Back to Journals » Clinical Ophthalmology » Volume 17
The Novel Optical Design and Clinical Classification of a Wavefront-Shaping Presbyopia-Correcting Intraocular Lens
Authors Kohnen T , Berdahl JP , Hong X, Bala C
Received 3 December 2022
Accepted for publication 21 April 2023
Published 18 August 2023 Volume 2023:17 Pages 2449—2457
DOI https://doi.org/10.2147/OPTH.S400083
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Supplementary video of "Wavefront-shaping presbyopia-correcting intraocular lens" [ID 400083].
Views: 530
Thomas Kohnen,1 John P Berdahl,2 Xin Hong,3 Chandra Bala4
1Department of Ophthalmology, Goethe University Frankfurt, Frankfurt am Main, Germany; 2Vance Thompson Vision, Sioux Falls, SD, USA; 3Alcon Vision LLC, Fort Worth, TX, USA; 4PersonalEYES, Sydney, NSW, Australia
Correspondence: Thomas Kohnen, Department of Ophthalmology, Goethe University, Theodor-Stern-Kai 7, Frankfurt, 60590, Germany, Email [email protected]
Purpose: To evaluate the clinical rationale of wavefront-shaping technology, describe how intraocular lenses (IOLs) using wavefront-shaping technology are differentiated from refractive or diffractive optical presbyopia-correcting designs, and describe the mode of action of this technology.
Methods: Extended depth of focus (EDoF) IOLs are the latest class of presbyopia-correcting IOLs addressing the growing demand of patients for reduced spectacle dependence. These use various optical technologies, including diffractive designs (eg, TECNIS Symfony ZXR00 and AT LARA 29 MP) and non-diffractive designs such as small aperture (eg, IC-8 IOL and XtraFocus Pinhole Implant), spherical aberration (eg, MINI WELL Ready and LuxSmart), and wavefront shaping (eg, AcrySof IQ Vivity DFT015 and Clareon Vivity CNWET0). Despite some improvement in visual acuity at intermediate and near distances, these technologies can still be associated with increased rate of visual disturbances or poorer distance vision compared with monofocal IOLs. One way to overcome such limitations is using a wavefront-shaping optical principle.
Results: Clinical data show that wavefront-shaping technology results in a continuous EDoF compared with a monofocal IOL while exhibiting a minimal halo, similar to an aspheric monofocal IOL. Clinically, this translates to a lens that has proven to exceed the American National Standards Institute/American Academy of Ophthalmology criteria for an EDoF IOL.
Conclusion: The novel wavefront-shaping optic technology allows patients to achieve a continuous range of vision from distance to functional near with low levels of visual disturbances comparable with aspheric monofocal IOLs.
Keywords: cataract surgery, visual disturbances, extended depth of focus, wavefront-shaping technology
Introduction
Multifocal intraocular lenses (IOLs) may be used to provide a refractive benefit at distance, as well as for intermediate and/or near, to patients undergoing cataract surgery or refractive lens exchange.1,2 Compared with monofocal IOLs that focus light on a single focal point on the retina, multifocal IOLs create two or more foci along the visual axis, resulting in a broader vision range for the patient from distance to near.3 However, each focus created by the multifocal IOLs can be associated with secondary out-of-focus images, resulting in visual disturbances for the patient, especially halos and glares.2 Recently, a new category of IOLs called extended depth of focus (EDoF) has emerged.4
Clinically, EDoF IOLs are intended to ensure a minimum threshold of good intermediate visual performance, while maintaining the distance vision and visual quality close to that of a monofocal IOL.3,4 Based on the recommendations of a task force from the American Academy of Ophthalmology (AAO), the US Food and Drug Administration defined several clinical criteria related to EDoF IOLs in the American National Standards Institute (ANSI)/AAO Standard Z80.35–2018.5 According to these criteria, EDoF IOLs should provide a monocular negative depth of focus at 0.2 logarithm of the minimum angle of resolution (logMAR) or Snellen 6/9.6 of at least 0.5 diopters (D) greater than a monofocal control. Additionally, monocular photopic distance-corrected intermediate visual acuity (VA) (66 cm) should be superior to that of a monofocal control IOL, and at least 50% of eyes should achieve a VA of 0.2 logMAR (Snellen 6/9.6) or better.5,6 Finally, mean monocular photopic best-corrected distance VA should be non-inferior to that of a monofocal control IOL. Monocular mesopic contrast sensitivity and visual symptoms questionnaires are required to be assessed; however, no specific criteria relating to visual quality or visual disturbances have been detailed.5,6
To provide a range of vision that could meet ANSI/AAO EDoF criteria without increasing the risk for visual disturbances, new optical principles of non-diffractive and diffractive IOLs were explored. The non-diffractive wavefront-shaping technology was found to be a potential optical design that could be applied to an IOL to meet this unmet need for patients. This novel optical principle shapes the wavefront so that the focal point is altered to form a continuous extended focal range, and it has been clinically proven to meet the design intent.5–8
Understanding the main optical principles of EDoF IOLs is crucial to ensure appropriate lens selection and, ultimately, postoperative patient satisfaction. This article describes the different main optical principles of LENTIS Comfort MF15 (Teleon Surgical BV, Spankeren, the Netherlands), ACUNEX Vario (Teleon Surgical BV, Spankeren, the Netherlands), AT LARA (Carl Zeiss Meditec, Jena, Germany), TECNIS Symfony (Johnson & Johnson Vision, Santa Ana, CA, USA), LuxSmart (Bausch + Lomb, Berlin, Germany), AcrySof IQ Vivity and Clareon Vivity (Alcon Laboratories, Inc., Fort Worth, TX, USA), IC-8 IOL (AcuFocus, Irvine, CA, USA), and XtraFocus Pinhole Implant (MORCHER GmbH, Stuttgart, Germany). Great focus is given in explaining the mode of action of the wavefront‑shaping technology. Furthermore, the mode of action of this technology could be further understood by watching the Supplementary video. We also compared this non-diffractive technology with IOLs utilizing other optical technologies to extend the depth of focus.
Overview of the Different Optical Principles of EDoF IOLs
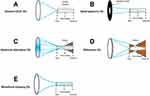
The ideal EDoF lens would concentrate light down to a point that would remain focused over an extended range, like a line of light along the visual axis that puts distance and near objects, as well as everything in between, in sharp focus (Figure 1A).5 Although such lenses may be physically impossible to manufacture because of the limitations of the properties of light, the evolution of EDoF technology should converge toward this ideal solution.3–5 However, if the strict requirements of the line focus are reduced slightly, and the light entering the eye can be focused to a narrow channel that is stretched over an extended range, some of the goals of the ideal EDoF lens can be achieved because longer channels lead to more extended vision range, and smaller diameter channels create sharper images.5 This is because light that strikes the retina within that channel is in focus and contributes to the perception of a sharp image, whereas light that strikes the retina outside that channel is spread around and may be perceived as either halos or blur.5 Over the last decade, various optical principles have been utilized to create the channel of EDoF IOLs, including small aperture, spherical aberration, wavefront shaping, and diffractive optics.4,5 Small aperture, spherical aberration, and wavefront shaping are all non-diffractive lens technologies.
Non-Diffractive Technologies
Small Aperture
Small-aperture IOLs create an EDoF via the use of an opaque mask (also called the pinhole effect), which blocks peripheral light rays from entering the back of the eye (Figure 1B).3 The IC-8 IOL and XtraFocus Pinhole Implant are two examples of small-aperture IOLs currently available on the market.2,9,10 Similar to the small-aperture mode used in photography, small-aperture lenses do not increase the concentration of the light in the EDoF channel, but rather remove the light that would normally be outside the channel. This has the effect of increasing the depth of focus by reducing the total amount of light entering the eye, which can affect patient vision and contrast as has been observed with XtraFocus IOLs, especially in dim light conditions.5,9 The IC-8 IOL was also shown to reduce mesopic contrast sensitivity, which can be partly mitigated by monocular implant.10 This technology demonstrated improved vision in eyes with severe corneal irregularities or astigmatism, as well as those with iris trauma.9,11,12 However, some studies revealed that small‑aperture IOLs can induce halos in some patients, and that dysphotopsias and reduced depth of focus may occur with naturally large pupils.10,13
Spherical Aberration
Aspheric EDoF IOLs modulate the spherical aberration of the optic to increase depth of focus. They are made of zones, the curvature of which reduces continuously from the center to the periphery, resulting in a drop in optical power towards the edge: the greater the lens curvature, the greater the optical power.14,15 The different aspheric zones result in the focus of the light rays at different points along the vision axis forming an increased depth focus (ie, EDoF channel; Figure 1C). Depending on where the rays enter the lens, the light converges either to a focal point in the EDoF tunnel, or outside the channel, which can lead to halos. Examples of spherical aberration-based EDoF IOLs currently available on the market are MINI WELL Ready (SIFI S.p.A, Lavinaio, Italy) and LuxSmart (Bausch + Lomb, Berlin, Germany).3,16 MINI WELL Ready comprises three zones: a steep central zone with positive spherical aberration, a middle zone with negative spherical aberration, and an outer monofocal zone: EDoF results from the alternating positive and negative spherical aberrations of the central and middle zone.17 Some clinical studies reported that MINI WELL can provide satisfactory intermediate VA with a favorable visual disturbance profile.18,19 However, contrast sensitivity at high spatial frequency was found lower for these IOLs compared with monofocal IOLs, and halos perception was increased for MINI WELL Ready compared with a monofocal IOL.19
Diffractive Technologies
Diffractive technologies have already been used in multifocal IOLs to create multiple foci and improved near and/or intermediate vision.2 This IOL design is usually associated with higher levels of visual disturbances when compared with other types of design.5 More recently, diffractive designs with low addition power have been used to develop EDoF IOLs.3 TECNIS Symfony ZXR00 and AT LARA 29 MP are two examples of diffractive EDoF IOLs currently available on the market.3 In this case, some of the rays come to focus within the EDoF channel at the distinct foci, while the remaining rays lay outside of the channel, resulting in halos (Figure 1D).5 By decreasing the near optical power to an add power below +2.0 D,20 diffractive EDoF IOLs can create a more continuous range of vision from distance to intermediate and into the near range; however, these are typically still associated with an increased level of visual disturbances compared with a monofocal IOL.5 The ReSTOR +2.5 D (Alcon Laboratories, Inc., Fort Worth, TX, USA) can be classified between a low-addition multifocal IOL and a traditional bifocal diffractive IOL intended to provide good near vision.21,22 The lens was designed to have a +2.5 D add power, which equates to moderate near vision, and an enhancement to intermediate vision (Hany Michail, Alcon, personal communication, August 2013).22,23 However, ReSTOR +2.5 D may not meet the EDoF criteria of providing a monocular EDoF >0.5 D over a monofocal at 0.2 logMAR, because the add power is high enough that the defocus curve dips between distance and intermediate.24
Wavefront-Shaping IOLs
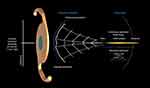
Despite the large offering on the market, current EDoF technologies present some disadvantages, especially with regard to visual disturbances and quality of vision.3,4 To overcome these limitations while still meeting the range-of-vision criteria for EDoF IOLs, a novel EDoF IOL optical technology called wavefront shaping has been developed (Figure 1E). A similar optical concept is applied to laser technology, especially for laser manufacturing: the principle is to modify or shape the wavefront of the light to change its spatial propagation, so that when the light reaches the retina, it is confined to a region within the EDoF channel.4 The wavefront-shaping technology is located on the anterior surface of the lens in the central 2.2-mm region of the lens and uses two smooth-surface transition elements that work effectively to alter the travel distance of the light entering the eye, producing a continuous extended range of vision from distance to functional near (Figure 2 and Supplementary video).5,25 The first element is a slightly elevated plateau (≈1 µm), and the second element is a small curvature change across the entire 2.2-mm central optic, which enables most of the light energy to be used.5,25 After light passes through the surface transition elements, the emergent wavefront takes the same shape as the anterior side of the IOL (Supplementary video). Parts of the wavefront advance, while the central portion of the wavefront is delayed, resulting in a continuous extended focal range.5 Notably, the very central portion of the wavefront-shaping optic is not elevated. This was designed specifically for pupils smaller than 2.2 mm,25 so the lens can still provide an EDoF through the pinhole effect in these patients (Figure 2). Unlike diffractive optics, wavefront-shaping technology does not “waste” light energy at high diffractive orders, and therefore maximizes the efficiency of the transmitted light across the range of vision.26
Clinical Performance
The optical design performance attributes of wavefront-shaping technology IOLs have demonstrated excellent clinical results. The technology has proven to meet the EDoF criteria in two trials while maintaining a monofocal visual disturbances profile.27,28 The data of two prospective, 6-month, multicenter, randomized, parallel-group trials (NCT03010254 and NCT03274986) indicated superior mean monocular distance-corrected VA at intermediate and near (both <0.001), and non-inferior corrected distance VA in subjects (n = 282 and n = 218, respectively) after bilateral implantation of wavefront-shaping EDoF versus monofocal IOL.27,28 EDoF at 0.2 logMAR was increased with the wavefront-shaping technology by at least 0.50 D.27,28 The enhanced intermediate vision with wavefront-shaping technology was reflected in patient-reported outcomes, showing higher rates of spectacle independence than that of a monofocal design.27,28 Furthermore, reports of severe glares, halos, and starbursts were uncommon, with similar rates for wavefront-shaping and monofocal lenses (3.8% vs 2.5%, 0.9% vs 0%, and 3.8% vs 2.5%, respectively).27 The results on VA and subject-reported visual disturbances were also corroborated by other, smaller studies.7,8
Residual Surface Elevation
The wavefront-shaping optic is minimal in structure compared with diffractive optics that use multiple rings. Taking diffractive-EDoF IOLs as an example, whose diffractive rings have a profile change in height up to 6 µm, residual surface elevation maps showed that these are approximatively fivefold higher compared with the slightly elevated plateau of approximately 1 µm of the non-diffractive structure (elements 1 and 2 combined) of the wavefront-shaping IOL (Jessie Hull, Alcon, personal communication, June 2021 (Figure 3).29
Through-Focus Point Spread Function
As previously noted, an ideal EDoF IOL would provide a continuously focused light beam from distance to near vision.5 The point spread function (PSF) cross sections for each IOL eye model were simulated with a pupil size of 3.0 mm and at various levels of defocus, ranging from +0.5 D to –2.5 D, to make image formation comparisons between different IOLs (Kevin Baker, Alcon, personal communication, May 2020). The through-focus PSF was created by combining the transverse intensity at each defocus plane and computed using the Fast Fourier Function (Kevin Baker, Alcon, personal communication, May 2020). Figure 4 shows that both diffractive EDoF IOLs exhibited several breaks along the range of focus, indicating the presence of areas where the light is not focused. These breaks are because of the diffractive design and can explain the presence of halos reported after implantation of these diffractive IOLs. Conversely, wavefront-shaping technology presents a continuous EDoF without unfocused light, which limits the risk of visual disturbances.5
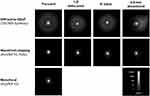
Laboratory Halo Imaging
Halo images of diffractive, wavefront-shaping, and aspheric monofocal IOLs were measured at the distance foci using the high dynamic range halo measurement system (Dan Varson, Alcon, personal communication, July 2020). IOLs were mounted in a model eye with 0.2-µm corneal spherical aberration and an external pupil scaled to provide a 4.5-mm effective aperture at the IOL plane. Images used to compute the area under the curve parameter were measured with a 10× microscope objective fitted to the camera, and a 400-µm diameter pinhole for the target (Kevin Baker, Alcon, personal communication, May 2020). Results showed reduced halos with wavefront-shaping technology at best focus (similar to the monofocal), 1-D defocused, 5° tilted, and 0.5-mm decentered compared with the diffractive EDoF IOL (Figure 5). The monofocal-like bench halo of this lens technology was confirmed by patients reporting low rates of halos, starbursts, and glares via validated questionnaires in two randomized, controlled trials, as well as in other studies of different nature (Jessie Hull, Alcon, personal communication, June 2021).7,8,27,28,30
Patient Selection
Patient selection is an important aspect in conducting well-controlled scientific clinical trials. However, the broader, more-diverse population of patients that surgeons meet and work with in clinical practice introduces greater variability, which warrants a holistic approach to IOL/patient matching that goes beyond inclusion and exclusion criteria typical of a randomized study.31 First, objective evaluations of ocular characteristics (such as qualitative and quantitative assessment of corneal astigmatism, overall ocular surface health, and presence of comorbidities) need to be carried out to ensure the patient’s eligibility. Within this context, the surgeon should also take into account confounding factors, such as changes in the corneal curvature, which may hinder the accuracy of preoperative lens power calculations and negatively affect postoperative refractive outcomes.32 These anatomical and physiological assessments should then be followed by a discussion with the patient, to understand their goals and expectations as well as compromises they would agree to accept.33 Although there is no definitive guidance as to how to choose the “perfect” IOL, active involvement of the patient in the consultation journey is paramount to optimize outcomes and ensure high levels of satisfaction after implantation.
Conclusion
The increased demand from patients for reduced spectacle dependence has led to the development of new presbyopia-correcting IOLs, such as EDoF IOLs.4 Based on ANSI/AAO criteria, EDoF IOLs should provide patients with superior distance‑corrected intermediate vision, an increased minimum depth of focus, and best‑corrected distance vision similar to that offered by a monofocal.5,6 However, there is no mention of the level of visual disturbances in the criteria. In the past decade, several EDoF optical technologies have been developed and made available, with each exhibiting different clinical performances with associated visual disturbances.
The wavefront-shaping technology meets the ANSI/AAO EDoF criteria while limiting the level of visual disturbances. In this review, we explained the mode of action of the wavefront-shaping technology and showed that, unlike diffractive EDoF designs, this technology results in a continuous EDoF from distance to functional near without splitting light, which usually causes increased visual disturbances. Clinical data from two pivotal clinical trials assessing the visual performance of the wavefront-shaping technology showed similar results when compared with bench studies, namely showing a good distance, intermediate, and functional near vision with a visual disturbance profile similar to a monofocal IOL.27,30 Wavefront-shaping technology represents an important advancement in the field of ophthalmic surgery: when utilized appropriately, following an accurate patient selection process that holistically takes into account the recipient’s objective ocular assessment alongside their preferences and visual goals, this technology can improve the overall quality of vision of those undergoing IOL implantation.
Acknowledgments
The authors thank James Schwiegerling, PhD, Val P. Injev, MBA, P.E., and Jessie Lemp-Hull, PhD for their critical review to this manuscript and supporting video, as well as Chameleon Communications for editorial assistance in the preparation of the manuscript, with funding from Alcon Research LLC.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Thomas Kohnen – Consultant and Research for Abbott/Johnson & Johnson, Alcon/Novartis, Avedro, Lensgen, Oculentis, Oculus, Presbia, Santen, SCHWIND, ZEISS. Consultant for Allergan, Bausch + Lomb, Dompé, Geuder, Med Update, Merck, Rayner, STAAR, Théa, TearLab, Thieme, Ziemer. Research for Avedro, Hoya. John Berdahl – Consultant for Alcon, Bausch + Lomb, Johnson & Johnson, RxSight and ZEISS. He also reports personal fees from AbbVie, Aerie, Aerpio, Aldeyra, Aurea Medical, Aurion Biotech/CorneaGen, Dakota Lions Eye Bank, Elios Vision INC, Equinox, Expert Opinion, Glaukos, Gore, Iprimis, iRenix, Iacta Pharmaceuticals, Kala, Kedalion, MELT Pharmaceuticals, MicroOptx, New World Medical, Ocular Surgical Data, Omega Ophthalmic, Ocular Theraputix, Orasis, Oyster Point, Santen, Sight Sciences, Surface Inc, Tarsus, Tear Clear, Vertex Ventures, ViaLase, Vittamed, Vance Thompson Vision, Verana Health, Versa Biologics, Visionary Ventures, and Visus, outside the submitted work. Xin Hong is an employee of Alcon. Chandra Bala is a speaker bureau member for Alcon, Johnson & Johnson and involved in clinical trial for Johnson & Johnson. He is also a consultant for Hoya. The authors report no other conflicts of interest in this work.
References
1. de Silva SR, Evans JR, Kirthi V, Ziaei M, Leyland M. Multifocal versus monofocal intraocular lenses after cataract extraction. Cochrane Database Syst Rev. 2016;12:CD003169. doi:10.1002/14651858.CD003169.pub4
2. Salerno LC, Tiveron MC, Alio JL. Multifocal intraocular lenses: types, outcomes, complications and how to solve them. Taiwan J Ophthalmol. 2017;7:179–184. doi:10.4103/tjo.tjo_19_17
3. Kanclerz P, Toto F, Grzybowski A, Alio JL. Extended depth-of-field intraocular lenses: an update. Asia Pac J Ophthalmol. 2020;9(3):194–202. doi:10.1097/APO.0000000000000296
4. Kohnen T, Suryakumar R. Extended depth-of-focus technology in intraocular lenses. J Cataract Refract Surg. 2020;46(2):298–304. doi:10.1097/j.jcrs.0000000000000109
5. Schwiegerling J, Gu X, Hong X, Lemp-Hull J, Merchea M. Optical principles of extended depth of focus IOLs. Available from: https://us.alconscience.com/sites/g/files/rbvwei1736/files/pdf/Optical-Principles-of-EDOF-US-CAT-2000006.pdf.
6. American National Standard for Ophthalmics. American National Standard for Ophthalmics - Extended depth of focus intraocular lenses. Available from: https://webstore.ansi.org/standards/vc%20(asc%20z80)/ansiz80352018.
7. Kohnen T, Petermann K, Böhm M, et al. Nondiffractive wavefront-shaping extended depth-of-focus intraocular lens: visual performance and patient-reported outcomes. J Cataract Refract Surg. 2022;48(2):144–150. doi:10.1097/j.jcrs.0000000000000826
8. van Amelsfort T, Webers VSC, Bauer NJC, Clement LHH, van den Biggelaar FHM, Nuijts RMM. Visual outcomes of a novel non-diffractive extended depth-of-focus intraocular lens targeted for mini-monovision: 3 month results of a prospective cohort study. J Cataract Refract Surg. 2021. doi:10.1097/j.jcrs.0000000000000825
9. Agarwal P, Navon SE, Subudhi P, Mithal N. Persistently poor vision in dim illumination after implantation of XtraFocus small-aperture IOL (Morcher). BMJ Case Rep. 2019;12(11):e232473. doi:10.1136/bcr-2019-232473
10. Dick HB, Piovella M, Vukich J, Vilupuru S, Lin L, Clinical Investigators. Prospective multicenter trial of a small-aperture intraocular lens in cataract surgery. J Cataract Refract Surg. 2017;43(7):956–968. doi:10.1016/j.jcrs.2017.04.038
11. Hooshmand J, Allen P, Huynh T, et al. Small aperture IC-8 intraocular lens in cataract patients: achieving extended depth of focus through small aperture optics. Eye. 2019;33(7):1096–1103. doi:10.1038/s41433-019-0363-9
12. Ho VWM, Elalfy M, Hamada S, Lake, D. One-year visual outcome of secondary piggyback pinhole device implantation in pseudophakic eyes with irregular corneal astigmatism and iris trauma. Eye. 2022;36(4):812–817. doi:10.1038/s41433-021-01537-7
13. Dick HB, Elling M, Schultz T. Binocular and monocular implantation of small-aperture intraocular lenses in cataract surgery. J Refract Surg. 2018;34(9):629–631. doi:10.3928/1081597X-20180716-02
14. Alió JL, Pikkel J. Multifocal Intraocular Lenses: The Art and the Practice. Springer; 2019.
15. Fannin TE, Grosvenor T. Clinical Optics.
16. Tahmaz V, Siebelmann S, Koch KR, Cursiefen C, Langernbucher A, Hoerster R. Evaluation of a novel non-diffractive extended depth of focus intraocular lens - first results from a prospective study. Curr Eye Res. 2022;47(8):1149–1155. doi:10.1080/02713683.2022.2074046
17. Tognetto D, Cecchini P, Giglio R, Turco G. Surface profiles of new-generation IOLs with improved intermediate vision. J Cataract Refract Surg. 2020;46(6):902–906. doi:10.1097/j.jcrs.0000000000000215
18. Savini G, Balducci N, Carbonara C, et al. Functional assessment of a new extended depth-of-focus intraocular lens. Eye. 2019;33(3):404–410. doi:10.1038/s41433-018-0221-1
19. Bellucci R, Cargnoni M, Bellucci C. Clinical and aberrometric evaluation of a new extended depth-of-focus intraocular lens based on spherical aberration. J Cataract Refract Surg. 2019;45(7):919–926. doi:10.1016/j.jcrs.2019.02.023
20. Millan MS, Vega F. Extended depth of focus intraocular lens: chromatic performance. Biomed Opt Express. 2017;8(9):4294–4309. doi:10.1364/BOE.8.004294
21. Vega F, Alba-Bueno F, Millan MS, Varón C, Gil MA, Buil JA. Halo and through-focus performance of four diffractive multifocal intraocular lenses. Invest Ophthalmol Vis Sci. 2015;56(6):3967–3975. doi:10.1167/iovs.15-16600
22. Hayashi K, Ogawa S, Manabe S, Hirata A. Visual outcomes in eyes with a distance-dominant diffractive multifocal intraocular lens with low near addition power. Br J Ophthalmol. 2015;99(11):1466–1470. doi:10.1136/bjophthalmol-2014-306476
23. Unsal U, Baser G. Evaluation of different power of near addition in two different multifocal intraocular lenses. J Ophthalmol. 2016;2016:1395302. doi:10.1155/2016/1395302
24. Alcon Vision LLC. AcrySof® IQ ReSTOR® +2.5 Multifocal IOL [US Product Information]. Alcon Vision LLC; 2015.
25. Hong X, Choi M-T, Karakelle M, Zhang X, Simpson MJ, Zhang Y, inventors; Novartis AG, assignee. Extended depth of focus (EDOF) lens to increase pseudo-accommodation by utilizing pupil dynamics. United States reissued patent US RE45,969 E. 2016 Apr 12.
26. Alcon. AcrySof IQ [Product Information]. Alcon. 2015
27. Bala C, Poyales F, Guarro M, et al. Multicountry clinical outcomes of a new nondiffractive presbyopia-correcting IOL. J Cataract Refract Surg. 2022;48(2):136–143. doi:10.1097/j.jcrs.0000000000000712
28. McCabe C, Berdahl J, Reiser H, et al. Clinical outcomes in a U.S. registration study of a new EDOF intraocular lens with a nondiffractive design. J Cataract Refract Surg. 2022;48(11):1297–1304. doi:10.1097/j.jcrs.0000000000000978
29. Loicq J, Willet N, Gatinel D. Topography and longitudinal chromatic aberration characterizations of refractive-diffractive multifocal intraocular lenses. J Cataract Refract Surg. 2019;45(11):1650–1659. doi:10.1016/j.jcrs.2019.06.002
30. Alcon Research LLC. AcrySof™ IQ Vivity™ extended vision intraocular lens – FDA summary of safety and effectiveness data. 2020. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf/P930014S126B.pdf.
31. Sharma A, Palaniappan L. Improving diversity in medical research. Nat Rev Dis Primers. 2021;7(1):74. doi:10.1038/s41572-021-00316-8
32. Savini G, Hoffer KJ. Intraocular lens power calculation in eyes with previous corneal refractive surgery. Eye Vis. 2018;5:18. doi:10.1186/s40662-018-0110-5
33. Yeu E, Cuozzo S. Matching the patient to the intraocular lens: preoperative considerations to optimize surgical outcomes. Ophthalmology. 2021;128(11):e132–e141. doi:10.1016/j.ophtha.2020.08.025
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.