Back to Journals » Clinical Interventions in Aging » Volume 17
The Median Effective Dose and Bispectral Index of Remimazolam Tosilate for Anesthesia Induction in Elderly Patients: An Up-and-Down Sequential Allocation Trial
Authors Liu M, Sun Y, Zhou L, Feng K, Tianlong Wang, Feng X
Received 26 February 2022
Accepted for publication 16 May 2022
Published 20 May 2022 Volume 2022:17 Pages 837—843
DOI https://doi.org/10.2147/CIA.S364222
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Miao Liu,1– 3 Yuan Sun,1– 3 Lingxue Zhou,1– 3 Kunpeng Feng,1– 3 Tianlong Wang,1– 3 Xuexin Feng1– 3
1Department of Anesthesiology, Capital Medical University Xuanwu Hospital, Beijing, People’s Republic of China; 2Center for Sleep and Consciousness Disorders, Capital Medical University, Beijing, People’s Republic of China; 3Institute of Brain Disorders, Collaborative Innovation Center for Brain Disorders, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Xuexin Feng, Department of Anesthesiology, Capital Medical University Xuanwu Hospital, No. 45 Changchun St., Xicheng District, Beijing, 100053, People’s Republic of China, Tel +86-1083798277, Email [email protected]
Purpose: Remimazolam is a new type of ultrashort benzodiazepine drug with an unclear optimal dose for general anesthesia induction in elderly patients aged > 60 years. Therefore, this study aimed to determine the effective dose of remimazolam tosilate induction and explore its correlation with the bispectral index (BIS).
Patients and Methods: A total of 42 elderly patients were divided into two age groups: 60– 69 (group A) and 70– 85 (group B) years. An initial dose of 0.1mg/kg(Group A) and 0.05 mg/kg(Group B) remimazolam tosilate was administered, and the Modified Observer’s Assessment of Alertness/Sedation scale was used to assess adequate responses. The dose was calculated using the up-and-down allocation technique based on the previous response. The sequential formula and probit regression model were used to calculate ED50 and BIS50. ED95 was determined using the probit regression model.
Results: The ED50 of remimazolam tosilate for anesthesia induction were 0.088 mg/kg (95% confidence interval [CI] 0.071– 0.108) and 0.061 mg/kg (95% CI 0.053– 0.069) in groups A and B, respectively. ED95 was 0.118 mg/kg (95% CI 0.103– 0.649) and 0.090 mg/kg (95% CI 0.075– 0.199) in groups A and B, respectively. The remimazolam tosilate administration could decrease BIS. BIS50 was 86.0 (95% CI 83.7– 88.6) and 85.4 (95% CI 84.1– 86.8) in groups A and B, respectively.
Conclusion: During the induction process, patients’ consciousness should be observed. The dose of remimazolam tosilate could be chosen after careful consideration of individual variations.
Keywords: remimazolam, anesthesia induction, geriatric, up-and-down sequential allocation
Introduction
Remimazolam is a new type of ultrashort benzodiazepine drug that is increasingly applied in clinical anesthesia. Several previous studies reported that benzodiazepines have been confirmed to function as a gamma-aminobutyric acid-A (GABA-A) receptor in the amygdala and reticular activating system binding sites and to change the conformation of chloride channels, resulting in hyperpolarization that inhibits its effects in the central nervous system.1,2 Midazolam was the classic benzodiazepine used for sedation and as an anticonvulsant since 1982.3 However, the long-term use of midazolam is associated with disadvantages, namely, drug accumulation and prolonging sedation time.4 Remimazolam is a midazolam derivative added with an ester moiety, and besylate and tosylate forms were developed.5 It is metabolized by nonspecific tissue esterase enzymes, and the binding ability of its main metabolite CNS7054 to GABA-A receptor decreased significantly.5 Studies have shown that its sedative effects can be quickly reversed by flumazenil.6 The advantages of remimazolam include a fast onset of action, short recovery time, and stable hemodynamics.1 Currently, a clinical study of remimazolam mainly focused on the induction and maintenance of non-elderly patients and endoscopic anesthesia.7 The optimal doses required for general anesthesia induction in elderly patients aged >60 years remain unclear. The bispectral index (BIS) is a method used to monitor the hypnotic state, which has been demonstrated to correlate well with sedation levels following induction with propofol. The effect of remimazolam on BIS during induction in elderly patients is not completely understood.
Therefore, this study aimed to determine the effective dose of remimazolam in elderly patients aged 60–85 years using a sequential design and Dixon up-and-down methods and to evaluate the effect of remimazolam on BIS scores.
Materials and Methods
This study was registered in the Chinese Clinical Trial Registry (ChiCTR2100046482). Ethical approval was provided by the Ethics Committee of Capital Medical University, Beijing, China, on April 26, 2021 (No. [2021] 022). This study was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients who participated in this study.
Inclusion and Exclusion Criteria
Due to the physiological deterioration in elderly patients, the efficacy of sedative drugs may be different in various age groups.8 Therefore, they were subdivided into two groups according to age: 60–69 (group A) and 70–85 (group B) years. From June to December 2021, 42 elderly patients undergoing elective hepatobiliary surgery were enrolled in this trial. All patients were induced general anesthesia with endotracheal intubation mechanical ventilation. Inclusion criteria were as follows: patients with American Society of Anesthesiologists status of I–III and body mass index (BMI) of 18–30 kg/m2. Exclusion criteria were as follows: emergency surgery; hypovolemia; shock; need to be combined with other anesthesia methods; infectious heart disease; need for cardiopulmonary bypass; need for heart, liver, or kidney transplantation; severe liver or kidney impairment; anemia or thrombocytopenia; severe heart disease; poor blood pressure or blood glucose control; difficulty in respiratory tract management; a history of drug abuse and/or alcohol abuse; mental system diseases; or history of allergy to benzodiazepine.
Anesthesia Procedure and Statistical Analysis
Upon entering the operating room, standard vital signs monitoring, including the heart rate, noninvasive blood pressure, peripheral pulse oxygen saturation, anesthesia depth (BIS), and body temperature monitoring were performed. Invasive monitoring can be performed based on the clinical needs. Before the induction of anesthesia, an 18- or 20-G intravenous catheter was placed in the basilic vein, and 3 mL/kg of lactated Ringer’s solution was administered as a precharge liquid. Before induction, adequate mask oxygen inhalation was administered. BIS values when awake (BISawake) were recorded after allowing patients to lay down for 5 min. Remimazolam tosilate was administered according to the protocol, and the Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale was also assessed. After successful sedation, sufentanil of 0.1–0.3 ug/kg and rocuronium of 0.6–1.0 mg/kg were administered consecutively. Endotracheal intubation was performed when analgesia and muscle relaxation were achieved. Propofol was titrated to maintain BIS between 40 and 60, and continuous pump infusion of remifentanil was maintained at 0.1–0.3 μg/kg/min. In this study, statistical analyses were performed using Microsoft Office Excel 2016 (Microsoft Inc., Washington, USA). ED50 and BIS50 values were determined according to a sequential formula using SPSS software version 25.0 (IBM SPSS Statistics Inc., Chicago, IL) to perform probit regression analysis, paired t-test, and Pearson’s linear correlations. Figures were prepared using GraphPad Prism version 5.0 (GraphPad Software Inc. San Diego, CA, USA). Statistical significance was set at the p value of <0.05.
Identification of ED50 and ED95
The dose distribution was determined using Dixon’s modified up-and-down method.9–11 The administration regimen was as follows: the initial dose of remimazolam tosilate (Hengrui Pharmaceutical Co., Ltd, Jiangsu China) was set at 0.1 mg/kg in group A, and the dose was designed following the geometric progression with a ratio of 2. The initial dose was injected within 1 min (±5 s) and the MOAA/S score was performed after 1 min. “Successful sedation” is defined as the sedation level maintained at MOAA/S score of 0. Then, the next patient received a low-level dose. Conversely, the MOAA/S score of ≥1 was defined as “failed sedation,” which the next patient received a high-level dose. In group B, the initial dose of remimazolam tosilate was set at 0.05 mg/kg and designed based on the geometric progression with a ratio of 1.5. Patients with failed sedation at the initial dose would be given half of the initial dose and then scored again. The maximum observation time was 5 min. Until this time, patients who still had failed sedation would take remedial measures by injecting propofol of 0.5–1 mg/kg. The observation was stopped when seven crossover points were observed between successful and failed sedation. Using Dixon’s up-and-down method data, ED50 was determined using two methods, sequential formula, and probit regression model, and only using the probit regression model to determine ED95. These data were expressed as mean and 95% confidence interval (CI).
Determination of BIS50
In this experiment, patients taking remedial measures would be excluded. BIS50 was defined as the BIS associated with 50% of patients who fell asleep. We recorded the lowest BIS in this procedure. The lowest BIS was determined based on an analogy with drug doses and divided into geometric progression for each group. BIS50 could be determined by substituting the geometric progression data into the sequential formula and probit regression model.12 We still recorded the BIS when the MOAA/S score was 0, which was considered as BIS when the patient is asleep (BISasleep). A paired t-test was used to compare changes between BISawake and BISasleep. The relationship between the total dose of remimazolam tosilate, and the decline of BIS was investigated using Pearson’s linear correlations.
Results
The patient characteristic data are listed in Table 1. A total of 43 elderly patients were assessed for study eligibility. One patient in group B was excluded because he refused to participate. Finally, 42 patients were enrolled (Figure 1).
 |
Table 1 The Characteristic Data |
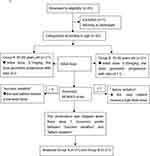 |
Figure 1 Flow diagram of included participants. |
ED50 and ED95
In an effective dose experiment, ED50 of remimazolam tosilate for anesthesia induction was 0.088 mg/kg (95% CI 0.071–0.108) and 0.061 mg/kg (95% CI 0.053–0.069) in groups A and B, respectively, using the sequential method. Then, using the probit regression model, the ED50 of remimazolam tosilate was 0.090 mg/kg (95% CI 0.071–0.107) and 0.063 mg/kg (95% CI 0.049–0.085) in groups A and B, respectively. The ED95 was 0.118 mg/kg (95% CI 0.103–0.649) and 0.090 mg/kg (95% CI 0.075–0.199) in groups A and B, respectively. The Dixon up-and-down plots for each group are shown in Figure 2A and B. The dose-response curve of remimazolam tosilate induction in each group is shown in Figure 3.
 |
Figure 2 The Dixon up-and-down plots demonstrating dose reported as successful sedation (point) and failed sedation (fork) in groups A (A) and B (B). |
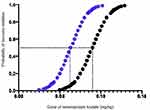 |
Figure 3 The dose-response curve of remimazolam tosilate induction in each group. Black point curve represents Group A and blue point curve represents Group B. |
BIS50
No patient has taken remedial measures. In the study of the correlation between remimazolam tosilate induction and BIS value, the BIS50 was 86.0 (95% CI 83.7–88.6) and 85.4 (95% CI 84.1–86.8) in groups A and B using the sequential method. Calculated by probit regression, the BIS50 was 88.1 (95% CI 80.4–92.8) and 87.2 (95% CI 80.1–91.5) in groups A and B, respectively. According to the paired t-test, the BIS value decreased after remimazolam tosilate administration in both groups (mean ± standard error [19.87 ± 1.56]), p < 0.001 in group A and [16.05 ± 1.09], p < 0.001 in group B).
The remimazolam tosilate administration could decrease BIS. However, no strong correlations were found between the total dose of remimazolam tosilate and the decline of BIS (Pearson’s correlation coefficient, 0.005, p = 0.055 in group A; Pearson’s correlation coefficient, −0003, p = 0.990 in group B).
Table 2 summarizes the results of ED50, ED95, and BIS50 in both groups. Appendix 1 shows the specific calculation process used to derive the abovementioned results.
 |
Table 2 Summary List of Results Between the Two Groups |
Discussion
Remimazolam is a new ultra-short-acting benzodiazepine intravenous sedation agent.1,2 It is a water-soluble and tosilate salt that has been approved for procedural sedation in China.13 The experimental drug used in this study was in tosilate form. At present, it is mainly used for endoscopic procedure sedation, such as colonoscopy and upper gastrointestinal endoscopy.7 Only a few reports applied remimazolam for general anesthesia, and the inclusion population of these reports focused on non-elderly patients.14,15 Therefore, clinical medication experience and recommended effective dose are lacking for elderly patients. As already known, elderly patients with reduced reserves of various physiological functions have poor anesthesia tolerance, and the risk of anesthesia increases to a certain extent.16 Thus, anesthesia should be more individualized and cautiously administered. In this study, the ED50 and ED95 of remimazolam tosilate for general anesthesia induction were explored in elderly patients aged >60 years for the first time and set the initial dose to 0.1mg/kg or 0.05 mg/kg. Furthermore, to obtain a more accurate and effective induction dose, elderly patients were also grouped according to age, and ED50, ED95, and BIS50 were determined for patients aged 60–69 and 70–85 years, respectively. Liu administered an initial dose of 0.15 mg/kg of remimazolam tosilate for sedation in elderly patients undergoing colonoscopy and obtained a procedural success rate of 96.52%.17 This is consistent with our ED95 and its CIs. Moreover, results calculated using two statistical methods are similar. Therefore, our data are relatively reliable and are hoped to provide effective information for the use of remimazolam tosilate in inducing general anesthesia in elderly patients. The results may pave the way for more clinical research and follow-up studies in the future. The safety and efficacy of remimazolam tosilate will be the focus of our follow-up studies.
In this study, BIS values of remimazolam tosilate were also analyzed. BIS monitoring is widely used clinically as a monitoring indicator to quantify the extent of sedation.18 Previous studies have shown that propofol is rapidly metabolized in patients and that changes in its plasma concentration or effector compartment concentration are strongly correlated with sedation changes noted through BIS monitoring.19–21 A prospective trial focusing on the propofol concentration in Chinese patients showed that BIS associated with a 50% probability of loss of consciousness was 58 (58–59).22 In our study, although remimazolam tosilate injection for induction could reduce BIS, BIS50 was higher using remimazolam tosilate than that using propofol. Therefore, BIS cannot be used as an index to judge whether patients fall asleep during the remimazolam tosilate administration to induce general anesthesia.
The present study has certain limitations. For instance, only a few studies have reported the efficacy and safety of remimazolam tosilate to induce general anesthesia in elderly patients. Further studies should aim to elucidate the appropriate dose in different populations. Therefore, our study should be considered exploratory. Furthermore, individual differences in elderly patients might cause variations in drug metabolism. Finally, the study was limited to the relatively healthy elderly patients undergoing elective surgeries. Further research must focus on highly at risk elderly people.
Conclusion
In conclusion, the ED50 of remimazolam tosilate for anesthesia induction was 0.088 mg/kg (95% CI 0.071–0.108) and 0.061 m/kg (95% CI 0.053–0.069) in elderly patients aged 60–69 and 70–75 years, respectively. The ED95 was 0.118 mg/kg (95% CI 0.103–0.649) and 0.090 mg/kg (95% CI 0.075–0.199) in elderly patients aged 60–69 and 70–85 years, respectively. Only a slight correlation was observed between the decline of BIS value and falling asleep when inducing general anesthesia using remimazolam tosilate. BIS value cannot be used as an indicator of patients falling asleep. During the induction process, patients’ consciousness should be observed. The remimazolam tosilate dose could be selected after carefully considering individual variations.
Data Sharing Statement
Data related to this study can be obtained by contacting the corresponding author if reasonable.
Acknowledgments
The authors thank Hengrui Pharmaceutical Co., Ltd for providing Remimazolam Tosilate for Injection.
Disclosure
The authors declare no conflict interests. The Hengrui Pharmaceutical Co., Ltd had no role in the conduct and analysis of the trial. This manuscript was supported by grants from Beijing Municipal Health Commission (code: Jing2019-2).
References
1. Noor N, Legendre R, Cloutet A, et al. A comprehensive review of remimazolam for sedation. Health Psychol Res. 2021;9(1):24514. doi:10.52965/001c.24514
2. Kilpatrick GJ. Remimazolam: non-clinical and clinical profile of a new sedative/anesthetic agent. Front Pharmacol. 2021;12:690875. doi:10.3389/fphar.2021.690875
3. Kanto J, Klotz U. Intravenous benzodiazepines as anaesthetic agents: pharmacokinetics and clinical consequences. Acta Anaesthesiol Scand. 1982;26:554–569. doi:10.1111/j.1399-6576.1982.tb01817.x
4. Wesolowski AM, Zaccagnino MP, Malapero RJ, et al. Remimazolam: pharmacologic considerations and clinical role in anesthesiology. Pharmacotherapy. 2016;36(9):1021–1027. doi:10.1002/phar.1806
5. Kilpatrick GJ, McIntyre MS, Cox RF, et al. CNS 7056: a novel ultra-short-acting benzodiazepine. Anesthesiology. 2007;107(1):60–66. doi:10.1097/01.anes.0000267503.85085.c0
6. Worthington MT, Antonik LJ, Goldwater DR, et al. A phase ib, dose-finding study of multiple doses of remimazolam (CNS 7056) in volunteers undergoing colonoscopy. Anesth Analg. 2013;117:1093–1100. doi:10.1213/ANE.0b013e3182a705ae
7. Wang F, Zhou Q, Shen M, et al. Efficacy and safety of remimazolam in procedural sedation and analgesia: a protocol for systematic review and meta-analysis. Medicine. 2020;99:e20765. doi:10.1097/MD.0000000000020765
8. Su WY. The characteristics of medication in elderly patients and the principles of rational drug application (In Chinese). J Pract Med. 2008;15:3957–3958.
9. Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev. 1991;15:47–50. doi:10.1016/S0149-7634(05)80090-9
10. Dixon WJ. The up-and-down method for small samples. J Am Stat Assoc. 1965;60:967–978. doi:10.1080/01621459.1965.10480843
11. Pace NL, Stylianou MP, Warltier DC. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology. 2007;107:144–152. doi:10.1097/01.anes.0000267514.42592.2a
12. Xing XU, Xiulan WEI. Determination of BIS50 and ED50 for propofol during induction by sequential experimental method. Chin J Anesthesil. 2001;21:7–9.
13. Wang W, Sun Q. Novel targeted drugs approved by the NMPA and FDA in 2019. Signal Transduct Target Ther. 2020;5:65. doi:10.1038/s41392-020-0164-4
14. Sheng X-Y, Liang Y, Yang X, et al. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur J Clin Pharmacol. 2020;76:383–391. doi:10.1007/s00228-019-02800-3
15. Doi M, Morita K, Takeda J, et al. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34(4):543. doi:10.1007/s00540-020-02788-6
16. Evered L, Scott DA, Silbert B. Cognitive decline associated with anesthesia and surgery in the elderly: does this contribute to dementia prevalence? Curr Opin Psychiatry. 2017;30:220–226. doi:10.1097/YCO.0000000000000321
17. Liu X, Ding B, Shi F, et al. The efficacy and safety of remimazolam tosilate versus etomidate-propofol in elderly outpatients undergoing colonoscopy: a prospective, randomized, single-blind, non-inferiority trial. Drug Des Devel Ther. 2021;15:4675–4685. doi:10.2147/DDDT.S339535
18. Kerssens C, Klein J, van der Woerd A, et al. Auditory information processing during adequate propofol anesthesia monitored by electroencephalogram bispectral index. Anesth Analg. 2001;92(5):1210–1214. doi:10.1097/00000539-200105000-00024
19. Wang D, Song Z, Zhang C, et al. Bispectral index monitoring of the clinical effects of propofol closed-loop target-controlled infusion: systematic review and meta-analysis of randomized controlled trials. Medicine. 2021;100(4):e23930. doi:10.1097/MD.0000000000023930
20. Rüsch D, Arndt C, Eberhart L, et al. Bispectral index to guide induction of anesthesia: a randomized controlled study. BMC Anesthesiol. 2018;18(1):66. doi:10.1186/s12871-018-0522-8
21. Glass PS, Bloom M, Kearse L, et al. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86(4):836–847. doi:10.1097/00000542-199704000-00014
22. Zhipeng X, Liu F, Yue Y, et al. C50 for propofol-remifentanil target-controlled infusion and bispectral index at loss of consciousness and response to painful stimulus in Chinese patients: a multicenter clinical trial. Anesth Analg. 2009;108:478–483. doi:10.1213/ane.0b013e31818f8a30
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
