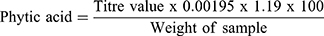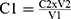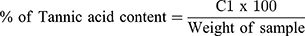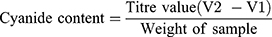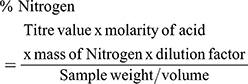Back to Journals » Journal of Experimental Pharmacology » Volume 12
The Pharmacobiochemical Effects of Ethanol Extract of Justicia secunda Vahl Leaves in Rattus Norvegicus
Authors Onochie AU , Oli AH, Oli AN , Ezeigwe OC , Nwaka AC, Okani CO , Okam PC, Ihekwereme CP, Okoyeh JN
Received 28 June 2020
Accepted for publication 9 September 2020
Published 2 November 2020 Volume 2020:12 Pages 423—437
DOI https://doi.org/10.2147/JEP.S267443
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Bal Lokeshwar
Anthony U Onochie,1 Adaobi Helen Oli,1,2 Angus Nnamdi Oli,3 Obiajulu Christian Ezeigwe,4 Andrew C Nwaka,1 Chukwudi O Okani,5 Princeston Chukwuemeka Okam,6 Chibueze P Ihekwereme,7 Jude Nnaemeka Okoyeh8
1Department of Biochemistry Faculty of Natural Sciences, Chukwuemeka Odumegwu Ojukwu University, Uli, Anambra State, Nigeria; 2HIV Lab, HIV Care Unit, Nnamdi Azikiwe University Teaching Hospital, Nnewi, Anambra State, Nigeria; 3Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Agulu, Anambra State, Nigeria; 4Department of Applied Biochemistry, Faculty of Biosciences, Nnamdi Azikiwe University, Awka, Anambra State, Nigeria; 5Department of Histopathology, Faculty of Clinical Medicine, Chukwuemeka Odumegwu Ojukwu University Teaching Hospital Amaku, Awka 420108, Anambra State, Nigeria; 6Department of Pharmacology and Therapeutics, College of Health Sciences, Faculty of Medicine, Nnamdi Azikiwe University, Nnewi, Anambra State, Nigeria; 7Department of Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Awka, Anambra State, Nigeria; 8Department of Biology and Clinical Laboratory Science, Division of Arts and Sciences, Neumann University, Aston, PA 19014-1298, USA
Correspondence: Angus Nnamdi Oli
Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, Agulu, Nnamdi Azikiwe University, Anambra State, Nigeria
Tel +234 8056306927
Email [email protected]
Purpose: This study evaluated the biochemical effects of ethanol leaves extract on Wistar rats and also shed light on its constituents and phytonutrients.
Methods: The ethanolic extract of J. secunda leaves was prepared using conventional methods. Then, proximate and phytochemical analyses of the extracts were carried out using several methods previously reported in the literatures. The biochemical studies were also carried out as reported in previous literatures.
Results: The ethanolic leaves extract contains appreciable quantities of phytonutrients and micronutrients as well as phytochemical constituents. The LD50 of the extract was determined to be 3800mg/kg body weight. There was a dose-dependent elevation of the blood sugar in comparison with the control. There was no significant increase on the bilirubin and liver enzymes levels or on the haematological parameters of the lab animals. The extract significantly elevated the lipid profile (P value < 0.0001), the glomerular filtration rate (increased creatinine and blood urea levels – P value < 0.0001), the serum electrolytes and the animals’ weight. There was a significant decrease in the anion gap (P value < 0.01).
Conclusion: The ethanol leaf extract of Justicia secunda has negative cardiac and renal effects on Wistar rats, causing increased lipid profile values, creatinine and blood urea levels in the experimental animals compared with control. The LD50 is below the safety level. Caution should be exercised as the biochemical profiles of cardiac and renal effects do not seem to be promising and the LD50 is below the safety level.
Keywords: liver function, kidney function, cardiac function, serum electrolytes, Justicia secunda
Introduction
Medicinal plants not only serve as food but are the major sources of trado-medicines. Notwithstanding the improvements in healthcare delivery systems using western medicines, medicinal plants still constitute an indispensable part of human and animal healthcare systems with approximately 80% of the global population still depending on them for basic healthcare needs.1,2
The species Justicia secunda Vahl belongs to the family – Acanthaceae – together with up to 600 other shrubs and herbs species.3 The plants in the family are reported to have CNS effects which are exploited in the management of epilepsy, other mental disorders, headache and fever, probably due to their sedative and analgesic properties.3,4 Other uses of the plants in folk-medicine are: wound healing, and management of anaemia and abdominal cramps.5 A good number of Justicia species have remained useful in folk medicine, being applied in the treatment and management of respiro-gastrointestinal diseases as well as inflammation.4,5 In folk medicine, every part of the Justicia plant is utilized. Extracts made from only the leaves are the most frequently used, followed by root extracts.
Justicia secunda Vahl can grow 90–200cm tall. The leaves are prepared as a decoction for the management of anaemia and as an antioxidant in stress management.5–7 Earlier studies confirmed the haematinic, anti-sickling, anti-hypertensive and antimicrobial potentials of the plant.6,8–10 Studies on the phytochemistry of the plant revealed the presence of several bioactive drug-lead compounds.11,12 Previous studies investigated the in vitro bioactivity of the plant’s leaves extracts and their fractions and demonstrated their Gram-positive activities with no activity against Gram-negative strains of enterobacteriaceae.13,14 Zambrano et al12 showed that quindoline, a constituent of J. secunda stem, has only very minimal acetylcholinesterase inhibitory activity in vitro and so can help in muscle activation and contraction. Theiler et al15 established the scientific basis for the antidiabetic effects of the leaves extract by identifying the α-Glucosidase Inhibiting compounds in the extract.
The animal model - Rattus norvegicus - gives an excellent and cheap model for aspects of human physiology which cell-based studies do not offer at the moment.16 Renal, cardiac and hepatic markers as well as inflammatory parameters in Rattus norvegicus are excellent predictive of the biomarkers in humans.17 Rattus norvegicus is relatively easy to manage. Factors such as food intake, temperature and humidity and then, lighting conditions can readily be controlled compared to human studies. These obvious factors informed our choice of Wistar rat (Rattus norvegicus) as the model animal for the study. The findings of the current study are translatable to the human species and systems because of similarities of Rattus norvegicus physiology with that of humans.
Notwithstanding the variously reported medicinal uses of J. secunda in folkloric medicine and authenticated scientific evidences, little or nothing is known about its effect on the body chemistry. Studies on its biochemical effects on laboratory animal or on humans are totally lacking. This study was based on the hypotheses that the Justicia secunda Vahl leaves extracts affect vital organs like the heart, liver, kidney and the blood, and contain important phytochemical constituents and phytonutrients. Using an animal model (Wistar rat - Rattus norvegicus), this study will aim to establish how the plant affects vital organs and shed light on the constituents and phytonutrients of the plant which underlie its numerous medicinal and pharmacological properties.
Materials and Methods
Plant Collection and Identification
J. secunda leaves were obtained from a farmland in Nnewi, Anambra Sate and identified by a taxonomist – Mbazulike Akwuba – of the Department of Pharmacognosy and Traditional Medicine, Faculty of Pharmaceutical Sciences of Nnamdi Azikiwe University. A specimen is deposited in the department’s Herbarium with a reference number PCG/474/A/027.
Preparation of the J. secunda Ethanol Extract
The J. secunda leaves harvested from a farmland in Nnewi, Anambra Sate of Nigeria were first washed and shed dried for 3 weeks. After mechanical pulverization, 250g of the powder were soaked in 1 L of 70% ethanol for 24 h and thereafter sieved and filtered through a Whatman number one filter paper. The filtrate was later concentrated (using a rotary evaporator at 60°C) to a jelly-like dark brown solid and stored in a universal bottle in a refrigerator for future use.
Phytochemical and Proximate Analyses of the Leaf Extract
Phytochemical Analysis
This was done as previously reported.18–20 0.2g of the leaves extract were weighed into a 250mL conical flask and covered with 2% conc. HCl (100mL) to soak for 3 hr. Thereafter, the sample was filtered and 50mL of filtrate poured into another 250mL beaker. The volume was made up to 250mL with distilled water and titrated with standard FeCl3 solution (containing 0.00195g iron per 1mL) using 0.3% ammonium thiocyanate solution (10mL) as an indicator. Phytic acid content was calculated as20
The Follins Dennis titrating method as modified by Akajiaku et al21 and Ezeonu and Ejikeme22 was used. 20g of the leaves extract were placed in a 250 mL conical flask containing petroleum ether (100mL) and covered tightly. After 24 h, the content was filtered out and the filtrates left to stand for 15 mins to enable the petroleum ether evaporate. Re-extraction was done by re-soaking in 100mL of 10% acetic acid in ethanol for another 4h. After filtration, the tannin alkaloid was precipitated out using 25mL NH4OH and later heated in electric hot plate to a total volume of 33mL. To 5mL of this was added 20mL ethanol; then titrated with 0.1M NaOH using phenolphthalein indicator. A pink-coloured solution was taken as the end point. “Tannin content was then calculated in % as (C1V1=C2V2) molarity.21 Where,
C1 = Conc. of Tannic acid, V1 = volume of Tannic acid, C2 = Conc. of base and V2 = Volume of base”
A method suggested in literature23 was used with some modifications. 2.5g of sample were first dissolved in 200mL of distilled water; then 5mL each of 10% sulphuric acid and 0.1M Nitric acid were added in succession. After agitation for 3 mins, the mixture was filtered and the filtrate titrated with 0.2M sliver nitrate using ammonium molybdate as indicator until a faint but permanent turbid colour was obtained.
The method suggested by Obadoni and Ochuko24 and Akajiaku et al21 was used with modifications. Five grams of sample were placed in a 250mL conical flask and 100mL of 20% acetic acid in ethanol added. The flask was kept undisturbed for 24 h on a water bath of temperature 50°C. After filtration, the filtrate was concentrated (in a water bath) to 25% of the original volume and saponin precipitated with concentrated NH4OH (added in a drop-wise fashion). After allowing it to settle for 20 minutes, it was filtered and the filtrate weighed. The saponin content was calculated in percentage as21
This was carried our according to the method reported in the literature25,26 with some modifications. 0.5g of extract were placed in a 250mL conical flask and 100mL of 80% ethanol was added and spun at 2235g for 20 min. The supernatant was poured out, while the residue was evaporated to dryness and later dissolved in 10mL of distilled water. Then, 0.5mL of the dissolved solute was mixed with 1:10 Folin Ciocalteu reagent (2.5mL) and 20% Na2CO3 (1.5mL) and left to stand at 25°C for 30 min. Absorbance was taken at 517nm using UV–Vis spectrophotometer (Santa Clara, US). Using Gallic acid as the standard, the total phenolic contents were stated in mg/g Gallic Acid equivalents (GAE).
The gravimetric method suggested in the literature27 was used with modifications (22). Five grams (5g) of samples were placed in 50mL of 10% ethanolic acetic acid solution. After vortexing for 5 min, the mixture was allowed to stand for 4 h and then filtered. The filtrate was evaporated to 25% of its initial volume and the alkaloid precipitated with conc. NH4OH (added in drop-wise manner). The precipitate was placed in a clean filter paper, washed with 1% NH4OH solution and then dried at 60°C in an electric oven for 30 min and reweighed. The alkaloid content is expressed as a fraction of the sample weight analyzed.
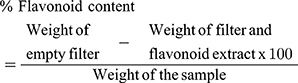
With some modifications, this was performed as previously described in the literatures.25,26 Five grams (5g) of extract were placed in a conical flask containing 50mL distilled water and 2mL HCl. After boiling for 30 min and cooling, the content was filtered using Whatman filter paper No 42 (Camlab, Cambridge, United Kingdom (UK)) and re-dissolved in 10mL ethyl acetate. A repeat filtration using previously weighed Whatman filter paper No. 42 (Camlab, Cambridge, UK) was done and the residue then dried at 60°C in an oven. After cooling in a dissector and weighing, the quantity of flavonoid was determined using the formula;
Proximate Analyses
This was done in accordance with The Official Method of Analysis of Official Methods of Analysis of AOAC INTERNATIONAL.28
Determination of Moisture Content
Five grams (5 g) of extract were further dried to constant weight in an oven (Gen. Lab Oven MINO/30 WIDNES, Cheshire England UK) at 75°C for 6 h. The loss in weight obtained after cooling and reweighing represented the moisture content, and was expressed in percentage.
Determination of Ash Content
A 1.0 g of the extract sample was placed in a dried porcelain crucible of known weight and fed into a muffle furnace (Vestar Furnace Type EF3 Chesterfield UK). The temperature of the furnace was allowed to rise slowly to 450°C and then maintained for 4 h. The crucible was then transferred to a desiccator, cooled to 25°C and weighed. The percentage loss on ignition from weight loss during combustion was calculated.
Determination of the Crude Protein Content
This was determined by the micro Kjeldahl method as explained in the literature.29 A 1.0g of the extract sample was placed into a 500 mL Kjeldahl flask and triturated with 2.0g of digestion catalyst (K2SO4 and H2O mixture) and 20 mL of conc H2SO4. The flask was gently heated until frothing subsided. The heat was increased until a colorless or pale green digest was obtained. After cooling and diluting to 5mL with distilled water, 20mL of the diluted digest was placed in the distillation apparatus. A volume of 25mL of 4M NaOH solution was added and the apparatus switched on for distillation to proceed. The distillate was collected in the receiver containing 10mL of boric acid of indicator solution. A 0.1M HCl solution was used to titrate to pale end point.
The nitrogen content was estimated using the formula:
The Crude Protein contents were calculated by multiplying the % Nitrogen value with a conversion factor of 6.25
Determination of Total Lipids
Five grams (5 g) of extract were weighed into a soxhlet extraction thimble. The thimble was transferred into a 60 mL capacity soxhlet extractor. A clean, dry 250mL flat–bottomed flask (boiling flask) containing black beads was accurately weighed. 20mL of ether was added to the flask which was connected to the extractor and extraction was continued for 4–6 h. Thereafter, the flask was removed and placed in a water bath, and the ether was allowed to evaporate using a stream of nitrogen. The flask was left in a vacuum oven at 40°C for 30 min, cooled in desiccator and then reweighed. The total lipid (fat) was then calculated.
Determination of Crude Fiber Content
One gram (1 g) of extract was weighed into a 200mL pyrex flask. After adding 1.25% H2SO4 and covering with a watch glass, the content was gently boiled on a hot plate for 30 min. The residue was decanted under a sintered glass crucible into a beaker. The content of the beaker was mixed with 200mL solution of 1.25g carbonate-free NaOH and gently boiled on a hot plate for 30 min. After removing the alkali, the residue was washed twice with 50 mL of boiling water. The content of the beaker was washed into a sintered glass crucible and was then dried, incinerated, cooled and weighed to constant weight. The difference divided by the sample weight expressed in percentage gives the fiber content.
Determination of Carbohydrate Content
This was calculated as a difference of 100 from the other values above.
ie, 100 – Moisture Content + Ash + Protein + Fat + fiber = % CHO
Method for Metal Analysis
Using a Varian AA240 Atomic Absorption Spectrophotometer (AAS), the metal content of an extract sample was evaluated as described by the American Public Health Association.30 The samples aspirated into flame are split into its constituent atoms as the AAS’s light beam falls incident upon the flame and forms a monochromatic light characteristic of the elements. This is directed onto an attached detector to quantify the light absorbed by the atomized element in the flame. The energy of the characteristics wavelength absorbed in the flame is directly proportional to the sample’s elemental contents.
Justicia secunda leaves extract were first digested according to earlier reports30,31 with some modifications. Briefly, 2g of extract were weighed and placed in a 200mL digestion flask containing 20mL of mixture of 650mL conc. HNO3, 20mL conc. H2SO4 and 80mL HClO4. The flask was heated until a clear digest was obtained; then diluted with distilled water up to the 100mL mark. Dilutions were then made for each element.
Preparation of Reference Solution
A series of known metals in their optimum concentration range was first prepared to serve as the stock solutions. Fresh reference solutions of the various metals were then prepared by diluting the stock solutions with water containing 1.5mL conc HNO3 per liter.
A calibration blank was prepared using all the reagents except for the metal stock solutions. Calibration curves for each metal were prepared by plotting the absorbance of standards versus their concentrations.30
Experimental Animals
Thirty-seven (37) Rattus norvegicus rats of both sexes (median weight = 103g and range = 100–105 g) and aged 8 weeks were sourced from the animal house of the Department of Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, Chukwuemeka Odumegwu Ojukwu University, Igbariam Campus, Anambra State. The animals were first acclimatized for 2 weeks by housing them in aluminum cages at temperature: 26 ± 2 °C, relative humidity: 45 ± 2% and under normal day/night cycles. They were provided generously with clean drinking water and standard commercial pelleted feed (Vital feed® Nigeria). The Rattus norvegicus were maintained and used for the study in compliance with existing local and international guidelines32–34 for care and use of laboratory animals. The study protocol was approved by the Ethical Board for Animal Studies/Proposal vetting committee of the Faculty of Natural Sciences, Chukwuemeka Odumegwu Ojukwu University in Uli Campus of Anambra State. ETHICAL NUMBER: EBAS/FNS/COOU/005.
The cardinal reason for the 3Rs (replacement, refinement or reduction) in animal use in scientific experiments is to abolish distress to the animals.35 The method of animals handling was refined by ensuring that they were handled by experienced personnel and housed in the approved animal house of the university with standard environmental conditions and according to approved guidelines.32–34 The study also employed humane sacrificing (treatment with isoflurane) and disposal of these animals as explained in the later part of the method section. The study reduced the number of animals as much as possible to accomplish ethical as well as scientific goals, by using the fewest animals that could generate valid and reproducible data. The nature of the study does not warrant the use of non-animal models and there was no room for the replacement of the model as there was no reported in vitro study.
Estimation of the Median Lethal Dose (LD50) of Justicia secunda Leaves
A previously reported method36 was used with some modifications. Thirteen (13) rats were used to determine the median Lethal Dose (LD50) of Justicia secunda leaves extracts. Test animals were randomly divided into 6 groups. The first 3 groups which contain 3 animals each were given 10mg/kg, 100mg/kg and 1000mg/kg body weight of the ethanolic extract of J. secunda leaves. The J. secunda extracts were administered orally and the animals were monitored for 24 h. The last 3 group which contain one animal per group were then given 1600mg/kg, 2900mg/kg and 5000mg/kg body weight of the ethanolic extract of J. secunda leaves and were observed for 24 h. Preliminary studies informed the choice of all the doses used in the experiment. There was no need of a control group. Animals’ allocation into groups was such that the mean weights of the groups were equal (or near equal) and there were equal number of each sex per group. The extract administration, the observation of the effects of the extracts and result recording were all done by the investigator. The lowest dose which had killed one animal and the highest dose which had not killed any animal were noted. The animals that survived were euthanized by placing them in glass jars containing cotton balls soaked with isoflurane (just sufficient to effect death in 2 minutes) and their remains buried in a nearby bush. Death was confirmed by pupillary non-responsiveness to light. The geometric mean of the doses for 0/1 and 1/1 was determined and the LD50 calculated mathematically using the formula: LD50 = √Highest None Lethal Dose (HNL) x Least Lethal Dose (LLD) = √2900 x 5000 = 3800mg/kg body weight.
Biochemical Study
This was done according to the modified method of Onoja et al.6 The twenty-four (24) rats used for this study were made to fast overnight but allowed free access to water prior to the study. They were randomly divided into four groups (A–D) of 6 rats each such that the mean weights of the groups were equal and there were equal number of each sex per group. Group A, serving as control, received orally 10 mL/kg/day of distilled water while Groups B–D were administered orally 100, 200 and 400 mg/kg/day of the plant extract respectively for 30 days. Preliminary studies informed the choice of the doses used. All treatments were carried out in the animal house in the morning period. The oral route was chosen to mimic the natural means of administration of Justicia secunda in humans. All the animals were allowed free access to feed and clean water throughout the experimental period. The initial weights of the animals were recorded and subsequently, every week. On the 30th day, animals’ blood samples were collected in sterile Eppendorf tube using a method described previously37 with some modifications by inserting heparinized capillary tube just below the eye ball of the various animals. The animals were treated humanely during the blood collection by first placing them in glass jars containing cotton balls soaked with isoflurane (just sufficient to induce sleep) and covered for 2.5 minutes. Later, the animals were euthanized by returning them to airtight glass jars containing cotton balls soaked with isoflurane (just enough to effect death in 2 minutes) and buried in a nearby bush. For the biochemical tests, the blood samples collected were first centrifuged and the serum were used for testing as described previously in the literatures38–41 using kits from RANDOX, Diamond Road Crumlin, Co. Antrim, United Kingdom. For the haematological and random blood glucose tests, whole blood was used without centrifugation. It was Random Blood glucose Test in the sense that the animals were feed normally in the morning and blood collection and testing were done later in the day.
Statistical Analysis
The results were presented as figures and Tables, and data analyzed using Graph Pad Prism version 5.00 for Windows, GraphPad Software, Inc. San Diego California USA, www.graphpad.com. One-way ANOVA with Dunnett’s post-test was “used to compare continuous variables in Groups”. Two-way ANOVA was “used to compare how the continuous variables responded to the effect of two factors”37 (length of treatment and the different treatments Groups). Dunnett’s Tests of Multiple Comparison was used to determine the effects between the groups. The p values were taken for two-tailed tests and level of significance taken at α = 0.05. D’Agostino Omnibus normality test was performed on the data sets to check if data follows Gaussian distribution.
Results
Figure 1 shows the plant studied as a flowering evergreen perennial herb, shrub or subshrub having more or less woody stems. Leaves present epidermal cell wall outgrowths called cystoliths and have leaf stalk (the petiole) and full blade margins. The terminal or axillary flowers are conspicuous. The bracts with their bracteoles are equally conspicuous and arranged such that they overlap.
 |
Figure 1 The Justicia secunda plant. |
The proximate analysis (Table 1) shows that the plant is mostly rich in carbohydrate followed by protein when compared to its composition of other macronutrients; It also has an appreciable composition of micronutrients (Zn, Mg, Fe, Ca, Na, K and Mn), the highest being Na followed by Zn and then Mn. The Phytochemical studies also revealed that the plant contains more flavonoids and alkaloids than other phytochemicals. However, it also contains some quantity of cyanide.
 |
Table 1 Composition of the Plant Extract |
The results of the acute toxicity study (LD50) are shown in Table 2. The animals that were administered the different doses of the ethanolic extract of J. secunda survived after 24 h of administration except the one that was given 5000mg/kg body weight. The LD50 value was determined to be 3800mg/kg body weight.
 |
Table 2 Effect of the Ethanolic Extracts of J. secunda on the Rats (Acute Toxicity Study) |
In the experiment to evaluate the animals’ biochemistry, it was observed that one animal in group D died on the 29th day of the experiment and that accounted for the consistent loss of data that would have been obtained from that animal. No protocol modification was made and all of the remaining animals survived to the 30th day of the experiment.
Figure 2 shows that the plant extract significantly increased (P value < 0.05) the random blood glucose of the lab animals in a dose-dependent manner. The significant increase was much more in Group B and then a small increase in Group D animals when compared with the Control Group A.
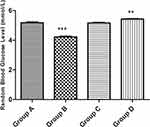 |
Figure 2 Random blood glucose. |
Figure 3 shows the result of the Liver function Test. The plant extracts did not significantly increase (P value > 0.05) the bilirubin (both direct and total) levels of the lab animals when compared with the control group.
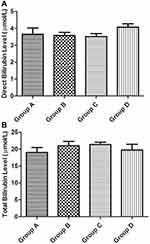 |
Figure 3 Liver function test (bilirubin levels). (A) Direct (unconjugated) bilirubin and (B) total bilirubin. |
Figure 4 shows the liver function test (Liver enzymes levels). The plant extracts did not significantly increase (P value > 0.05) the liver enzyme (Alkaline phosphatase, Aspartate Transferase and Alkaline Transaminase) levels of the lab animals. The results of the liver function tests show that the plant extract has an effect on the lab animals’ liver comparable with the effect of water.
 |
Figure 4 Liver function test (liver enzymes levels). (A) Alkaline phosphatase, (B) aspartate transferase and (C) alkaline transaminase. |
Figure 5 shows the result of the lipid profile. The plant extract significantly elevated (P value < 0.0001) the Total Cholesterol levels of the lab animals in a dose-dependent manner. The increase was more obvious in the Groups B and D animals (p value < 0.05) but not significant in the Group C animals when compared with the Group A (Control) animals. A similar increase was observed in the Triglyceride levels (P value < 0.0001). The increase was similarly highly significant in the Test Groups (B-D) animals (p value < 0.05) when compared with the Group A (Control) animals. Similar changes to the Triglyceride levels were observed with the High Density Lipoprotein levels. Similar effects to those on the High Density Lipoprotein levels were observed in the Low Density Lipoprotein levels, with minor differences. The differences seen in Groups B and C were similar and smaller than that seen in Group D animals. The results of the lipid profile, in general, showed that the extracts significantly elevated the lipid profile of the lab animals (P value < 0.0001) above the effect of water.
 |
Figure 5 Lipid profile (A total cholesterol, B triglyceride, C high density lipoprotein, D low density lipoprotein). |
Figure 6 shows the kidney function result. The plant extracts showed a marked increased (P value < 0.0001) on the blood urea compared to the control (water). This significant effect was seen to a greater extent in the Group B and D animals compared to Group A (control). There was no significant effect observed in Group C animals when compared with the control. The extract also significantly elevated (P value < 0.0001) the Creatinine level of the lab animals. The significant difference was observed in all groups compared with the control. The Urea: Creatinine Ratio (UCR) was also elevated in the test group when compared with the control group.
 |
Figure 6 Kidney function (glomerular filtration). (A) Urea level, (B) creatinine level and (C) urea: creatinine ratio. |
Figure 7 shows the kidney function result. The plant extract significantly increased the [K+] and [Na+] in the animals’ blood (P value < 0.0001). Significant increases were observed in all groups. The effects of the extracts on the Rattus norvegicus’ blood chloride level showed some significant elevation compared to the control (P value = 0.0002). This difference was seen in Group B and was extremely obvious in Group D animals compared with the control. There was no difference was observed in Group C animals. There was significant decrease in the Anion Gap (AG) seen in the treatment and control group (P value < 0.0001). The decrease was observed only in Group C and D (P value < 0.05) when compared with the control Group. It was observed that increasing concentrations of the extract lowers the anion gap (ie, improved the neutrality of the lab animals’ blood and makes it equal to that of water – the control).
 |
Figure 7 Kidney function (serum electrolytes concentration). (A) Potassium level, (B) sodium level, (C) bicarbonate level, (D) chloride level and (E) anion gap. |
Figure 8 shows the haematological results. It was observed that the treatment duration with the extract did not produce significant increase on the haematological parameters (White Blood Cells, Hemoglobin and Packed Cell Volume) that were tested (P value > 0.05).
 |
Figure 8 Hematological result. (A) White blood cells, (B) hemoglobin and (C) packed cell volume. |
In Figure 9, it can be observed that the interaction between the treatment (doses of plant extract) and duration of treatment accounted for approximately 3.85% of the total increases seen in the animals’ weight with a P value = 0.8489. Plant extract treatment alone, accounted for approximately 23.06% of the total increases seen in animals’ weight with a P value < 0.0001 while the duration of treatment accounted for approximately 21.99% of the total weight increase with a P value < 0.0001. The plant extracts and the duration of treatment independently contributed to the observed increase in the animals’ weight gain. In Figure 9B, the interaction between the treatment (doses of Plant extract) and duration of treatment accounted for approximately 2.95% of the total increase seen in the animals’ change in weight with a P value = 0.8343. Plant extract treatment accounted for approximately 27.27% of the total increase seen in the animals’ change in weight with a P value < 0.0001 while the duration of treatment accounted for approximately 24.57% of the total variance seen in animals’ change in weight with a P value < 0.0001. The effect of treatment duration is, therefore, considered extremely significant. This significant effect was obvious only in Group D at the 2nd Week (difference = −18.27 and P < 0.05) and 4th Week (difference = −22.25 and P < 0.05) and much more obvious at the 3rd Week (difference = −26.33 and P<0.01).
 |
Figure 9 Weight results of the animals. (A) Animals’ weight and (B) animals weight. |
Discussion
We investigated the pharmacobiochemical effects of ethanol extract of J. secunda Vahl leaves on Wistar rats and also established its phytochemical constituents and phytonutrients. The major phytonutrients of this plant are carbohydrates, distantly followed by proteins. Notable phytomicronutrients and minerals include zinc, iron, calcium and manganese. Notable phytochemical constituents are flavonoids and alkaloids. The presence of cystoliths in the leaves is a confirmation of the presence of calcium as they (cystoliths) are usually composed of calcium carbonate compound.42,43
It is confirmed that chemicals/substances with LD50 ≥ 5000mg/kg body weight are practically non-toxic.44,45 The finding that the LD50 of the plant is below 5000mg/kg body weight confirms the cyanide constituent of the plant. The implication is that Justicia secunda Vahl leaf is not totally safe, potentially due to the cyanide content. This could be the reason behind the practice of first boiling the leaves before taking the infusion for the treatment of anemia.
The results of rats’ blood glucose level show a dose-dependent increase. This is predictable, considering the high carbohydrate content of the leaf. At high doses there is, therefore, the possibility of exceeding the threshold level leading to possible hyperglycaemia. While no study has evaluated the antidiabetic effect of Justicia secunda leave, previous study showed that some species of Justicia46 have some hypoglycaemic effects.
The study on the serum/plasma bilirubin levels and liver enzymes of the rats showed that the extract did not significantly increase levels when compared with the effect of water. It is understood that elevated bilirubin level is a mark of underlying liver pathology.47 From our study, the plant leaves extract does not predispose to liver disease at the doses tested. Also, an increase in serum/plasma liver enzymes is an indication of inflamed or damaged hepatocytes. Our study demonstrated that the plant leaves extract do not cause liver damage.
The effect on the lipid profile of the rat is very remarkable. The extract significantly increases the parameters measured in the lipid profile when compared with water. Generally, the extract increased the lipid profile values in a dose-dependent manner. It increased the blood levels of low-density lipoprotein (LDL) cholesterol and triglycerides. High levels of these substances increase the risk for developing heart disease. Although not much work has been done on the effect of any Justicia spp on lipid profile, the hyperlipidemic effect observed in this study may be due to an increase in the intracellular calcium concentrations. The proximate analysis confirmed the calcium content of the extract and increased intracellular calcium concentrations are associated with hyperlipidaemia and lipid abnormalities.48
Creatinine and urea blood levels are reflections of the glomerular filtration rate (GFR) or kidney function. When the plasma urea concentration is elevated, plasma creatinine will normally also increase due to reduction of GFR. This increase in plasma creatinine signifies renal pathology.49 Our study showed that the extract significantly elevated the creatinine and urea blood levels but reduced the Urea: Creatinine Ratio (UCR) when compared with water. These observations suggest that the extract may cause renal dysfunction due to possible accumulation in the kidney. The calculation of the UCR helps to establish a renal or non-renal cause of altered GFR. The results showed that the UCR was markedly reduced compared with the effect of water. Kidney pathology is associated with a reduction in the excretion of urea and creatinine with increase in creatinine and urea blood levels.50
A consideration of the animals’ serum electrolytes (Na+, K+, Cl−1 and HCO3−1) concentrations, revealed that the ethanolic extract significantly increased these parameters when compared with the effect of water (p value <0.05). Anion Gap (AG) = (Na+K) - (Cl + HCO3) is a measure of Metabolic Acidosis and provides information on the acidity of the blood and is useful in the clinical diagnosis of acid-base disorders and many other conditions.51 It indicates the concentration of other charged particles that must be in the blood to make it neutral. It was observed that increasing concentrations of the extract lowers the anion gap (ie, improved the neutrality of the lab animals’ blood, hence no negative haematic effect was observed). With low anion gap, there is an increase in the measured anions over measured cations. This increase is usually multi-factorial.51 When an anion gap is elevated, it is usually due to unmeasured anions that are retained in the serum.
The extract did not produce significant effects on the haematological parameters of non-anemic rat. This may be because the treatment was for a short duration (30 days) or more likely because of its low iron content.5 The low iron content may be as a result of the solvent (ethanol) used in the extraction process or due to geographical source of the plant material used. Methanolic extraction may give a higher iron content, better haematinic and other biological effects.6,52 It is possible that the extract may produce a significant effect on pathological anaemic, although this was not investigated.
The animals’ weight consistently increased over the period of the 30 days study. This infers that the plant extract did not have negative effects on the appetite (food in-take) of the animal. The increase in weight and the change in weight were comparable to the effect of water and normal food intake. Substances that depress the appetite will normally affect food in-take negatively and will lead to weight loss. It can also be concluded that the extract did not impact negatively on the taste and smell of the animals’ feed; otherwise, they would not be able to eat and would have lost weight. A previous study showed that these three factors (appetite, taste and smell or aroma) affect food in-take and satiety.53
Limitations
First, our study was limited to the effects of the ethanol extract on the lab animals and did not try to investigate the actual components of the extracts responsible for the observed effect. Secondly, no suitable control for the in vitro studies was available. It is, therefore, suggested that fingerprinting of this plant be undertaken as a future study. Thirdly, neither mechanistic nor molecular bases of the observed effects were investigated. Also, neither histopathological nor chronic toxicity investigations were carried out. Although the above-mentioned limitations exist, the study provides some scientific basis for why the plant leaves extract should not be consumed irrationally nor taken for a long period for any medical condition. An important source of bias in this study is that not all the data could be accounted for due to animal death and incomplete data recording, which could have led to imprecision in the results seen.
Conclusion
The ethanol leaf extract of Justicia secunda has negative cardiac and renal effects on Wistar rat, causing increased lipid profile, creatinine and blood urea levels in the experimental animal. The LD50 is below the safety level. Caution should be exercised as the biochemical profiles of cardiac and renal effects do not seem to be promising and the LD50 is below the safety level. This study offers a scientific basis as to why the plant extract should not be consumed irrationally nor taken for a long period for any medical condition.
Abbreviations
FC, Folin Ciocalteu; GAE, Gallic Acid equivalents; UK, United Kingdom; AOAC, The Association of Official Analytical Chemists; AAS, Atomic Absorption Spectrophotometer; APHA, The American Public Health Association; LD50, Median Lethal Dose; LLD, Least Lethal Dose; HNL, Highest None Lethal Dose; ANOVA, Analysis of Variance.
Acknowledgments
The authors wish to acknowledge Mbazulike Akwuba for taking time to authenticate the leaves used in the study. The authors also wish to express their profound gratitude to Dr. Rebecca Ashfield of Jenner Institute, University of Oxford, Old Road Campus Research Building, Roosevelt Drive, Oxford, OX3 7DQ, United Kingdom who painstakingly did copy-editing of the entire manuscript.
Funding
This study received no external funding and is part of the Master of Science thesis of AHO.
Disclosure
The authors declare that they have no competing interests.
References
1. Oli AN, Obaji M, Enweani IB. Combinations of Alchornea cordifolia, Cassytha filiformis and Pterocarpus santalinoides in diarrhoegenic bacterial infections. BMC Res Notes. 2019;12(1):649. doi:10.1186/s13104-019-4687-0
2. Sofowora A, Ogunbodede E, Onayade A. The role and place of medicinal plants in the strategies for disease prevention. AJTCAM. 2013;10(5):210–229.
3. Khan I, Jan SA, Shinwari ZK, Ali M, Khan Y, Kumar T. Ethnobotany and medicinal uses of folklore medicinal plants belonging to family acanthaceae: an updated review. MOJ Biol Med. 2017;1(2):34–38. doi:10.15406/mojbm.2017.01.00009
4. Verdam MCS, Guilhon-Simplicio F, Barbosa GS, et al. Anti-inflammatory action of Justicia acuminatissima leaves. Rev Bras Farmacogn. 2015;25(3):264–268. doi:10.1016/j.bjp.2015.05.002
5. Koné WM, Koffi AG, Bomisso EL, Tra BFH. Ethnomedical study and iron content of some medicinal herbs used in traditional medicine in Cote d’Ivoire for the treatment of anaemia. Afr J Tradit Complement Altern Med. 2011;9(1):81–87. doi:10.4314/ajtcam.v9i1.12
6. Onoja SO, Ezeja Maxwell I, Omeh YN, Onwukwe BC. Antioxidant, anti-inflammatory and antinociceptive activities of methanolic extract of Justicia secunda Vahl leaf. Alexandria J Med. 2017;53:207–213. doi:10.1016/j.ajme.2016.06.001
7. Anyasor GN, Okanlawon AA, Ogunbiyi B. Evaluation of anti-inflammatory activity of Justicia secunda Vahl leaf extract using in vitro and in vivo inflammation models. Clin Phytosci. 2019;5(49). doi:10.1186/s40816-019-0137-8
8. Carrington S, Cohall DH, Gossell-Williams M, Lindo JF. The antimicrobial screening of a Barbadian medicinal plant with indications for use in the treatment of diabetic wound infections. West Indian Med J. 2012;61(9):861–864. doi:10.7727/wimj.2011.223
9. Manda P, Abrogoua DP, Bahi C, Dano DS, Gnahoui G, Kablan BJ. Evaluation of the antihypertensive activity of total aqueous extract of Justicia secunda Valh (Acanthaceae). Afr J Pharm Pharmacol. 2011;5(16):1838–1845.
10. Kitadi JM, Lengbiye EM, Gbolo BZ, et al. Justicia secunda Vahl species: phytochemistry, pharmacology and future directions: a mini-review. Discovery Phytomedicine. 2019;6(4):157–171. doi:10.15562/phytomedicine.2019.93
11. Koffi EN, Le Guernevé C, Lozano PR, et al. Polyphenol extraction and characterization of Justicia secunda Vahl leaves for traditional medicinal uses. Ind Crops Prod. 2013;49:682–689. doi:10.1016/j.indcrop.2013.06.001
12. Zambrano Mora P, Bustamante Pesantes KE. Characterization and phytochemical study of Justicia secunda valh (sanguinaria, singamochilla, insulina). Rev Cuba Plantas Medicinales. 2017;22(1):1–8.
13. Khan SU, Anjum SI, Ansari MJ, et al. Antimicrobial potentials of medicinal plant’s extract and their derived silver nanoparticles: a focus on honey bee pathogen. Saudi J Biol Sci. 2019;26(7):1815–1834. doi:10.1016/j.sjbs.2018.02.010
14. Hernando H-M, Alfredo R-R, Oscar Crescente V. Biological activity of “Sanguinaria” (Justicia secunda) extracts. Pharm Biol. 2002;40:3206–212. doi:10.1076/phbi.40.3.206.5826
15. Theiler Barbara A, Stefanie I, Martin Z, et al. HPTLC bioautography guided isolation of α-glucosidase inhibiting compounds from Justicia secunda vahl (acanthaceae). Phytochem Anal. 2017;28(2):87–92. doi:10.1002/pca.2651
16. Shirani F, Teimoori A, Rashno M, Latifi SM, Karandish M. Using rats as a research model to investigate the effect of human adenovirus 36 on weight gain. ARYA Atheroscler. 2017;13(4):167–171.
17. Ellenbroek B, Youn J. Rodent models in neuroscience research: is it a rat race? Dis Model Mech. 2016;9(10):1079–1087. doi:10.1242/dmm.026120
18. Sivakumaran K, Kothalawala S. An overview of the analytical methods for food phytates. IJCS. 2018;6(1):2016–2020.
19. Abulude FO, Folorunso OR, Akinjagunla YS, Ashafa SL, Babalola JO. Proximate compositions, mineral levels and phytate contents of some alternative protein sources (Cockroach, Periplaneta americana, Soldier Ants Oecophylla sp. and Earthworm Lubricus terrestics) for use in animal feed formulation. Asian J Anim Vet Adv. 2007;2:42–45. doi:10.3923/ajava.2007.42.45
20. Yadav RC. The ultimate green irrigation practice by innovative application of scientific facts. World J Agron Food Sci Technol. 2015;2(1):1–30.
21. Akajiaku LO, Nwosu JN, Onuegbu NC, Njoku NE, Egbeneke CO. Proximate, mineral and anti-nutrient composition of processed (soaked and roasted) Tamarind (Tamarindus indica) seed nut. Curr Res Nutr Food Sci J. 2014;2(3):136–145. doi:10.12944/CRNFSJ.2.3.05
22. Ezeonu CS, Ejikeme CM. Qualitative and quantitative determination of phytochemical contents of indigenous nigerian softwoods. New J Sci. 2016;2016:9. doi:10.1155/2016/5601327
23. Delaney MF, Blodget C. Free cyanide forms during determination of free cyanide in drinking water. J Am Water Works Assoc. 2017;109(12):E514–E519. doi:10.5942/jawwa.2017.109.0120
24. Obadoni BO, Ochuko PO. Phytochemical studies and comparative efficacy of the crude extracts of some haemostatic plants in edo and delta states of Nigeria. Globl J Pure Appl Sci. 2002;8(2):203–208.
25. Baba SA, Malik SA. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J Taibah Univ Sci. 2015;9(4):449–454. doi:10.1016/j.jtusci.2014.11.001
26. Shi P, Du W, Wang Y, Teng X, Chen X, Ye L. Total phenolic, flavonoid content, and antioxidant activity of bulbs, leaves, and flowers made from Eleutherine bulbosa (Mill.) Urb. Food Sci Nutr. 2018;7(1):148–154. doi:10.1002/fsn3.834
27. Luyang L, Long W, Wan X, et al. Studies on quantitative determination of total alkaloids and berberine in five origins of crude medicine “sankezhen”. J Chromatogr Sci. 2015;53(2):307–311. doi:10.1093/chromsci/bmu060
28. Latimer GW; AOAC International. Official Methods of Analysis of AOAC INTERNATIONAL (2016). Vol. 01.
29. Mæhre HK, Dalheim L, Edvinsen GK, Elvevoll EO, Jensen I. Protein determination—method matters. Foods. 2018;7:5. doi:10.3390/foods7010005)
30. APHA: American Public Health Association. Standard Methods: For the Examination of Water and Wastewater, APHA, AWWA, WEF/1995. APHA Publication; 1995.
31. Adrian WJ. A comparison of a wet pressure digestion method with other commonly used wet and dry-ashing methods. Analyst (Lond). 1973;98(1164):213. doi:10.1039/an9739800213
32. Veterinary Surgeon Act Cap V3 LFN. Federal Republic of Nigeria. “The care and use of animals for scientific purposes”. 2004.
33. Animal Diseases (Control) Act. Cap A17 LFN. Federal Republic of Nigeria “the care and use of animals for scientific purposes”. 2004.
34. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals.
35. Singh J. The national centre for the replacement, refinement, and reduction of animals in research. J Pharmacol Pharmacother. 2012;3(1):87–89.
36. Garrido-Acosta O, Meza-Toledo SE, Anguiano-Robledo L, Valencia-Hernández I, Chamorro-Cevallos G. Adaptation of Lorke’s method to determine and compare ED50 values: the cases of two anticonvulsants drugs. J Pharmacol Toxicol Methods. 2014;70(1):66–69. doi:10.1016/j.vascn.2014.05.002
37. Oli AN, Agu RU, Oli UC, Nwoye CU, Ejiofor OS, Esimone CO. Safety evaluation in mice of the childhood immunization vaccines from two south-eastern states of Nigeria. Asian Pac J Trop Biomed. 2015;5(2):132–137. doi:10.1016/S2221-1691(15)30157-X
38. Ambade VN, Sharma YV, Somani BL. Methods for estimation of blood glucose: a comparative evaluation. Med J Armed Forces India. 1998;54(2):131–133. doi:10.1016/S0377-1237(17)30502-6
39. Li LH, Dutkiewicz EP, Huang YC, Zhou HB, Hsu CC. Analytical methods for cholesterol quantification. J Food Drug Anal. 2019;27(2):375–386. doi:10.1016/j.jfda.2018.09.001
40. Carl A, Edward RA, David EB. Uric Acid. Tietz Fundamentals of Clinical Chemistry.
41. Onyeabo C, Achi NK, Ekeleme-Egedigwe CA, Ebere CU, Okoro CK. Haematological and biochemical studies on Justicia carnea leaves extract in phenylhydrazine induced-anemia in albino rats. Acta Sci Pol Technol Aliment. 2017;16(2):217–230. doi:10.17306/J.AFS.2017.0492
42. Gal A, Hirsch A, Siegel S, et al. Plant cystoliths: a complex functional biocomposite of four distinct silica and amorphous calcium carbonate phases. Chemistry. 2012;18(33):10262–10270. doi:10.1002/chem.201201111
43. Ummu-Hani B, Noraini T. The structure of cystoliths in selected taxa of the genus Ficus L. (Moraceae) in Peninsular Malaysia. AIP Conf Proc. 2013;1571:372. doi:10.1063/1.4858686
44. Erhirhie EO, Ihekwereme CP, Ilodigwe EE. Advances in acute toxicity testing: strengths, weaknesses and regulatory acceptance. Interdiscip Toxicol. 2018;11(1):5–12. doi:10.2478/intox-2018-0001
45. Ihekwereme CP, Okoye FK, Agu SC, Oli AN. Traditional consumption of the fruit pulp of Chrysophyllum albidum (Sapotaceae) in pregnancy may be serving as an intermittent preventive therapy against malaria infection. Anc Sci Life. 2017;36:191–195. doi:10.4103/asl.ASL_208_16
46. Martínez‐Mora JA, Murillo‐Villicaña M, Salgado‐Garciglia R, et al. Relation of hypoglycemic activity and the antioxidant capacity of Justicia Spicigera leaf extracts in diabetic rats. FASEB J. 2017;31:944.
47. Vítek L. Bilirubin and atherosclerotic diseases. Physiol Res. 2017;66(Supplementum 1):S11–S20. doi:10.33549/physiolres.933581
48. Farhangi MA, Ostadrahimi A, Mahboob S. Serum calcium, magnesium, phosphorous and lipid profile in healthy Iranian premenopausal women. Biochem Med. 2011;21(3):312–320. doi:10.11613/BM.2011.042
49. Higgins C. Urea and creatinine concentration, the urea: creatinine ratio. Acute Care Test Hand. 2016;1–8.
50. Gounden V, Bhatt H, Jialal I. Renal function tests. [Updated 2020 Jul 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. Available from https://www.ncbi.nlm.nih.gov/books/NBK507821/.
51. Hopkins E, Sharma S. Physiology, acid base balance. [Updated 2020 Aug 16]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. Available from https://www.ncbi.nlm.nih.gov/books/NBK507807/.
52. Okoye EL, Uba BO, Uhobo PC, Oli AN, Ikegbunam MN. Evaluation of the antibacterial activity of methanol and chloroform extracts of Alchornea cordifolia leaves. J Sci Res Rep. 2014;3(1):255–262. doi:10.9734/JSRR/2014/4328
53. Yin W, Hewson L, Linforth R, Taylor M, Fisk ID. Effects of aroma and taste, independently or in combination, on appetite sensation and subsequent food intake. Appetite. 2017;114:265–274. doi:10.1016/j.appet.2017.04.005
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.