Back to Journals » OncoTargets and Therapy » Volume 15
The Predictive Value of Systemic Inflammatory Factors in Advanced, Metastatic Esophageal Squamous Cell Carcinoma Patients Treated with Camrelizumab
Authors Liu J , Gao D, Li J, Hu G, Liu J, Liu D
Received 19 July 2022
Accepted for publication 24 September 2022
Published 10 October 2022 Volume 2022:15 Pages 1161—1170
DOI https://doi.org/10.2147/OTT.S382967
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Jiang Liu,1,* Deyu Gao,2 Jiaheng Li,3 Guangyin Hu,1 Jianhua Liu,1 Degan Liu1,*
1The Affiliated Xinghua People’s Hospital, Medical School of Yangzhou University, Xinghua, People’s Republic of China; 2Department of Laboratory Medicine, Hefei BOE Hospital, Hefei, People’s Republic of China; 3Clinical Laboratory of Affiliated Hospital of Xuzhou Medical University, Xuzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiang Liu; Degan Liu, The Affiliated Xinghua People’s Hospital, Medical School of Yangzhou University, 419 Ying Wu Nan Road, Xinghua, Jiangsu, 225700, People’s Republic of China, Email [email protected]; [email protected]
Objective: Systemic inflammatory factors are independent risk factors in the formation and progression of various solid tumors. However, whether systemic inflammatory factors are associated with effect and prognosis of esophageal squamous cell carcinoma patients treated with immunotherapy remains unknown. The aim of this study is to assess the value of systemic inflammatory factors in the efficacy of camrelizumab for patients with advanced, metastatic esophageal squamous cell carcinoma.
Methods: We conducted a retrospective analysis of 90 patients with advanced, metastatic esophageal squamous cell carcinoma who received treatment with camrelizumab in Xinghua People’s Hospital between August 2019 and October 2021. The optimal cut-off values of platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR) and systemic immune-inflammation index (SII) for predicting efficacy and prognosis were identified based on the receiver operating characteristic (ROC) curve. Progression free survival (PFS) and overall survival (OS) were evaluated using the Kaplan–Meier method, and differences in PFS or OS between groups were compared by the Log rank test. Univariate and multivariate Cox proportional hazards regression models were performed to analyze prognostic values of each variable.
Results: The optimal cutoff values of PLR, NLR and SII predicted survival outcomes were 157.7, 3.84 and 750.8, respectively. Higher PLR, NLR and SII were associated with shorter PFS (HR for PLR = 2.899, P = 0.001; HR for NLR = 3.629, P < 0.001; HR for SII = 10.251, P < 0.001) and OS (HR for PLR = 4.583, P < 0.001; HR for NLR = 3.921, P < 0.001; HR for SII = 38.606, P < 0.001). Multivariate Cox regression analysis revealed that high PLR, NLR and SII were independent risk factors of PFS and OS in the advanced, metastatic esophageal squamous cell carcinoma patients receiving camrelizumab.
Conclusion: PLR, NLR and SII are potentially effective prognostic predictors in advanced, metastatic esophageal squamous cell carcinoma patients treated with camrelizumab.
Keywords: esophageal cancer, immunotherapy, prognostic predictors
Introduction
In China, esophageal cancer is one of the most common malignant tumors, and the fifth leading cause of cancer-related death. Esophageal cancer is mainly composed of esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC), and the former is the main pathological type in China. The vast majority of patients lose the chance of surgery at the time of diagnosis because of the lack of specific symptoms in the early stage of esophageal cancer, and the 5-year survival rate is lower than 30%.1 In recent years, immunotherapy has made great breakthroughs in treatment of cancers, including esophageal cancer. Studies on KEYNOTE-181,2,3 ATTRACTION-34 and ESCORT5 demonstrated that immunotherapy is effective in second-line treatment of esophageal cancer. In addition, the results of KEYNOTE-590,6 CHECKMATE-648,7 ESCORT-1st8 and ORIENT-159 studies further revealed that first-line immunotherapy combined with chemotherapy can significantly prolong mPFS and mOS in patients with advanced or metastatic esophageal cancer, accelerating the process of first-line immunotherapy for esophageal cancer. Although immunotherapy has brought significant survival benefits to the patients with esophageal cancer, a considerable number of patients still develop primary or acquired drug resistance. Hitherto, programmed death-ligand 1 (PDL-1), tumor mutation burden (TMB), mismatch repair (MMR) and tumor infiltrating lymphocytes (TILs) have been used to screen benefit population. However, due to different detection platforms, cutoff values and the lack of sufficient tumor tissues, they are not ideal indicators. Exploring biomarkers to predict tumor immunotherapy efficacy has an unmet clinical need.
Inflammation is considered to be closely related to the tumorigenesis, progression and metastasis and has important efficacy prediction and prognostic value in a variety of tumors.10–12 Hematological inflammatory parameters such as lymphocytes, neutrophils, platelets and monocytes can reflect the immune inflammatory state of the body and have important prognostic values for cancer patients. With the continuous understanding of cancer-related inflammation, systemic inflammatory biomarkers, such as platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), and lymphocyte-to-monocyte ratio (LMR), have been proved to be able to predict the survival outcomes of various tumor patients.13–17 Recently, some of studies have indicated that high NLR and PLR are also associated with poor clinical outcomes in some tumor patients receiving immunotherapy.18,19 Systemic immune inflammatory index(SII) is a novel inflammatory indicator that combines neutrophils, lymphocytes and platelets, which is an independent risk factor in the formation and progression of solid tumors.20 However, whether systemic inflammatory factors are associated with prognosis of ESCC patients treated with immunotherapy remains unknown. Therefore, our study aims to explore the correlation between systemic inflammatory factors and the efficacy and prognosis of immunotherapy in patients with advanced, metastatic ESCC.
Patients and Methods
Patient Selection
We collected the clinical information of 90 advanced ESCC patients who underwent camrelizumab therapy from Xinghua People’s Hospital between August 2019 and October 2021. Twenty-seven patients received first-line camrelizumab plus paclitaxel and cisplatin treatment. Sixty-three patients receiving second-line camrelizumab monotherapy previously underwent paclitaxel plus cisplatin chemotherapy. The inclusion criteria were as follows: (1) patients over 18 years old; (2) all patients whose pathologic diagnosis was ESCC; (3) patients with advanced or metastatic ESCC who were treated with camrelizumab; (4) patients with an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score at 0–2; (5) patients with complete peripheral hematological parameters and evaluable imaging data before treatment; (6) at least one cycle of treatment with camrelizumab; (7) patients in clinical stage IIIB or IV, according to the eighth edition of the American Joint Committee on Cancer staging manual. The exclusion criteria were as follows: (1) patients with incomplete clinicopathological data and follow-up information; (2) patients with cardiovascular or respiratory diseases; (3) patients with autoimmune or hematologic diseases; (4) patients with a history of using steroid within half a month; (5) patients with other primary carcinomas; (6) infectious diseases before camrelizumab treatment. In this study 32 patients were totally excluded and 90 patients were eventually enrolled. The whole enrollment process was clearly revealed in Figure 1. This study was approved by the Ethics Committee.
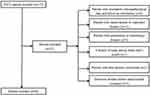 |
Figure 1 The flowchart of the enrollment process. |
Evaluation of Efficacy and Definition of SII, NLR and PLR
We performed a low‐dose computed tomography (LDCT) scan or barium enema examination every 8 weeks. Standard Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) were used for evaluation of response. We asked three radiologists to evaluate the efficacy according to the RECIST version 1.1. The primary endpoint of the study was OS which was defined as the time from randomization to death (from any reason). For subjects who had missed their follow-up visits prior to death, their last follow-up time was viewed as that of death. The secondary endpoint was PFS which was defined as the time from randomization to tumor progression (any aspect) or death (from any cause).
SII was defined as platelet count × neutrophil count/lymphocyte count, NLR as neutrophil/lymphocyte ratio, and PLR as platelets/lymphocyte ratio. Xisen Meikang XN9100 blood analysis instrument was used to perform blood testing. In order to ensure the accuracy of platelet, lymphocyte and neutrophil detection, we did internal quality control once a day and participated in the external quality assessment of the National Health Commission Clinical Inspection Center every six months.
Statistical Analyses
The ROC curve was applied to determine the optimal cut-off values for SII, NLR and PLR. Patients were divided into low SII/NLR/PLR groups and high SII/NLR/PLR groups according to the optimal cutoff values. The chi-square (X2) test was used to evaluate correlations between clinical parameters and blood inflammatory indicators. We used Kaplan–Meier (K-M) method and the log‐rank test to analyze the survival results (PFS and OS) between groups. The cox proportional hazard model was used to identify the prognostic factors of survival time. A two-sided P < 0.05 was considered statistically significant. The above statistical data were analyzed with IBM SPSS Statistic 26.
Results
Patient Characteristics
A total of 90 patients with advanced, metastatic ESCC patients treated with camrelizumab were enrolled in this study. The clinical characteristics of the patients, including age, gender, drinking history, ECOG PS, therapy lines, therapy regimen, tumor differentiation grade, tumor location, TNM stage, were obtained from the medical records. The median age was 67 years (range from 53 to 87 years). Male patients accounted for 62.2% of all participants. Patients with drinking history accounted for 58.9%. 70% of patients have received at least first-line treatment with paclitaxel plus cisplatin. Nearly 75% of patients were clinical stage IV. The median NLR, PLR and SII before treatment with camrelizumab were 4.32 (range, 1.63–11.98), 145.60 (range, 48.78–318.57) and 622.04 (range, 125.00–1874.67) respectively. By March 2022, the median follow-up time was 11.4 months (range 2.2–26 months).
Determination of Optimal Cut-Off Values for SII, NLR and PLR
As revealed in Figure 2, the areas under the ROC curve for PLR, NLR and SII were 0.862, 0.768 and 0.804 respectively. The optimal cut-off values of PLR, NLR and SII predicted survival results were 157.7, 3.84 and 750.8. Consequently, patients were separately divided into high and low groups in accordance with the optimal cut-off values.
 |
Figure 2 A ROC curve analysis for the optimal cut-off values of PLR, NLR and SII, respectively. The areas under the ROC curve of PLR, NLR and SII are indicated. |
Correlations Between the Clinical Features and SII, NLR and PLR
The relationship between the clinical features and SII, NLR and PLR is demonstrated in Table 1. The SII and NLR before treatment were associated with regimen (p < 0.001 and P = 0.042). The PLR before treatment was related to gender (p = 0.044).
 |
Table 1 Relationship Between SII, NLR as Well as PLR and Clinical Parameters of Patients with the Advanced, Metastatic Esophageal Squamous Cell Carcinoma |
Survival Analysis
We used the Kaplan–Meier method to conduct survival curves and the Log rank test to compare their differences. Compared with high SII group, patients before treatment in low SII group had longer PFS and OS (mPFS: 7.6 m vs 4.3 m, P < 0.001; mOS: 17 m vs 8 m, P < 0.001; Figure 3A and 3B). Compared with high NLR group, patients before treatment in low NLR group had longer PFS and OS (mPFS: 7.8 m vs 4.6 m, P < 0.001; mOS: 18 m vs 9 m, P < 0.001; Figure 4A and B). Compared with high PLR group, patients before treatment in low PLR group had longer PFS and OS (mPFS: 7.6 m vs 5.5 m, P = 0.001; mOS: 16.2 m vs 9.3 m, P < 0.001; Figure 5A and B).
Univariate and Multivariate Analyses
The Cox proportional hazards regression model was applied to conduct the univariate and multivariate analyses of PFS and OS. The results of univariate and multivariate analyses were revealed in Tables 2 and 3. In univariate analysis, we indicated that SII ≤ 750.8 before treatment, NLR ≤ 3.84 before treatment and PLR ≤ 157.5 before treatment were related to longer PFS and OS. To avoid the multicollinearity among SII, NLR and PLR, three independent Cox models were separately established in multivariate analysis, each of which only included one of the three indicators. The results of multivariate analyses displayed that SII ≤ 750.8, NLR ≤ 3.84 and PLR ≤ 157.5 were independently associated with longer PFS and OS.
 |
Table 2 Univariate and Multivariate Analyses of Progression-Free Survival in the Advanced, Metastatic Esophageal Squamous Cell Carcinoma Patients Treated with Camrelizumab |
 |
Table 3 Univariate and Multivariate Analyses of Overall Survival in the Advanced, Metastatic Esophageal Squamous Cell Carcinoma Patients Treated with Camrelizumab |
Analysis of Immune-Related Adverse Events
Immune-related adverse events (irAEs) occurred in 78 (86.7%) patients. Grades and categories of irAEs for different treatment regimens are demonstrated in Table 4. The most common irAEs were reactive cutaneous capillary endothelial proliferation (RCCEP). The vast majority of irAEs were grade 1–2 in the camrelizumab group, and Grade 3–4 irAEs were observed in only 2 case (6.1%). In the camrelizumab plus chemotherapy group, Grade 3–4 adverse events such as anemia, thrombocytopenia, leukopenia were obviously increased, which might be associated with chemotherapy. No adverse events led to termination of treatment. No irAE‐related deaths occurred.
 |
Table 4 Summary of irAEs (N = 90) |
Discussion
Esophageal cancer is one of the common malignant tumors threatening human life, especially in China. Currently, the main treatments for esophageal cancer, such as surgery, radiotherapy, chemotherapy and targeted therapy, fail to significantly improve the survival outcomes of patients. Immunotherapy, as an emerging treatment, brings hope to patients with esophageal cancer. Previous studies have shown that inflammatory response plays a role in the process of tumorigenesis and progression.21–23 In our study, a consecutive cohort of 90 advanced, metastatic ESCC patients who underwent treatment with camrelizumab was retrospectively analyzed. We demonstrated that high PLR, NLR and SII were independent risk factors for both PFS and OS in advanced, metastatic ESCC patients treated with camrelizumab. It was worth noting that our study primarily focused on the prognostic values of PLR, NLR and SII for survival outcomes of the advanced, metastatic ESCC patients treated with camrelizumab.
In recent years, immunotherapy has become one of the standard treatments for esophageal cancer. Continuous response and long-term survival benefits of immunotherapy have been reported in several studies. However, there is a significant proportion of patients who still do not benefit from this treatment, and knowing how to identify the populations most likely to benefit from the treatment remains an urgent problem presently. Consequently, exploring some effective biomarkers to predict the population benefitting the most from immunotherapy has important clinical significance, which is consistent with our research purpose.
Inflammatory response can affects tumor microenvironment by secreting bioactive molecules and inflammatory products, which may serve as potential biomarkers.24,25 Systemic inflammatory factors in peripheral blood, such as NLR, PLR and SII can reflect the body’s inflammatory reaction and host immune response. Pretreatment SII, NLR and/or PLR have been found to be independent prognostic predictors in various tumors where patients received immune checkpoint inhibitor therapy.19,26–28 Jingjing19 et al have suggested that pretreatment SII, NLR and PLR are significant prognostic predictors of PFS and OS in advanced NSCLC patients receiving nivolumab. Capone26 et al revealed that lower NLR is in line with superior PFS and OS in patients with stage IV melanoma treated with nivolumab. Another small sample retrospective study27 reported that in gastric cancer patients treated with nivolumab, high NLR might be associated with poor prognosis. Bilen28 et al demonstrated that lower NLR before treatment may be significantly correlated with superior PFS and OS in Metastatic renal-cell carcinoma patients with nivolumab treatment. The cut-off values of these studies are not the same, but similar. This may be associated with quality control in different laboratories and differences in the response of different tumors to immunotherapy. Several meta-analyses29,30 further confirm the above conclusion, which is consistent with our findings.
To the best of our knowledge, there are no data on the relationship between systemic inflammatory factors and the efficacy and prognosis of camrelizumab treatment in patients with advanced, metastatic ESCC. In the present study, this correlation was demonstrated for the first time. Furthermore, peripheral blood biomarkers used in our study, compared with PDL-1, TMB and MMR, have the highest benefit-cost ratio and are easily available in clinical practice. Nevertheless, our study had some limitations. For one thing, single center, small sample and retrospective analysis made our research unable to avoid selection bias. So, large sample, multi-center and prospective studies will be needed to validate these findings. In addition, although our findings were in accord with previous ones, it was difficult to determine our conclusions in other studies owing to the lack of standardized cut-off value. Therefore, it is necessary to find a unified test method to confirm the appropriate cut-off value.
Conclusions
In conclusion, the current research indicates that PLR, NLR and SII may be independent prognostic factors for advanced, metastatic ESCC patients treated with camrelizumab.
Ethics Statement
The study involving human participants was reviewed and approved by Ethics Committee of Medical School of Yangzhou University. As a non-intervention retrospective study, it has been approved to waive informed consent. Privacy and identity information of all patients kept confidential and kept in compliance with the Declaration of Helsinki.
Disclosure
All authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Kojima T, Shah MA, Muro K, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38(35):4138–4148. doi:10.1200/JCO.20.01888
3. Cao Y, Qin S, Luo S, et al. Pembrolizumab versus chemotherapy for patients with esophageal squamous cell carcinoma enrolled in the randomized KEYNOTE-181 trial in Asia. ESMO Open. 2022;7(1):100341. doi:10.1016/j.esmoop.2021.100341
4. Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, Phase 3 trial. Lancet Oncol. 2019;20(11):1506–1517. doi:10.1016/S1470-2045(19)30626-6
5. Huang J, Xu J, Chen Y, et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21(6):832–842. doi:10.1016/S1470-2045(20)30110-8
6. Kato K, Shah MA, Enzinger P, et al. KEYNOTE-590: phase III study of first-line chemotherapy with or without pembrolizumab for advanced esophageal cancer. Future Oncol. 2019;15(10):1057–1066. doi:10.2217/fon-2018-0609
7. Doki Y, Ajani JA, Kato K, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386(5):449–462. doi:10.1056/NEJMoa2111380
8. Luo H, Lu J, Bai Y, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326(10):916–925. doi:10.1001/jama.2021.12836
9. Shen L, Lu Z, Wang J, et al. LBA52 Sintilimab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced or metastatic esophageal squamous cell cancer: first results of the phase III ORIENT-15 study. Ann Oncol. 2021;32:S1330. doi:10.1016/j.annonc.2021.08.2132
10. Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12(10):584–596. doi:10.1038/nrclinonc.2015.105
11. Singel KL, Segal BH. Neutrophils in the tumor microenvironment: trying to heal the wound that cannot heal. Immunol Rev. 2016;273(1):329–343. doi:10.1111/imr.12459
12. Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126(5):582–588. doi:10.1182/blood-2014-08-531582
13. Zou ZY, Liu HL, Ning N, et al. Clinical significance of pre-operative neutrophil lymphocyte ratio and platelet lymphocyte ratio as prognostic factors for patients with colorectal cancer. Oncol Lett. 2016;11(3):2241–2248. doi:10.3892/ol.2016.4216
14. Guo W, Cai S, Zhang F, et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected non-small cell lung cancer. Thoracic Cancer. 2019;10(4):761–768. doi:10.1111/1759-7714.12995
15. Yılmaz U, Ozdemir O, Batum O, et al. The prognostic role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in patients with stage III non-small cell lung cancer treated with concurrent chemoradiotherapy. Indian J Cancer. 2018;55(3):276–281. doi:10.4103/ijc.IJC_624_17
16. Ji WH, Jiang YH, Ji YL, et al. Prechemotherapy neutrophil: lymphocyte ratio is superior to the platelet: lymphocyte ratio as a prognostic indicator for locally advanced esophageal squamous cell cancer treated with neoadjuvant chemotherapy. Dis Esophagus. 2016;29(5):403–411. doi:10.1111/dote.12322
17. Hirahara N, Tajima Y, Fujii Y, et al. A novel prognostic scoring system using inflammatory response biomarkers for esophageal squamous cell carcinoma. World J Surg. 2018;42(1):172–184. doi:10.1007/s00268-017-4144-y
18. Russo A, Russano M, Franchina T, et al. Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and outcomes with nivolumab in pretreated Non-Small Cell Lung Cancer (NSCLC): a large retrospective multicenter study. Adv Ther. 2020;37(3):1145–1155. doi:10.1007/s12325-020-01229-w
19. Liu J, Li S, Zhang S, et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal. 2019;33(8):e22964. doi:10.1002/jcla.22964
20. Fest J, Ruiter R, Mulder M, et al. The systemic immune-inflammation index is associated with an increased risk of incident cancer-A population-based cohort study. Int J Cancer. 2020;146(3):692–698. doi:10.1002/ijc.32303
21. Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
22. Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30:677–706. doi:10.1146/annurev-immunol-020711-075008
23. Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi:10.1038/nrc3611
24. Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274. doi:10.1038/nrc1586
25. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi:10.1016/j.cell.2010.01.025
26. Capone M, Giannarelli D, Mallardo D, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018;6(1):74. doi:10.1186/s40425-018-0383-1
27. Ogata T, Satake H, Ogata M, et al. Neutrophil-to-lymphocyte ratio as a predictive or prognostic factor for gastric cancer treated with nivolumab: a multicenter retrospective study. Oncotarget. 2018;9(77):34520–34527. doi:10.18632/oncotarget.26145
28. Bilen MA, Dutcher GMA, Liu Y, et al. Association between pretreatment neutrophil-to-lymphocyte ratio and outcome of patients with metastatic renal-cell carcinoma treated with nivolumab. Clin Genitourin Cancer. 2018;16(3):e563–e575. doi:10.1016/j.clgc.2017.12.015
29. Jin J, Yang L, Liu D, et al. Association of the neutrophil to lymphocyte ratio and clinical outcomes in patients with lung cancer receiving immunotherapy: a meta-analysis. BMJ Open. 2020;10(6):e035031. doi:10.1136/bmjopen-2019-035031
30. Yang T, Hao L, Yang X, et al. Prognostic value of derived neutrophil-to-lymphocyte ratio (dNLR) in patients with non-small cell lung cancer receiving immune checkpoint inhibitors: a meta-analysis. BMJ Open. 2021;11(9):e049123. doi:10.1136/bmjopen-2021-049123
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



