Back to Journals » Infection and Drug Resistance » Volume 15
The Potential Predictive Role of Tumour Necrosis Factor-α, Interleukin-1β, and Monocyte Chemoattractant Protein-1 for COVID-19 Patients Survival
Authors Kumboyono K , Chomsy IN , Iskandar A , Aryati A, Parwati I, Wihastuti TA
Received 8 November 2021
Accepted for publication 21 February 2022
Published 4 March 2022 Volume 2022:15 Pages 821—829
DOI https://doi.org/10.2147/IDR.S348392
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Kumboyono Kumboyono,1 Indah Nur Chomsy,2 Agustin Iskandar,3 Aryati Aryati,4 Ida Parwati,5 Titin Andri Wihastuti6
1Nursing Department, Faculty of Health Sciences, University of Brawijaya, Malang, 65151, Indonesia; 2Doctoral Program of Medical Science, Faculty of Medicine, University of Brawijaya, Malang, 65145, Indonesia; 3Department of Clinical Pathology, Faculty of Medicine, University of Brawijaya, Malang, 65145, Indonesia; 4Department of Clinical Pathology, Faculty of Medicine, Universitas Airlangga, Surabaya, 60131, Indonesia; 5Department of Clinical Pathology, Faculty of Medicine, Universitas Padjadjaran, Bandung, 40161, Indonesia; 6Basic Nursing Department, Faculty of Health Sciences, University of Brawijaya, Malang, 65151, Indonesia
Correspondence: Titin Andri Wihastuti, Basic Nursing Department, Faculty of Health Sciences, University of Brawijaya, Malang, 65151, Indonesia, Email [email protected]
Background: Tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and monocyte chemoattractant protein-1 (MCP-1) are early phase cytokines often encountered when the body is exposed to severe acute respiratory syndrome-associated-coronavirus-2. TNF-α, IL-1β, and MCP-1 are pro-inflammatory cytokines critical in the defence response against systemic infection and injury. Therefore, TNF-α, IL-1β, and MCP-1 are the most aggressive responses to viral infections in the acute phase, so they can be used to determine the survival of coronavirus disease 2019 (COVID-19) patients.
Purpose: The study aimed to determine the levels of TNF-α, IL-1β, and MCP-1 as predictors of survival for COVID-19 patients.
Patients and Methods: A prospective cohort study was conducted on confirmed COVID-19 by a reverse-transcriptase-polymerase-chain-reaction (RT-PCR) in 84 adults admitted to the hospital in Indonesia. TNF-α, IL-1β, and MCP-1 level were measured from serum subjects using the enzyme-linked immunosorbent assay.
Results: The results from logistic regression modelling of the survival status of COVID-19 patients based on TNF-α, IL-1β, and MCP-1 levels were significant (p-value=0.024). The predictors of all cytokines had P Wald < 0.05, so the three cytokines could be used simultaneously to predict the survival status of COVID-19 patients. MCP-1 has the most dominant risk relative value (2.76; 95% CI; 2.53– 4.68) compared to TNF-α and IL-1β in predicting patient survival.
Conclusion: TNF-α, IL-1β, and MCP-1 as markers of acute systemic inflammatory cytokines can be measured at the beginning of hospitalisation of COVID-19 patients for early diagnosis of disease severity so that healthcare professionals can determine clinical guidance needs for therapeutic programs.
Keywords: cytokine, survival predictors, COVID-19, SARS COV-2
Corrigendum for this paper has been published.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic is a global health crisis with very high morbidity and mortality rates in a short time. Activation of innate and adaptive immune responses by coronavirus infection causes an uncontrolled inflammatory response resulting in a cytokine storm in severe COVID-19 patients.1 Crosstalk between immune cells and endothelial cells during coronavirus infection impacts the activation of several inflammatory cascades, causing hyperproduction of pro-inflammatory cytokines. Cytokine storms have been shown to cause manifestations of acute respiratory distress syndrome and thromboembolism leading to vascular occlusions, such as in cases of acute ischemic stroke, myocardial infarction, encephalitis, acute kidney injury, vasculitis and sudden death.2,3 Significantly elevated pro-inflammatory cytokines suggest a poor prognosis in COVID-19 patients. Research shows that patients with severe COVID-19 tend to have higher concentrations of pro-inflammatory cytokines (such as Tumour Necrosis Factor-α (TNF-α), Interleukin-1β (IL-1β), and Monocyte Chemoattractant Protein-1 (MCP-1) than those with mild or moderate illness.4,5 This phenomenon shows a very close relationship between the severity of COVID-19 and the production of pro-inflammatory cytokines. Therefore, predicting the survival of COVID-19 patients using the three pro-inflammatory cytokines will allow clinicians to take prompt and appropriate treatment to prevent the severity of COVID-19 from reducing mortality.
Cytokines are now in the spotlight in various countries because of their essential roles in the pathogenesis of COVID-19. Proinflammatory cytokines such as IL1β, IL-2, IL-4, IL-6, IL-8, TNFa, IL-2, IL-4, IP-10 and MCP-1 are associated with an increased risk of mortality from acute respiratory distress syndrome (ARDS). Huang (2020) research stated that COVID-19 patients treated in the ICU had higher IL2, IL7, IL10, GSCF, IP10, MCP1, MIP1A, and TNFα than patients not admitted to the ICU.6 Meanwhile, a prospective cohort study in 24 COVID-19 patients revealed that levels of IL-4, IL-6, IL-8, IL-10, FGF, IP-10, MCP-1, and TNF correlated with 30-day mortality.7 In addition, Chen et al found that the levels of IP-10, MCP-1, and IL-6 were significantly higher in COVID-19 critically Ill patients.8 MCP-1 and IL-10 were not significantly associated with mortality but were associated with high levels of d-dimer associated with mortality.
Many studies have reported that acute respiratory distress syndrome (ARDS) is one of the leading causes of death related to COVID-19. ARDS is triggered by a cytokine storm that directly damages the respiratory epithelium, liver, heart, and kidneys, including TNF-α.9 The soluble form of TNF-α and the membrane protein form are active forms with different affinities for the TNF receptor. When it binds to the TNF receptor, TNF- will cause a pleiotropic effect or stimulate the secretion of other systemic cytokines, so TNF-α is known as an early-phase cytokine.10,11
Interleukin 1β is released by macrophages via inflammasomes and contributes to the cytokine storm as the most aggressive response to COVID-19.12 IL-1β is known to play a role in immunological dysregulation in severe patients with respiratory failure,13 which in cytokine storm of ARDS correlates with disease severity, also may be associated with organ failure.14,15 Yang et al (2020) found that increased IL-1 receptor antagonist as a marker of increased viral load and risk of death in 14 severe COVID-19 patients.16 Therefore, IL-1β is part of the cytokine storm generated by coronavirus infection.17 IL-1 functions after binding to the IL-1 receptor (IL-1R) and is known to transduce and activate one of the well-known inflammatory pathways, the NFkB pathway.18
MCP-1 is a potent monocyte chemotactic factor constitutively produced or induced by oxidative stress, cytokines, or growth factors.19 MCP-1 was associated with high levels of d-dimer associated with death.8 Furthermore, it has been reported that MCP-1 expression increases rapidly in the early phase of acute infection and then progressively decreases with disease progression.20
IL-1β, and MCP-1 are pro-inflammatory cytokines critical in the defence response against systemic infection and injury.17,21 IL-1β and MCP-1 respond as an emergent cytokine by initiating acute-phase protein synthesis. In addition to TNF-α, IL-1β and Monocyte Chemoattractant Protein-1 (MCP-1) are early phase cytokines often encountered when the body is exposed to coronavirus.4,21 Although there have been studies using similar cytokines to predict the survival of COVID-19 patients in various countries, there has been no reporting in Indonesia in the period of Delta variants of SARS-CoV-2. So, this study wants to confirm the research results on the survival of COVID-19 patients in Indonesia with various other countries.
Materials and Methods
Study Design and Setting
A prospective cohort study confirmed COVID-19 in adults admitted to the public hospitals to refer coronavirus disease patients in Indonesia. The grouping of patients as a risk factor for predicting the survival of COVID-19 patients is based on the severity level: mild, moderate, severe, and critically ill. The grouping of COVID-19 patients is divided into asymptomatic, mild, moderate, severe and critical.22
1. Mild: Patients with symptoms without evidence of viral pneumonia or hypoxia. Symptoms include fever, cough, fatigue, anorexia, shortness of breath, myalgia, nasal congestion, headache, diarrhoea, nausea and vomiting, loss of smell (anosmia), or loss of taste (ageusia).
2. Moderate: patients with clinical signs of pneumonia (fever, cough, shortness of breath, rapid breathing) but no signs of severe pneumonia, including SpO2 > 93% in room temperature, and no signs of severe pneumonia.
3. Severe/Severe Pneumonia: patients with clinical signs of pneumonia (fever, cough, shortness of breath, rapid breathing), with the addition of one of the following symptoms: respiratory rate > 30 times/minute, severe respiratory distress, or SpO2 < 93% on room air respiratory rate > 30 times/minute, severe respiratory distress, or SpO2 < 93% in room temperature.
4. Critically ill: Patients with ARDS, sepsis, and or septic shock.
Each group was then observed to determine the research’s outcome: survival or non-survival. There are three general hospitals in this study: Saiful Anwar Hospital Malang, Soetomo General Hospital Surabaya, and Hasan Sadikin General Hospital Bandung.
Study Subject and Data Collection
The subjects of this study were 84 inpatients at the three hospitals who were diagnosed as confirmed COVID-19, proven by a Reverse-Transcriptase–Polymerase-Chain-Reaction (RT-PCR) method. The inclusion criteria for this research subject are: patients who confirmed COVID-19 by the RT-PCR (nasopharyngeal or oropharyngeal swab); ages 17–60 years; and have onset time 14 days from the first time the patient has symptoms until confirmed as Covid-19 patient. This study’s subject exclusion criteria are pregnant or breastfeeding women, having a coagulation disorder before being diagnosed as COVID-19, and co-morbidities such as cancer, hepatitis, or HIV/AIDS.
Cytokine levels of TNF-α (ng/mL), IL-1β (pg/mL), and MCP-1 (ng/mL) were measured from serum subjects using the Enzyme-Linked Immunosorbent Assay (ELISA) examination procedure. The ELISA procedure was carried out according to the manual protocol of the Human IL-1β Bioassay Technology Laboratory (BT-Lab) ELISA kit (Cat. No. E0143Hu) and Human TNF-α BT-Lab ELISA Kit (Cat. No. E0082Hu) and, and Human MCP-1 BT-Lab ELISA Kit (Cat. No. E0124Hu). The ELISA procedure was carried out by adding 40μL of a serum sample to the sample well. After that, it added 50μL of streptavidin-HRP to the sample wells and standard wells. Covered well and incubated for 60 minutes at 37°C, then washed with Wash Buffer five times. First, 50μL of substrate solution. Then was attached to each well, and then 50μL of substrate solution B was added to each well. Next, it was incubated in the dark for 10 minutes at 37°C. After that, 50μL of Stop Solution was summed for each well. Each optical density (OD) value was appropriately read using a microplate reader at a wavelength of 450 nm within 10 minutes after adding the stop solution.
Ethical Clearance
The institutional ethics committee of University of Brawijaya approved the study (No. 209/EC/KEPK/07/2021) and also ethical committee of Saiful Anwar General Hospital (No. 4000/011/K.3/302/2021). All of the patients participate in this study provided informed consent that has been accordanced with the Declaration of Helsinki.
Data Analysis
The authors conducted the Mann–Whitney U-test to examine differences in TNF-α, IL-1B, and MCP-1 levels between survivors and non-survivors. The association between levels of TNF-α, IL-1B, and MCP-1 with survival status was multivariate analysed using logistic regression. In addition, the authors calculated values of the diagnostic test (sensitivity, specificity, positive predictive value, negative predictive value) and accuracy of the test (the area under Receiver Operator Characteristic curve) to describe TNF-α, IL-1B, and MCP-1 levels to predict mortality of COVID-19 patient. All statistical analyses were conducted with IBM® Statistical Product and Service Solution® for Windows version 25.0 with p-value <0.05 as a statistical significance level.
Results
Baseline Characteristic of the Research Subject
This study found 84 COVID-19 patients according to the inclusion and exclusion criteria from June 2020 to March 2021. There were 47 (55.9%) survivors of COVID-19 and 37 (44.1%) of COVID-19 patients who were died (non-survivors). Table 1 presents the characteristics of research subjects based on patient outcomes, namely survivors and non-survivors.
 |
Table 1 Characteristics of the Study Population |
TNF-α, IL-1β, and MCP-1 Levels in Survivors and Non-Survivors in COVID-19 Patients
Measurement of TNF-α, IL-1β, and MCP-1 levels in the serum of COVID-19 patients was carried out on the first day of hospitalisation (Table 2). The median levels of TNF-α, IL-1β, and MCP-1 in the non-survivor group of COVID-19 patients were significantly higher than those of COVID-19 survivors.
 |
Table 2 Levels of TNF- and IL-1β in the Survivors and Non-Survivors of COVID-19 |
Meanwhile, Table 3 shows logistic regression modelling of the survival status of COVID-19 patients based on TNF-α, IL-1β, and MCP-1 levels were significant (p-value=0.024). The predictors of all cytokines had P Wald <0.05, so the three cytokines could be used simultaneously to predict the survival status of COVID-19 patients. MCP-1 has the most dominant relative risk (RR) value compared to TNF-α and IL-1β in predicting patient survival.
 |
Table 3 Results of Multivariate Logistic Regression Analysis Between TNF-α, IL-1β, and MCP-1 Level with Survival Status Among COVID-19 Patients |
The accuracy of the three cytokines tests in predicting the death of COVID-19 patients can be seen in the following description.
The Ability of TNF-α as a Predictor of Mortality of COVID-19 Patients
ROC curve analysis of TNF-α levels was carried out to determine the ability of this biomarker as a predictor of mortality. The analysis results show that the area under the curve (AUC) is 0.685 with p-value=0.007 (Figure 1). Thus, the cut-off value of TNF- α to predict patient mortality is 37.88 ng/L, with a sensitivity of 62.07%, specificity 68.89%, positive predictive value (PPV) 56.25% and negative predictive value (NPV) 73.81%.
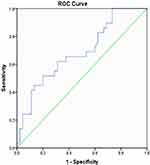 |
Figure 1 ROC curve of TNF-α levels on death of COVID-19 patients. |
The Ability of IL-1β as a Predictor of Mortality of COVID-19 Patients
The ability of IL-1β was determined as a predictor of COVID-19 patient’s mortality, an analysis of the ROC curve of IL-1β levels was carried out on the mortality of COVID-19 patients (Figure 2). The analysis results show that the area under the curve (AUC) is 0.645, with a significance value of p=0.035. Furthermore, the analysis results show that the area under the curve (AUC) is 0.645, with p-value=0.035. Thus, IL-1β levels predict the death of COVID-19 patients at the cut-off point of 761.54 pg/L with a sensitivity of 59.46%, specificity 70.59%, PPV 68.75% and NPV 61.54%.
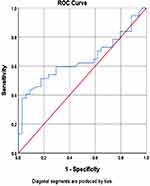 |
Figure 2 ROC curve of IL-1β levels on death of COVID-19 patients. |
The Ability of MCP-1 as a Predictor of Mortality of COVID-19 Patients
MCP-1 ability as a predictor of mortality in COVID-19 patients, an analysis of the ROC curve of MCP-1 levels on the mortality of COVID-19 patients was carried out (Figure 3). The analysis results show that the area under the curve (AUC) is 0.702 with a significance value of p=0.004. Therefore, MCP-1 levels predict the death of COVID-19 patients with a cut-off value of 51.31 PPV 52.9% and NPV of 71.1%.
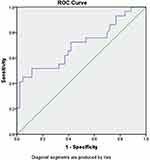 |
Figure 3 ROC curve of MCP-1 levels on death of COVID-19 patients. |
Discussion
Cytokine dysregulation has been identified as one of the major factors for poor prognosis in COVID-19.23 This cytokine storm produces an exaggerated inflammatory response, particularly in the lungs, leading to ARDS, pulmonary oedema, and multi-organ failure. Thus, pro-inflammatory factors play a central role in the severity of COVID-19, especially in patients with co-morbidities.24,25 An overactive and untreated immune response can be lethal in these situations, suggesting that death in COVID-19 cases is likely due to this virus-driven hyper inflammation.26
TNF-α is one of the cytokines that promote inflammation, including during SARS-CoV-2 infection. Of the 41 patients suffering from COVID-19, 13 were admitted to the ICU due to severe COVID-19. The patients treated in the ICU had higher IL2, IL7, IL10, GSCF, IP10, MCP-1, MIP1A, and TNF than non-ICU patients.27 According to a cohort study conducted by Del Valle et al, 1484 COVID-19 patients were followed up to 41 days after admission with a median of 8 days.23 They found that high serum levels of IL-6, IL-8 and TNF-α at the time of hospitalisation were strong and independent predictors of patient survival. In addition, the investigators obtained a cut-off of 35 ng/L, where values above the cut-off value had a high mortality rate.28
We followed the patient’s condition for up to 28 days so that we got the patient outcomes in the form of survivors and non-survivors. TNF-α was calculated on the first day the patient was admitted. The optimal cut-off value of TNF-α obtained statistically was 37.88 ng/L. This cut-off value becomes a reference for calculating sensitivity, specificity, positive and negative predictive values, and relative risk in the TNF-α examination. In this study, the median TNF-α in non-survivor patients was higher than in survivors. The increase in TNF-α levels in non-survivor patients in this study supports the results of a study conducted by Huang et al and Valle et al, where high levels of TNF-α were found in both ICU-treated and deceased COVID-19 patients. In addition, TNF-α can also be an independent predictor of severity in COVID-19 patients.28,29
Cytokine storm is positively correlated with disease severity. Cytokine storm conditions in COVID-19 patients have been shown to increase the severity of COVID-19 disease so that it can increase the risk of death in COVID-19.30 However, few studies prove the relationship of IL-1β with the death of COVID-19 patients. In this study, the mean levels of IL-1β were higher in the non-survivor group of COVID-19 patients than in the survivor group, which is consistent with previous studies. The study by Nile et al on 27 severe COVID-19 patients analysed nine biomarkers (IL-1β, IL-2R, IL-6, IL-8, IL-10, TNF-, CRP, Ferritin) and lymphocytes as pro-inflammatory biomarkers in COVID-19.31 This study found that pro-inflammatory cytokines were increased in patients with severe COVID-19.
In addition, a study by Shang et al in 55 COVID-19 (mild-severe) patients admitted to the general ward and ICU showed an increase in IL-1β levels.32 The increase in IL-1β is significant and correlates with worsening the patient’s condition, namely damage to lung tissue, respiratory failure, and death.27 Thus, an increase in IL-1 levels in COVID-19 patients can potentially be a predictor of death, wherein this study, IL-1β levels 761.54 pg/L had a sensitivity of 59.46%, specificity 70.59%, positive predictive value 68.75%, a negative predictive value of 61.54% for death and an increase in IL-1β levels of 1.12 times can increase the risk of death of COVID-19 patients.
Cytokine storm conditions in COVID-19 patients have been shown to increase the severity of COVID-19 disease so that it can increase the risk of death in COVID-19.33,34 However, few studies have proven the relationship between MCP-1 and the mortality of COVID-19 patients. In this study, the mean MCP-1 level was higher in the non-survivor group of COVID-19 patients than in the survivor group, consistent with previous studies. A study by Abers et al, on 175 COVID-19 patients (mild-critical) analysed 66 soluble biomarkers with the severity of symptoms and mortality in COVID-19.35 CCL2/MCP-1 as a biomarker associated with monocyte/macrophage activation and NFκB signalling increased COVID-19 mortality compared to moderate-critical cases and healthy controls, and increased MCP-1 was associated with mortality independently. In addition, a study by Anderberg et al, on 24 confirmed COVID-19 patients admitted to the ICU showed that MCP-1 levels were correlated with the occurrence of respiratory failure, acute renal failure and death.7 Thus, an increase in MCP-1 levels can predict death in COVID-19, wherein this study, MCP-1 levels 51.31 ng/L had a sensitivity of 62.1%, specificity 62.8%, the positive predictive value of 52.9%, a negative predictive value of 71.1% for mortality and an increase in MCP-1 levels 2.71 times increased the risk of death of COVID-19 patients. This study found that the significant difference was obtained from the level of severity, not based on the risk factors and morbidity in COVID-19 patients.
Conclusion
The levels of TNF-α, IL-1β, and MCP-1 showed significant differences between survivors and non-survivors in COVID-19 patients. Therefore, the levels of all three have strong potential as predictors of outcome for COVID-19 patients. In addition, TNF-α, IL-1β, and MCP-1 as markers of acute systemic inflammatory cytokines can be measured at the beginning of hospitalisation of COVID-19 patients for early diagnosis of disease severity so that healthcare professionals can determine clinical guidance needs for therapeutic programs. Finally, the recovery of COVID-19 patients is expected to increase.
Acknowledgments
The authors are thankful for the Ministry of Culture and Education; the Republic of Indonesia sponsored this research financially through “Program Penelitian Kolaborasi Indonesia” World Class University with grant numbers: 342.2/UN10.C10/PN/2021.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med. 2020;383(23):2255–2273. doi:10.1056/NEJMra2026131
2. Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. 2020;194:101–115. doi:10.1016/j.thromres.2020.06.029
3. Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8(6):e46–e47. doi:10.1016/S2213-2600(20)30216-2
4. Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75.
5. Darif D, Hammi I, Kihel A. The pro-inflammatory cytokines in COVID-19 pathogenesis: what goes wrong? Microb Pathog. 2021;153:104799. doi:10.1016/j.micpath.2021.104799
6. Huang C, Wang Y, Li X, Ren L. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
7. Bülow Anderberg S, Luther T, Berglund M, et al. Increased levels of plasma cytokines and correlations to organ failure and 30-day mortality in critically ill Covid-19 patients. Cytokine. 2021;138:155389. doi:10.1016/j.cyto.2020.155389
8. Chen Y, Wang J, Liu C, et al. IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Mol Med. 2020;26(1):97. doi:10.1186/s10020-020-00230-x
9. Leija-Martínez JJ, Huang F, Del-Río-Navarro BE, et al. IL-17A and TNF-α as potential biomarkers for acute respiratory distress syndrome and mortality in patients with obesity and COVID-19. Med Hypotheses. 2020;144:109935. doi:10.1016/j.mehy.2020.109935
10. Kany S, Vollrath JT, Relja B. Cytokines in Inflammatory Disease. Int J Mol Sci. 2019;20(23):6008.
11. Idriss HT, Naismith JH. TNF alpha and the TNF receptor superfamily: structure-function relationship(s). Microsc Res Tech. 2000;50(3):184–195.
12. Birra D, Benucci M, Landolfi L, et al. COVID 19: a clue from innate immunity. Immunol Res. 2020;68(3):161–168. doi:10.1007/s12026-020-09137-5
13. Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020;27(6):992–1000.e3. doi:10.1016/j.chom.2020.04.009
14. Song P, Li W, Xie J, Hou Y, You C. Cytokine storm induced by SARS-CoV-2. Clin Chim Acta. 2020;509:280–287. doi:10.1016/j.cca.2020.06.017
15. Zhang X, Li S, Niu S. ACE2 and COVID-19 and the resulting ARDS. Postgrad Med J. 2020;96(1137):403–407. doi:10.1136/postgradmedj-2020-137935
16. Yang Y, Shen C, Li J, et al. Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. Infect Dis. 2020. doi:10.1101/2020.03.02.20029975
17. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J Infect. 2020;80(6):607–613.
18. Zhong J, Tang J, Ye C, Dong L. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol. 2020;2(7):e428–e436.
19. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–326. doi:10.1089/jir.2008.0027
20. Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-A review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727–732. doi:10.1080/22221751.2020.1746199
21. Lorkiewicz P, Waszkiewicz N. Biomarkers of Post-COVID Depression. J Clin Med. 2021;10(18):4142.
22. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; 2020. Available from: https://www.covid19treatmentguidelines.nih.gov/.
23. Kim JS, Lee JY, Yang JW, et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11(1):316–329.
24. Ni M, Tian FB, Xiang DD, Yu B. Characteristics of inflammatory factors and lymphocyte subsets in patients with severe COVID-19. J Med Virol. 2020;92(11):2600–2606. doi:10.1002/jmv.26070
25. Heriansyah T, Chomsy IN, Febrianda L, Farahiya Hadi T, Andri Wihastuti T. The Potential Benefit of Beta-Blockers for the Management of COVID-19 Protocol Therapy-Induced QT Prolongation: a Literature Review. Sci Pharm. 2020;88(4):55. doi:10.3390/scipharm88040055
26. de la Rica R, Borges M, Gonzalez-Freire M. COVID-19: in the Eye of the Cytokine Storm. Front Immunol. 2020;11:1–11.
27. McElvaney OJ, McEvoy NL, McElvaney OF, et al. Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am J Respir Crit Care Med. 2020;202(6):812–821.
28. Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643.
29. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506.
30. Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 Cytokine Storm; What We Know So Far. Front Immunol. 2020;11:1446.
31. Nile SH, Nile A, Qiu J, Li L, Jia X, Kai G. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi:10.1016/j.cytogfr.2020.05.002
32. Shang W, Dong J, Ren Y, et al. The value of clinical parameters in predicting the severity of COVID-19. J Med Virol. 2020;92(10):2188–2192. doi:10.1002/jmv.26031
33. Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine Storm in COVID-19: the Current Evidence and Treatment Strategies. Front Immunol. 2020;11:1708.
34. Wu F, Zhao S, Yu B, et al. Author Correction: a new coronavirus associated with human respiratory disease in China. Nature. 2020;580(7803):E7.
35. Abers MS, Delmonte OM, Ricotta EE, et al. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight. 2021;6(1):e144455.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
