compounds in Ajwa dates.10–12 Ajwa date fruit is also rich in active flavonoids including quercetin, isoquercetin, luteolin, apigenin, and rutin.32 Both gallic acid11 and quercetin12 proved effective in treating diclofenac toxicity. Our data agreed with these reports and confirmed that ADFE is very rich in both gallic acid and quercetin (Figure 3A and B). Our data agreed with Khalid et al who reported that more than 50% of the polyphenols in ADFE is gallic acid and more than 50% of the flavonoids in ADFE is quercetin.10–12,32
In this study, acute diclofenac toxicity caused significantly (p<0.001) increased serum oxidants, ie ATD caused increased serum MDA and H2O2 concentration denoting increased oxidative stress. This is in agreement with the report by Esmaeilzadeh et al where diclofenac toxicity caused increased serum MDA and decreased serum catalase and that was corrected upon treatment with gallic acid.11 This explains that diclofenac-induced colonic tissue damage is due to oxidative stress. Such oxidative stress was completely and significantly prevented (p<0.001) by giving ADFE before administering a toxic dose of diclofenac in the toxicity prevention group (group IV). Likewise, serum H2O2 levels significantly decreased after treating diclofenac toxicity with ADFE. Biochemical oxidative stress is in line with the tissue damage induced by diclofenac toxicity, denoting that acute diclofenac poisoning induces oxidative tissue damage to the colon.
Our data illustrated the mechanism of acute diclofenac toxicity-induced tissue damage to be due to increased oxidants versus decreased serum antioxidants as TAC and serum catalase activity, denoting decreased antioxidant power versus increased oxidative stress effects. This further explains the mechanisms of diclofenac-induced colonic tissue damage. This agreed with a previous report.11 Such a decrease in antioxidant power was completely and significantly prevented upon giving ADFE before giving a toxic dose of diclofenac (prevention group). Likewise, serum catalase activity levels significantly increased after treating diclofenac toxicity with ADFE. Biochemical oxidative stress goes in line with the tissue damage induced by acute diclofenac toxicity, denoting that diclofenac poisoning induces oxidative tissue damage to the colon that can be completely prevented and significantly treated by the antioxidants contained in ADFE.
Our data (Figure 7) agreed with previous publications33 that destruction of the colonic crypts architecture and patchy loss of the crypts causing ulcers and damage are induced by acute diclofenac toxicity. Diclofenac is commonly prescribed for treating geriatric conditions, where a relatively large dose of diclofenac (50 mg four times daily) may be given for treating osteoarthritis. Diclofenac toxicity caused colonic ulcerations, positive stool tests for occult blood, anaemia, minimal antral gastritis (by esophagogastroduodenoscopy), colonic ulceration of the ascending colon, cecum ulcerations and perforation of the terminal ileum. All that was reported. A biopsy of the colonic mucosa showed focal necrosis and an acute inflammatory infiltrate. This improved after stopping diclofenac intake.33
In agreement with our data (Figure 4A–D), Aycan et al reported that subchronic diclofenac toxicity caused increased generation of reactive oxygen/nitrogen species and decreased TAC in the tissues resulting in gastrointestinal lesions, kidney lesions and increased fibrosis in rats. Thymoquinone, the major active antioxidant ingredient in Nigella Sativa, significantly alleviated diclofenac-induced haematological and tissue damage in the stomach and duodenum. Diclofenac treatment induced a decrease in TAC in the kidneys, stomach and duodenal tissue. Thymoquinone administration increased gastrointestinal and renal TAC in diclofenac-treated rats, suggesting that thymoquinone can alleviate the gastrointestinal and renal toxicity induced by diclofenac poisoning.34 Ajwa dates contain seed, moisture, fructose, glucose, soluble protein, and fiber and strongly inhibit lipid peroxidation, and cyclooxygenase enzymes and exert potent antioxidant and anti-inflammatory effects.35,36 This agreed with our data that ADFE is rich in polyphenols and flavonoids that exert potent antioxidant effects (Figure 3A and B).
Our study agreed with the previous reports35,36 where examination of colonic sections of the control group (group I) revealed normal histological architecture of the mucosa, demonstrating numerous, regularly arranged, evenly spaced and tightly packed crypts. The crypts occupied the whole thickness of the mucosa resting on the muscularis mucosa. The surface epithelium and crypt lining are formed mainly of simple columnar absorptive cells and goblet cells. The simple columnar absorptive cells become fewer and deeper into the crypts, while goblet cells become more numerous. Connective tissue lamina propria houses normally resident immune cells, mainly lymphocytes.
Moreover, examination of colon sections of group II (acute diclofenac toxicity group) showed many features of mucosal affection as distorted mucosa, absence of foldings, loss of columnar surface cells and goblet cells, malformed crypts and even patchy loss of crypts. The lumen of some crypts showed cellular debris. There was heavy inflammatory infiltration in the mucosa and in the widened submucosa together with the presence of polymorph nuclear leukocytes, lymphocytes and eosinophils in the lamina propria. Also, areas of mucosal haemorrhage, submucosal exudates and dilated congested blood vessels in submucosa were found. This is in agreement with the report by Khan et al who reported damaged architecture of hepatocytes exposed to hepatocarcinogens.18
Examination of colon sections of group III (acute diclofenac toxicity followed by treatment with ADFE) showed apparently normal histological architecture. Colonic mucosa exhibited preserved crypts with intact continuous surface epithelium and numerous goblet cells. Examination of colon sections of group IV (rats that received ADFE then exposed to ATD) showed marked improvement and restored the normal colon histological architecture. Colonic mucosa exhibited preserved crypts which were lined with columnar epithelium and numerous goblet cells. This is in agreement with the report by Khan et al who reported a significant improvement of the damaged architecture of hepatocytes exposed to hepatocarcinogens upon treatment using Ajwa date fruit.18 A graphical abstract (that summarized our methodology and findings) is illustrated (Figure 10).
 |
Figure 10 A graphic indicating the promising therapeutic and preventive effects induced by Ajwa date fruit against diclofenac-induced colon toxicity. |
Novelty of our data came from using Ajwa date fruit to prevent and treat colon toxicity induced by diclofenac. This is the first report on that. To the best of our knowledge, this study is novel. No similar studies were reported using Ajwa date fruit to treat colonic effects of diclofenac toxicity. In the future, we plan to expand the course of this study to include genomic expression studies.
Conclusion
ADFE is rich in polyphenols as gallic acid and flavonoids as quercetin that have potent antioxidant and antitoxic effects. ADFE maximally and significantly prevented and treated diclofenac toxicity-induced biochemical and colonic damage resulting in improved histology of the colon towards normal. Ajwa date fruit extract is strongly recommended for both the prevention and treatment of diclofenac toxicity owing to its general antitoxic effects.
Acknowledgments
The authors are grateful to the Medical Research Centre and the Deanship of Scientific Research at Taibah University, Saudi Arabia for kindly supporting the kits and research materials for this article in the research project #8016. We are so grateful to the memory of the two late professors Professor Mohamed El-Kablawy (professor of pathology, Taibah University, Al-Madina, Saudi Arabia) and Professor Osama Bashir (professor of pharmacognosy, Taibah University, Al-Madina, Saudi Arabia) for their efforts and support to this work.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in relation to this work.
References
1. Jan M, Singh G, Zarfishan A, et al. Pre-emptive analgesic efficacy of intravenous diclofenac versus paracetamol in orthopedic surgery. Bahrain Medical Bull. 2021;43(1):354–358.
2. Franceschi F, Saviano L, Petruzziello C, et al. Safety and efficacy of low doses of diclofenac on acute pain in the emergency setting. Eur Rev Med Pharmacol Sci. 2016;20(20):4401–4408.
3. Moore N, Scheiman JM. Gastrointestinal safety and tolerability of oral non-aspirin over-the-counter analgesics. Postgrad Med. 2018;130:188–199. doi:10.1080/00325481.2018.1429793
4. Inan A, Koca C, Sen M. Effects of diclofenac sodium on bursting pressures of anastomoses and hydroxyproline contents of perianastomotic tissues in a laboratory study. Int J Surg. 2006;4:222–227. doi:10.1016/j.ijsu.2006.01.002
5. Niu X, de Graaf IA, Langelaar-Makkinje M, et al. Diclofenac toxicity in human intestine ex vivo is not related to the formation of intestinal metabolites. Arch Toxicol. 2015;89:107–119. doi:10.1007/s00204-014-1242-6
6. Munipalle PC, Garud T, Light D. Diaphragmatic disease of the colon: systematic review. Colorectal Dis. 2013;15(9):1063–1069. doi:10.1111/codi.12218
7. Reuter BK, Cirino G, Wallace JL. Markedly reduced intestinal toxicity of a diclofenac derivative. Life Sci. 1994;55(1):PL1–PL8. doi:10.1016/0024-3205(94)90083-3
8. Park WS, Kim SW, Lee S, Lee ST, Park HS. Multiple ileal perforations due to regular diclofenac sodium injections: a case report. BMC Res Notes. 2013;6:129. doi:10.1186/1756-0500-6-129
9. Shahzad N, Maqbool A, Gauhar TM, et al. Colonic perforation induced by short term use of slow release diclofenac. J Coll Physicians Surg Pak. 2012;22:328–329. doi:05.2012/JCPSP.328329
10. Khalid S, Khalid N, Khan RS. A review on chemistry and pharmacology of Ajwa date fruit and pit. Trends Food Sci Technol. 2017;63:60e69.
11. Esmaeilzadeh M, Heidarian E, Shaghaghi M. Gallic acid mitigates diclofenac-induced liver toxicity by modulating oxidative stress and suppressing IL-1β gene expression in male rats. Pharm Biol. 2020;58(1):590–596. doi:10.1080/13880209.2020.1777169
12. Nouri A, Heidarian E, Amini-khoei H. Quercetin through mitigation of inflammatory response and oxidative stress exerts protective effects in rat model of diclofenac induced liver toxicity. J Pharm Pharmacogn Res. 2019;7(3):200–212.
13. Aljuhani N, Elkablawy MA, Elbadawy HM. Protective effects of Ajwa date extract against tissue damage induced by acute diclofenac toxicity. J Taibah Univ Med Sci. 2019;14(6):553–559. doi:10.1016/j.jtumed.2019.10.002
14. Al Jaouni SK, Hussein A, Alghamdi N. Effects of Phoenix dactylifera Ajwa on infection, hospitalization, and survival among pediatric cancer patients in a University Hospital: a nonrandomized controlled trial. Integr Cancer Ther. 2019;18:1534735419828834. doi:10.1177/1534735419828834
15. Qasim N, Shahid M, Yousaf F. Therapeutic potential of selected varieties of phoenix dactylifera L. Against microbial biofilm and free radical damage to DNA. Dose Response. 2020;18(4):1559325820962609. doi:10.1177/1559325820962609
16. Elsadek B, El-Sayed ES, Mansour A, Elazab A. Abrogation of carbon tetrachloride-induced hepatotoxicity in Sprague-Dawley rats by Ajwa date fruit extract through ameliorating oxidative stress and apoptosis. Pak J Pharm Sci. 2017;30(6):2183–2191.
17. Alqarni MMM, Osman MA, Al-Tamimi DS, et al. Antioxidant and antihyperlipidemic effects of Ajwa date (Phoenix dactylifera L.) extracts in rats fed a cholesterol-rich diet. J Food Biochem. 2019;43(8):e12933. doi:10.1111/jfbc.12933
18. Khan F, Khan TJ, Kalamegam G, et al. Anti-cancer effects of Ajwa dates (Phoenix dactylifera L.) in diethylnitrosamine induced hepatocellular carcinoma in Wistar rats. BMC Complement Altern Med. 2017;17:418. doi:10.1186/s12906-017-1926-6
19. Albukhari MBI, Al-Bukhari S. Treatment of magic using Al-Ajwa. In: Book of medicine. 1st ed. Lebanon: Ibn Kathir Publishing House; 2002(10):249.
20. Khan M, Siddiqui N. Role of Ajwa in the treatment and prevention of ischaemic heart disease. J Pak Med Assoc. 2017;67(12):1954.
21. Saeed N, Khan MR, Maria Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012;12:221. doi:10.1186/1472-6882-12-221
22. Kim DO, Jeong SW, Lee CY. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003;81:321–326. doi:10.1016/S0308-8146(02)00423-5
23. Park Y-S, Jung S-T, Kang S-G, et al. Antioxidants and proteins in ethylene-treated kiwifruits. Food Chem. 2008;107:640–648. doi:10.1016/j.foodchem.2007.08.070
24. Elshopakey GE, Elazab ST. Cinnamon aqueous extract attenuates diclofenac sodium and oxytetracycline mediated hepato-renal toxicity and modulates oxidative stress, cell apoptosis, and inflammation in male albino rats. Vet Sci. 2021;8:9. doi:10.3390/vetsci8010009
25. Perry C, Chung JY, Ylaya K. A buffered alcohol-based fixative for histomorphologic and molecular applications. J Histochem Cytochem. 2016;64:425–440. doi:10.1369/0022155416649579
26. Wang SL, Yang SF, Chan CY, et al. Computerized morphometric study of thyroid follicular carcinoma in correlation with known prognostic factors. Kaohsiung J Med Sci. 2005;21:65–69. doi:10.1016/S1607-551X(09)70279-7
27. Razali NM, Wah YB. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov lilliefors and Anderson-Darling tests. JOSMA. 2011;2(1):21–33.
28. Amelin AV, Tereshchenko NM, Gotovchikov AA. Clinical experience with the use of a fixed combination of diclofenac and orphenadrine in the treatment of acute pain syndrome. Zh Nevrol Psikhiatr Im S S Korsakova. 2021;121(8):83–86. doi:10.17116/jnevro202112108183
29. Cooper C, Chapurlat R, Al-Daghri N, et al. Safety of oral non-selective non-steroidal anti-inflammatory drugs in osteoarthritis: what does the literature say? Drugs Aging. 2019;36(Suppl 1):15–24. doi:10.1007/s40266-019-00660-1
30. Kean WF, Rainsford KD, Kean IR. Management of chronic musculoskeletal pain in the elderly: opinions on oral medication use. Inflammopharmacology. 2008;16(2):53–75. doi:10.1007/s10787-008-1623-7
31. Arya N, Hawe MJ, Ozo C. Diclofenac suppositories and acute ischaemic proctitis. Ulster Med J. 2004;73(1):63–64.
32. Hamad I, AbdElgawad H, Al Jaouni S, et al. Metabolic analysis of various date palm fruit (Phoenix dactylifera L.) cultivars from Saudi Arabia to assess their nutritional quality. Molecules. 2015;20:13620e13641. doi:10.3390/molecules200813620
33. Aloysius MM, Kaye PV, Lobo DN. Non-steroidal anti-inflammatory drug (NSAID)-induced colonic strictures and perforation: a case report. Dig Liver Dis. 2006;38:276–278. doi:10.1016/j.dld.2005.09.003
34. Aycan İÖ, Elpek Ö, Akkaya B, et al. Diclofenac induced gastrointestinal and renal toxicity is alleviated by thymoquinone treatment. Food Chem Toxicol. 2018;118:795–804. doi:10.1016/j.fct.2018.06.038
35. Zhang CR, Aldosari SA, Vidyasagar PS, Nair KM, Nair MG. Antioxidant and anti-inflammatory assays confirm bioactive compounds in Ajwa date fruit. J Agric Food Chem. 2013;61:5834–5840. doi:10.1021/jf401371v
36. Gonçalves Junior I, Naresse LE, Rodrigues MA, etal. Diclofenac sodium and imipenem action on rat intestinal mucosa: a biomechanical and histological study. Acta Cir Bras. 2012;27:131–136. doi:10.1590/S0102-86502012000200006
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.