Back to Journals » Journal of Asthma and Allergy » Volume 14
The Patients’ Experience of Severe Asthma Add-On Pharmacotherapies: A Qualitative Descriptive Study
Authors Clark VL , Gibson PG, McDonald VM
Received 8 December 2020
Accepted for publication 25 January 2021
Published 15 March 2021 Volume 2021:14 Pages 245—258
DOI https://doi.org/10.2147/JAA.S296147
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Vanessa L Clark,1,2 Peter G Gibson,1,3 Vanessa M McDonald1– 3
1National Health and Medical Research Council Centre for Research Excellence in Severe Asthma and the Priority Research Centre for Healthy Lungs, The University of Newcastle, New Lambton Heights, NSW, Australia; 2School of Nursing and Midwifery, The University of Newcastle, New Lambton Heights, NSW, Australia; 3Department of Respiratory and Sleep Medicine, John Hunter Hospital, Hunter Medical Research Institute, New Lambton Heights, NSW, Australia
Correspondence: Vanessa M McDonald Email [email protected]
Purpose: Add-on therapies for severe asthma are continually emerging with proven efficacy in randomised controlled trials. To date, however, there are no qualitative studies exploring patients’ experiences with these treatments. We aimed to understand the experience of patients who were treated with an add-on therapy for their severe asthma.
Patients and Methods: A qualitative descriptive study was conducted, participants were recruited from the respiratory clinics and databases of a tertiary referral hospital. Participants with treatment-refractory severe asthma (n=20) prescribed an add-on therapy for > 4 months (75% mepolizumab; 25% omalizumab, and 25% macrolide) were recruited. Qualitative semi-structured interviews were conducted, with interviews thematically analysed.
Results: Participants’ mean (SD) age was 59.5 (15.3) years, and 50% were male. Participants reported 4.5 (2.3) exacerbations in the past year. Asthma Control Questionnaire score was 2.0 (1.4). The monoclonal add-on therapies had been prescribed for a median (IQR) of 12.5 (7.0, 24.0) months. Experience was captured in four emergent themes: “Life is just easier” provided an overall message that the add-on therapy made the participants’ life easier in terms of increasing participation, levelling out symptoms, providing more energy and reducing healthcare use. “Prednisone: A necessary evil” was discussed, particularly in terms of dose and dependence and damaging side effects. The theme “worry and hope for the future” referenced treatment non-response or cessation of effect which was discussed by some participants. Finally, “holistic care” was centred on the sentiment that the participant’s asthma management and overall health were not related to one aspect or medication alone.
Conclusion: Patients with severe asthma experience vast improvements in quality-of-life and life participation with add-on therapies, but there remains a significant burden related to oral corticosteroids and incomplete treatment responses. Addressing this residual burden is an important area for future research.
Keywords: severe asthma, asthma medications, monoclonal antibody therapies, quality of life, patient experience
Introduction
Severe asthma is a high burden disease with an increased risk of morbidity and mortality,1–4 and is associated with quality-of-life impairment.5,6 The burden of severe asthma pervades throughout many aspects of life, leading to impairments across physical, emotional, economic and social domains.5,7,8 Confounding the treatment of severe asthma is the heterogeneous nature of the disease, with its variable response to treatment, multiple comorbidities and risk factors.9
In recent years, the emergence of add-on therapies for severe asthma has improved clinical outcomes for some people with severe asthma.10 Monoclonal antibody therapies (mAb) targeting Type 2 inflammatory asthma11–13 improves health-related quality-of-life (HRQoL) and asthma control, and reduces acute attacks.12,14 Additionally, azithromycin in severe asthma leads to reduced attacks and improved HRQoL.15,16 Although these treatments have been shown to be efficacious, residual burden from asthma attacks, ongoing asthma symptoms and quality-of-life impairment remain.17
Whilst the patient experience of living with severe asthma has been recently explored7,8 the experience of people using these medications is not described. Understanding these experiences will aid communication of the potential benefits and limitations of the medications. Therefore, in this study, we aimed to understand the experience of patients who were treated with an add-on therapy for their severe asthma.
Patients and Methods
Study Design
A qualitative descriptive study exploring the experiences of adults with severe asthma in relation to add-on asthma therapies was conducted. Data were collected using semi-structured, in-depth face-to-face interviews with a researcher (VLC), a behavioural scientist, who had no prior relationship with the participants and was not involved in their medical care. Ethical approval was obtained (Hunter New England Human Research Ethics 16/05/8/5.02). This study was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent prior to study commencement. Participants were informed as part of the consent process that anonymised quotes will be used in possible publications.
Setting
Participants were recruited from the respiratory research database and clinics of a tertiary referral centre in New South Wales, Australia between May 2018 and December 2018.
Participants
Adult (>18 years) participants (n=20), who had a prior confirmed doctor diagnosis of severe persistent asthma, were purposefully recruited. The eligibility for severe asthma was based on the American Thoracic Society/European Respiratory Society taskforce,18 including the requirement for maximal high-dose inhaled corticosteroids and a long-acting β2-agonist, or requiring frequent oral corticosteroids. A further criterion was the prescription of a novel add-on therapy (omalizumab or mepolizumab or azithromycin) for at least 4 months prior to study entry.
Data Generation
Baseline demographic data were collected prior to the interview including age, age of asthma diagnosis, acute attacks and medical history, and asthma control was assessed using the Asthma Control Questionnaire (ACQ).19
Semi-structured interviews were conducted to understand the patient experience of using add-on severe asthma medication. Free-speaking was facilitated by open-ended questioning and probing where required. The interview guide was developed with reference to the study aims and a review of the literature20–23 by the research team, including expert severe asthma multidisciplinary clinicians (PGG and VMMcD) and a behavioural scientist (VLC). The interview guide covered six core aspects aimed at understanding the experience of add-on therapies in severe asthma (see supplement).
Participants were assured of confidentiality and informed at the beginning of the interview that they may ask for the recording to be stopped at any time. All interviews took place in a private room, the mean (SD) duration was 37.65 (16.00) minutes. Interviews were stored via password-protected encrypted storage. Interviews were continually evaluated using an iterative process to ensure additional components did not need to be incorporated into the interview guide. Audio-recorded interviews were conducted face-to-face. Pseudonyms were used in place of participant’s names to ensure confidentiality.
Analysis
Recordings were transcribed verbatim, anonymised, and entered into NVivo version 12 (QSR International, Doncaster, Australia) for data coding. Thematic analysis24 was performed using an inductive approach. The first analytical step involved familiarising the data by reading and re-reading the transcripts and making initial notes. Following, these data were initially descriptively coded, and then line-by-line using inductive coding. In the third step, codes were categorized and merged based on similar codes from the initial inductive coding. Data were then synthesised into themes and subthemes. Themes and subthemes were discussed, reviewed and confirmed with co-authors, once consensus was reached, the themes were named to reflect the theme content. Additionally, the emergent themes and subthemes were continually checked against the codes and the original transcripts to ensure they were representative of the interview content.
Results
Participants were mean (SD) 59.50 (15.27) years and 50% male (Table 1). Most (75%) were prescribed Mepolizumab, 25% prescribed omalizumab and 25% using azithromycin (Table 1). The median (IQR) duration of monoclonal antibody therapy was 12.50 (7.00, 24.00) months. Of all participants, 60% had poor symptom control determined by an ACQ>1.5. Half of the included participants were prescribed maintenance oral corticosteroids (OCS), at a mean (SD) dose of 29.45 (38.16) mg.
 |
Table 1 Patient Demographics |
There were four emergent themes. These themes and their subthemes are displayed in Figure 1. Exemplar quotes associated with each emergent theme and their associated subthemes are summarised in Tables 2–5.
 |
Table 2 Theme 1, “Life is Just Easier” |
 |
Table 3 Theme 2, “Prednisone: A Necessary Evil” |
 |
Table 4 Theme 3, “Worry and Hope for the Future” |
 |
Table 5 Theme 4, “Holistic Approach” |
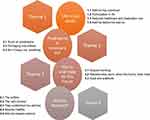 |
Figure 1 Themes (circles) and subthemes (boxes) for understanding the experience of add-on therapies in severe asthma. |
Theme 1: “Life is Just Easier”
The theme “life is just easier” provided an overall message that the add-on therapy made the participants’ life easier in terms of levelling out symptoms, providing more energy and reducing healthcare use. Participants described three main subthemes in which the add-on therapies had impacted their life. These were “asthma has stabilised”, “participation in life” and “reduced healthcare and medication use” (Table 2). Participants also contrasted their experience of “asthma before the add-on therapy” (Table 2).
Subtheme 1.1: Asthma Has Stabilised
Participants described how the add-on therapy had stabilised their asthma. They described a levelling out of their symptoms, with reductions in the severity of their attacks, and a reduction in time taken to recuperate from an attack (Table 2). Participants still talked about having symptoms of asthma, including congestion and chest tightness, but that these symptoms were more manageable.
Subtheme 1.2: Participation in Life
Participants described a substantial improvement in quality-of-life. They reported improvements in energy, ability to participate in life and the ability to undertake household chores, which they were unable to complete prior to their medication commencement. Reduction in asthma symptoms was credited by several participants for their quality-of-life improvement (Table 2). “The other thing is just the quality of life is just so much better. Sleeping better. Just not having to be restricted because I am constantly coughing and spluttering away.” Howard, Male, 62.
In addition, an improvement in energy also contributed to the positive experience of the add-on therapies “I feel more alert, I think. I feel bouncier, I’ve got more energy” William, 72, male. The combination between symptom reduction and increased energy enabled participants to take part in more activities, including going for walks, travelling and doing work around the house. Further, Ian, age 63, male described his experience “In terms of impacting my life, well it means that I can live a normal life, essentially”.
Subtheme 1.3: Reduced Healthcare and Medications Use
Reductions in healthcare and medication use were described by the majority of the participants (Table 2), which was attributed as a positive response to the medication. Participants described the need for fewer emergency department and general practitioner visits. They also talked about less Salbutamol and Prednisone use (Table 2). However, not all participants were able to reduce their oral corticosteroids (examined in the theme “Prednisone- A necessary evil”).
Subtheme 1.4: Asthma Before the Add-On
Participants described what life was like before they were commenced on their add-on therapy (Table 2). They described the need for high OCS use, frequent attacks and limitations to life.
Theme 2: “Prednisone – A Necessary Evil”
Despite the perceived effectiveness of add-on therapies prednisone use was a concern for the majority of participants, with subthemes emerging around the frustration of being “stuck on prednisone”, concerns for the “damaging side-effects”, and acceptance “but it keeps me breathing” (Table 3).
Subtheme 2.1: Stuck on Prednisone
The inevitability of having to continue to take prednisone was discussed. Some participants described unsuccessful reduction attempts, and being caught in a cycle of reducing prednisone, then increasing again (Table 3). When asked about the medication they were currently taking, Rhonda, female, 56 said
… I’ve been stuck with [prednisone] for the five and a half years, that I just can’t get off. We even have problems getting me below 20 milligrams … below 20 usually causes an exacerbation. Even though you technically can cut by five milligrams, supposedly if we try and cut me at five milligrams I can end up in hospital.
Subtheme 2.2: Damaging Side-Effects
Concerns around the damaging side-effects of prednisone were described by over three quarters of the participants. Exemplars are presented in Table 3. Side-effects were described across multiple body systems - “I have osteoporosis, the broken bones, the cataracts, mood swings, puffiness, the works … I need it to keep me alive, but it’s not a nice thing.” James, male, 45. Furthermore, participants expressed how side-effects impaired their quality-of-life, with one participant explaining
I hate steroids. They’re just brutal. I’m so against them. Just how you feel on them, you’re just - you can’t sleep. Your body’s [SIC] so exhausted from being sick with asthma, but you’re just awake; the fluid in my legs, my feet, that’s also there. I don’t know the long-term side-effects of steroids, that scares me a lot. They’re just - to me, I call them a Band-Aid.
Naomi, female 34.
Subtheme 2.3: But It Keeps Me Breathing
They acknowledged, however, a trade-off between the side-effects of prednisone for the benefits. Participants acknowledged that prednisone was an effective medication, and implied that often it was their only option “But it’s a necessary evil so I put up with it”. Anthony, male, 72. Additionally, prednisone was described as a “safety net” by one participant and another described it as a “last resort” (Table 3).
Theme 3: Worry and Hope for the Future
The theme “worry and hope for the future” centred around the anxiety that some participants felt when their current add-on therapies did not provide or sustain the disease control that they wanted. Several participants described how their add-on therapies had “stopped working”. Additionally, some felt like this medication was their last hope and expressed feelings of “hopelessness, worry about the future, or false hope”. However, others remained hopeful that there would be new asthma discoveries.
Subtheme 3.1: Stopped Working
Reduced efficacy or treatment non-response was discussed by some participants (Table 4). James, male, 45, expressed frustration at not being able to reduce the prednisone. “No. That’s [the problem]. We haven’t been able to reduce it [the prednisone]. We tried to not long ago [to] step down. I – we tried – as far as I got was 20 milligrams from 25 but I got sick, so we had to put it back up.”
Naomi, female, 34, who had expressed that the add-on therapy had enabled her to lead a normal life, indicated that the medication was no longer helping her asthma, “I think, for the first eight months, I think it changed a lot. I was quite able to – I felt that I was kind of living a normal life, which was amazing. But in the recent three, four months, I don’t think it’s helped me a lot at all.”
Subtheme 3.2: Helplessness, Worry About the Future, False Hope
Some participants expressed worry about the unknown future around some aspects of their medication, for example, not knowing how long they needed to be on the medication. They feared that the medication was losing or would lose its effectiveness (Table 4), with one participant stating,
“At this stage – certain periods through your life, they give you these drugs and say is it going to work? It appears to work at the time … [then they don’t work anymore]”, William, male, 72.
Further, Jane, female, 64 described, “I’d like to know that there’s something in the pipeline, but that’s a security issue … I have concerns, very real and very consistent … ” There was additional concern around not knowing what the future will hold in terms of their severe asthma, and this was limiting to their life.
“Just not knowing, like you want to get a full-time job but not knowing if you can hold it because you don’t know if you’re going to get sick that night or you’re going to be well” Rebecca, female, 21.
Subtheme 3.3: Hope and Gratitude
Some participants expressed a sense of hope for the future in terms of new discoveries (Table 4). Helen, female, 76, recalled “When I started to get it years ago, asthma, there was no medication. I was told that was all in my mind and I was just stressing too much, even though I was crawling up the back steps. The fact that you have medication, I bow to the medical world”.
Theme 4: Holistic Approach
“Holistic approach” (Table 5) was centred around participants expressing that it is more than their add-on therapies that contribute to their disease management. The subthemes describe the role of other medications (use of their “puffers”), as well as the getting “the right cocktail” of medication. In addition to their pharmacological treatments, participants also expressed the importance of having access to the right healthcare team, and knowledge of what they can do to control their asthma and stay healthy.
Subtheme 4.1: The Puffers
Participants talked about the importance of their other asthma medications in addition to their add-on therapies. Overall, the majority of people felt that the puffers kept them going and the add-on therapy gave them the extra boost.
Subtheme 4.2: The Right Cocktail
Whilst participants were enthusiastic about the effect their add-on therapy had on their asthma symptoms and life (Table 2), they also recognised that finding “the right cocktail” of medication was important. There was a recognition that their add-on therapies provided add-on value as part of their existing asthma management strategies (Table 5). Ian, male, 63 illustrates, “I don’t believe I could just survive on mepolizumab alone. Clearly, Prednisone on its own at 5 milligrams is not enough. It probably would be if I stuck at 50 milligrams a day, but that would be a pretty horrific dose. Yeah, so I think it’s all part of a collective.”
Subtheme 4.3: They Understood My Asthma
Some participants expressed the importance of having access to expert clinicians for their overall severe asthma management, as well as being able to get access to new treatments (Table 5). They credited that it was important to “find the right doctor” and how important their healthcare team was in understanding their asthma.
Subtheme 4.4: Staying Healthy
Some of the participants talked about the importance of staying healthy. Participants spoke about the importance of diet, exercise, medication adherence, as well as being informed about their disease (Table 5).
Subtheme 4.5: Attitude Towards Asthma
Some participants demonstrated a stoic view towards their asthma (Table 5). This included people not wanting others to perceive them as sick, or impaired. “Then yeah, it’s really important to me to be normal. I don’t want anybody to ever perceive me as being an invalid. I don’t like to be seen as a person with a weakness or a sickness, or whatever label you want to put on it. I have to manage it so that you don’t know.” Jane, female, 64.
Discussion
This study encapsulated the patient experience of using new add-on therapies for the treatment of severe asthma. Overwhelmingly participants spoke about how their add-on therapy had positively improved their life. These novel data provide insight into the patient experience beyond that of patient and clinician-reported outcome measures used in clinical trials. We highlight the positive response to treatment and describe the ongoing concerns of patients. Importantly, patients described how the new treatments integrate as a component of a holistic asthma management approach.
The improvements in patient-perceived quality-of-life reported in this study are consistent with the clinical trials of add-on therapies; however, improvements in quality of life were only modest in randomised controlled trials,12,14,16 whereas the experiential description from patients suggests a far greater improvement. The reasons for this are unclear. It may be that quality-of-life questionnaires used in clinical trials25 do not adequately capture the experience of people with severe asthma, or may not ask the questions that are of most important to people with severe asthma. The use of fit for purpose severe asthma patient-reported outcome measures may provide greater sensitivity.20
The theme “participation in life” may also provide important insights in this regard, as participation, meaning increased participation in everyday activities, such as social and physical activities and improved relationships, was highly valued by participants. In another study exploring outcomes that matter to patients with severe asthma, wanting to be more physically active was rated as one of the most important outcomes from a patients’ perspective;26 however, this outcome is infrequently assessed. Physical activity is a complex construct and can relate to participation as well as activity/functional limitations.27
Participants in this study contrasted their experience of living with severe asthma before they received their add-on therapy. These limits to life have been described in previous studies.7,8,20 The themes described in this study extend current knowledge about the patient experience by specifically exploring the impact of novel add-on asthma therapies from a patient’s perspective. From their perspectives, these therapies were able to bring back a normal level of function, such as participating in simple household tasks, like mowing the lawn. Additionally, in previous literature, participants have focused on the negative emotional and physical side-effects of severe asthma treatment.7,8 In the current study, the side-effects of add-on therapies were scantly mentioned.
The use of oral corticosteroids however remained a major concern for people with severe asthma.20,28,29 In this regard, our study mirrored the previously outlined concerns of patients and illustrated that oral corticosteroids use remains a significant problem despite add-on therapies.30 In this current study, participants detailed the damaging consequences of long-term oral corticosteroid use and the inability to wean from this treatment despite mAbs or macrolide therapies. This caused frustration for some as they felt deflated about being “still stuck on prednisone”. Whilst participants described the negative aspects of oral corticosteroids in terms of their side-effects, it was acknowledged by some participants that it was an essential part of their severe asthma management. These data highlight the importance of oral corticosteroid minimisation and the need for oral corticosteroid stewardship in treating severe asthma.31
The impact of treatment and future options was an important consideration. A subset of participants described that their severe asthma add-on therapy had just “stopped working” or had reduced efficacy. Fear and panic in relation to living with asthma has been previously described;20 however, in this study, some participants expressed hopelessness in relation to the likelihood of new medication discoveries, with some “starting to give up a bit” that new medications would come along. Lack of response to treatment is a frustration that is shared by other chronic respiratory diseases, such as chronic obstructive pulmonary disease.32 Additionally, some participants resigned that they were “pretty well the way I’m going to be all the time”. This view was not shared by all, with some people expressing hope for new discoveries. Engaging in discussions with patients about their expectations of treatment, their perception of efficacy and potential new developments may help allay some of these concerns.
Another insightful finding was that participants acknowledged their add-on therapies were only a component of their disease management. They talked about the importance of holistic management and having access to the right clinical team and their other asthma medications in managing their disease, describing the add-on severe asthma therapy as providing additional support. This is consistent with current guidelines for the management of severe asthma.33,34 A systematic review using qualitative data7 highlighted the importance of autonomy from a patient’s perspective in managing their symptoms, acquiring knowledge on treatment options and their relationship with health care providers. The findings of this study are complementary to those of Eassey et al7, with participants describing the importance of what they can do to manage their disease as well as the importance of the right health care team. Given the reported rates of poor treatment adherence in severe asthma35 and the limited use of self-management strategies, such as written action plan,36 the recognition from participants that a comprehensive programme of care is necessary is important and further understanding from patients with severe asthma, may improve these critical aspects of care.
This study provides important understandings of the patient experience of novel add-on therapies. Using rich qualitative data we have provided detailed experiences from a diverse range of participants to assist in understanding the real-world experiences of people with severe asthma with add-on therapies. Our data present a diverse range of experiences from n =20 participants, while this sample is small in quantitative research it is considered appropriate for qualitative designs. We also recognise some limitations. At the time of this study, there were only two mAb therapies available for prescription in Australia. Since this study was conducted an additional mAb therapy has become available, with several others available overseas. We, therefore, have been unable to capture the patient experience of all available mAbs. Further, as it was the aim of the study to understand the experience of patients who were treated with an add-on therapy for their severe asthma we have not sampled to cater for subgroup analyses that may provide further novel information. Further research could focus on sampling to include subgroups of interest, such as different types of mAbs, duration of medication or age of prescription. A strength of this study was that there were a broad range of participants interviewed, who were at varying stages since treatment initiation.
Conclusion
This study provides insight into the patient’s experience of add-on severe asthma therapies which has previously not been described, and cannot be gained from efficacy trials. Understanding these experiences will aid communication of the potential benefits and limitations of these add-on medications. Discussion points between clinicians and patients can include, what a patient can expect from their new treatment based on real-world experiences, whether the treatment will be able to reduce their oral corticosteroids, and the place of these therapies in the asthma management strategy. They can also guide discussion around alternative treatment in cases of reduced treatment efficacy or non-response. These data also highlight the importance of effectively capturing the patient experience.
Acknowledgments
The authors would like to thank the study participants involved in this study. Special thanks to Leonie Jones and Jenny Darcy (Severe Asthma Outpatient Clinic-John Hunter Hospital, Newcastle, Australia), Kelly Steel, Amber Smith and Paola Urroz (Priority Research Centre for Healthy Lungs, University of Newcastle) for their assistance with recruitment.
Funding
This work was supported by the Centre of Research Excellence in Severe Asthma.
Disclosure
Dr Vanessa Clark received a fellowship from the National Health and Medical Research Council, Centre of Research Excellence in Severe Asthma, and reports personal speaking fees from Astra Zeneca for work outside the submitted manuscript. Prof. Gibson reports personal fees from AstraZeneca, GlaxoSmithKline, Novartis, grants from AstraZeneca, GlaxoSmithKline, outside the submitted work. Prof. McDonald reports grants from Hunter Medical Research Institute, grants from National Health and Medical Research Council, grants from John Hunter Hospital Charitable Trust Research Grants, during the conduct of the study; grants and personal fees from GSK from Menarini for educational content, grants and personal fees from Astra Zeneca, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet. 2018;391(10118):350–400.
2. McDonald VM, Gibson PG. Exacerbations of severe asthma. Clin Exp Allergy. 2012;42(5):670–677. doi:10.1111/j.1365-2222.2012.03981.x
3. McDonald V, Kennington E, Hyland M. Understanding the experience of people living with severe asthma. In: Chung K, Gibson P, editors. Severe Asthma (ERS Monograph). Sheffield: European Respirtory Society; 2019.
4. McDonald VM, Hiles SA, Godbout K, et al. Treatable traits can be identified in a severe asthma registry and predict future exacerbations. Respirology. 2019;24(1):37–47. doi:10.1111/resp.13389
5. Stubbs M, Clark V, McDonald V. Living well with severe asthma. Breathe. 2019;15(2):e40–e49. doi:10.1183/20734735.0165-2019
6. McDonald VM, Hiles SA, Jones KA, Clark VL, Yorke J. Health-related quality of life burden in severe asthma. Med J Austr. 2018;209(S2):S28–S233. doi:10.5694/mja18.00207
7. Eassey D, Reddel HK, Foster JM, et al. I’ve said I wish I was dead, you’d be better off without me”: a systematic review of people’s experiences of living with severe asthma. J Asthma. 2019;56(3):311–322. doi:10.1080/02770903.2018.1452034
8. Foster JM, McDonald VM, Guo M, Reddel HK. “I have lost in every facet of my life”: the hidden burden of severe asthma. Eur Respir J. 2017;50(3):1700765. doi:10.1183/13993003.00765-2017
9. Ten Brinke A, Sterk PJ, Masclee AA, et al. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J. 2005;26(5):812–818. doi:10.1183/09031936.05.00037905
10. Upham J, Lp C. Optimising treatment for severe asthma. Med J Austr. 2018;209(S2):S22–S27. doi:10.5694/mja18.00175
11. Fricker M, Heaney LG, Upham JW. Can biomarkers help us hit targets in difficult-to-treat asthma? Respirology. 2017;22(3):430–442. doi:10.1111/resp.13014
12. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi:10.1016/S0140-6736(12)60988-X
13. Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, Phase 3 trials. Lancet Respir Med. 2015;3(5):355–366. doi:10.1016/S2213-2600(15)00042-9
14. Niebauer K, Dewilde S, Fox-Rushby J, Revicki DA. Impact of omalizumab on quality-of-life outcomes in patients with moderate-to-severe allergic asthma. Ann Allergy Asthma Immunol. 2006;96(2):316–326. doi:10.1016/S1081-1206(10)61242-2
15. Hiles SA, McDonald VM, Guilhermino M, Brusselle GG, Gibson PG. Does maintenance azithromycin reduce asthma exacerbations? An individual participant data meta-analysis. Eur Respir J. 2019;54:1901381. doi:10.1183/13993003.01381-2019
16. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10095):659–668. doi:10.1016/S0140-6736(17)31281-3
17. Harvey E, Langton DL, Powell H, Gibson PG. Clinical response to mepolizumab in patients with severe eosinophilic asthma. Eur Respir J. 2019;54(suppl 63):PA541.
18. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. European Respiratory Journal. 2014;43(2):343–373.
19. Juniper EF, Bousquet J, Abetz L, Bateman ED. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100(4):616–621. doi:10.1016/j.rmed.2005.08.012
20. Hyland ME, Whalley B, Jones RC, Masoli M. A qualitative study of the impact of severe asthma and its treatment showing that treatment burden is neglected in existing asthma assessment scales. Qual Life Res. 2015;24(3):631–639. doi:10.1007/s11136-014-0801-x
21. Katsaounou P, Odemyr M, Spranger O, et al. Still Fighting for Breath: a patient survey of the challenges and impact of severe asthma. ERJ Open Res. 2018;4(4):00076–02018. doi:10.1183/23120541.00076-2018
22. Lempp H, Hofmann D, Hatch SL, Scott DL. Patients’ views about treatment with combination therapy for Rheumatoid Arthritis: a comparative qualitative study. BMC Musculoskelet Disord. 2012;13(1):200. doi:10.1186/1471-2474-13-200
23. Marshall NJ, Wilson G, Lapworth K, Kay LJ. Patients’ perceptions of treatment with anti-TNF therapy for rheumatoid arthritis: a qualitative study. Rheumatology. 2004;43(8):1034–1038. doi:10.1093/rheumatology/keh237
24. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. doi:10.1191/1478088706qp063oa
25. Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax. 1992;47(2):76–83. doi:10.1136/thx.47.2.76
26. Clark V, Gibson P, McDonald V. What severe asthma treatment outcomes matter to patients? Respirology. 2019;24(Suppl.1):44.
27. Paterson DH, Warburton DER. Physical activity and functional limitations in older adults: a systematic review related to Canada’s Physical Activity Guidelines. Int J Behav Nutr Phys Act. 2010;7(1):38. doi:10.1186/1479-5868-7-38
28. Walsh LJ, Wong CA, Oborne J, et al. Adverse effects of oral corticosteroids in relation to dose in patients with lung disease. Thorax. 2001;56(4):279–284. doi:10.1136/thorax.56.4.279
29. Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk in patients with chronic lung diseases treated with bronchodilator drugs and inhaled and oral corticosteroids. Chest. 2007;132(5):1599–1607. doi:10.1378/chest.07-1092
30. Ramsahai JM, Wark PA. Appropriate use of oral corticosteroids for severe asthma. Med J Aust. 2018;209(S2):S18–s21. doi:10.5694/mja18.00134
31. McBrien CN, Menzies-Gow A. Time to FOCUS on oral corticosteroid stewardship in asthma management. Respirology. 2019;24(4):304–305. doi:10.1111/resp.13494
32. Harb N, Foster JM, Dobler CC. Patient-perceived treatment burden of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:1641–1652. doi:10.2147/COPD.S130353
33. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013
34. Holguin F, Cardet JC, Chung KF, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society Guideline. Eur Respir J. 2019;1900588.
35. Lee J, Tay TR, Radhakrishna N, et al. Nonadherence in the era of severe asthma biologics and thermoplasty. Eur Respir J. 2018;51(4):1701836. doi:10.1183/13993003.01836-2017
36. Tan DJ, Burgess JA, Perret JL, et al. Non-pharmacological management of adult asthma in Australia: cross-sectional analysis of a population-based cohort study. J Asthma. 2020;57(1):105–112. doi:10.1080/02770903.2018.1545030
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
