Back to Journals » Journal of Pain Research » Volume 16
The Interaction Between Psychosocial Factors and Exercise-Induced Hypoalgesia in Pain-Free Nurses
Authors Johnsen K , Owen PJ , Tagliaferri SD, Van Oosterwijck J , Fitzgibbon BM , Ford JJ, Belavy DL, Miller CT
Received 16 August 2022
Accepted for publication 16 December 2022
Published 17 February 2023 Volume 2023:16 Pages 529—541
DOI https://doi.org/10.2147/JPR.S386440
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jonathan Greenberg
Kristian Johnsen,1 Patrick J Owen,1 Scott D Tagliaferri,1 Jessica Van Oosterwijck,2– 4 Bernadette M Fitzgibbon,5 Jon J Ford,6,7 Daniel L Belavy,1,8 Clint T Miller1
1Institute for Physical Activity and Nutrition (IPAN), School of Exercise and Nutrition Sciences, Deakin University, Geelong, VIC, Australia; 2Spine, Head and Pain Research Unit Ghent, Department of Rehabilitation Sciences, Faculty of Medicine and Health Sciences, Ghent University, Ghent, Belgium; 3Department of Rehabilitation Sciences and Physiotherapy, Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium; 4Research Foundation – Flanders (FWO), Brussels, Belgium; 5School of Public Health and Preventive Medicine, Faculty of Medicine, Nursing and Health Sciences, Monash University, Melbourne, VIC, Australia; 6Advance HealthCare, Boronia, VIC, Australia; 7Low Back Research Team, College of Science, Health and Engineering, La Trobe University, Bundoora, VIC, Australia; 8Department of Applied Health Sciences, Division of Physiotherapy, Hochschule für Gesundheit, Bochum, Germany
Correspondence: Clint T Miller, Institute for Physical Activity and Nutrition (IPAN), School of Exercise and Nutrition Sciences, Deakin University, 221 Burwood Highway, Burwood, VIC, 3125, Australia, Tel +61 3 9244 6605, Email [email protected]
Purpose: This cross-sectional study aimed to investigate whether psychosocial factors were predictive for exercise-induced hypoalgesia (EIH) in pain-free adults.
Methods: A sample of 38 pain-free nurses with a mean (SD) age of 26 (6) years were included in this study. Participants completed psychosocial questionnaires prior to physical tests. Pressure pain threshold (PPT) was assessed bilaterally at the calves (local), lower back (semi-local) and forearm (remote) before and immediately after a maximal graded cycling exercise test. Separate linear mixed effects models were used to determine change in PPT before and after cycling exercise (EIH). Multiple linear regression for all psychosocial variables and best subset regression was used to identify predictors of EIH at all locations.
Results: The relative mean increase in PPT at the forearm, lumbar, calf, and globally (all sites pooled) was 6.0% (p< 0.001), 10.1% (p< 0.001), 13.9% (p< 0.001), and 10.2% (p=0.013), respectively. Separate best subset multiple linear regression models at the forearm (predictors; Multidimensional Scale of Perceived Social Support (MSPSS) total), lumbar (predictors; MSPSS total, Pain Catastrophizing Scale (PCS) total, Depression Anxiety Stress Scale (DASS) depression), calf (predictors; MSPSS friends, PCS total), and global (predictors; MSPSS friends, PCS total) accounted for 7.5% (p=0.053), 13% (p=0.052), 24% (p=0.003), and 17% (p=0.015) of the variance, respectively.
Conclusion: These findings confirm that cycling exercise produced EIH in young nurses and provided preliminary evidence to support the interaction between perceived social support, pain catastrophizing and EIH. Further investigation is required to better understand psychological and social factors that mediate EIH on a larger sample of adults at high risk of developing chronic musculoskeletal pain.
Keywords: aerobic exercise, acute exercise, pain pressure threshold, perceived social support, kinesiophobia
Introduction
Acute exercise can lead to decreased pain sensitivity, which is referred to as exercise-induced hypoalgesia (EIH).1–3 This form of endogenous pain modulation, which occurs predictably in healthy pain-free populations, is characterised by a reduction in pain sensitivity, expressed as a reduction in self-reported pain intensity and a heightened pain threshold and tolerance to a noxious stimulus (such as pressure, thermal or electro-cutaneous stimuli).1–3 EIH can persist for up to 30 minutes after aerobic, isometric or isotonic exercise.1–3 The hypoalgesic response appears smaller and less consistent in non-exercised versus exercised body parts,4,5 and varies between individuals.1–3 Although mechanisms of EIH are not completely understood, endogenous opioids and endocannabinoids likely activate endogenous pain inhibitory pathways to mediate EIH.1,6–8 These physical factors can be released at peripheral, spinal, and supraspinal brain sites,7 which attenuate ascending sensory information relayed towards pain-processing brain regions.6,9–11 However, pain is more complex than physical factors alone,12–15 and the pain experiences are shown to be moderated by psychological and social factors.12,14–19
Psychosocial factors may play a mediating role in EIH for both pain and pain-free populations. Negative psychological features, such as depression, anxiety, and pain catastrophizing, influence endogenous pain modulation via descending pain inhibitory pathways.20 For instance, conditioned pain modulation, a paradigm that measures endogenous pain modulation, may be reduced by the presence of these psychological features.18,20 There is also evidence that social support in the form of verbal support and touching or viewing of an intimate other can moderate pain.19 However, research investigating the relationship between psychosocial factors and EIH is limited and conflicting.21–23 This is important because psychosocial factors such as depression, kinesiophobia, and pain catastrophising may influence endogenous pain modulation, and increase the risk of pain onset, development of chronic pain, and increase pain intensity.21 A better understanding of the association between psychosocial factors with endogenous pain modulation may therefore assist with identifying individuals at greater risk of developing musculoskeletal pain conditions, or predict which individuals develop persistent pain.
Physically and psychologically demanding occupations such as nursing have a high prevalence of both mental illness and musculoskeletal injury.24 Nurses confront potential exposure to infectious diseases, toxic substances, back injuries due to patient lifting and challenging postures, and radiation. They are also subject to hazards such as stress, shift work, working long hours and overtime, and violence in the workplace.25,26 Nursing, a female-dominant occupation, has the second highest incidence of non-fatal occupational injury in the United States,27 and of these, 25% become chronic.28 Chronic pain is associated with the development of dysfunctional pain processing pathways29 and comorbid psychosocial health impairment.24 However, it is not clear whether endogenous pain modulation is already impaired in the nursing population when there is no current or previous history of serious musculoskeletal pain. Although considered important,1,21 few studies have investigated the influence of psychosocial factors on EIH in pain-free populations, and none have been conducted in a population known to be at high risk of developing musculoskeletal pain. Therefore, the aim of this study was to determine the effect of aerobic exercise on EIH, and the association of psychosocial factors with EIH in nurses without a history of chronic pain.
Methods
Study Design
This cross-sectional study was performed as a secondary analysis of a prospective cohort study investigating causative factors in the development of low back pain from August 2018 to August 2019 at Deakin University (Victoria, Australia). This project complies with the Declaration of Helsinki, and ethical approval was provided by the Deakin University Human Research Ethics Committee (URBAN; project number 2018–221). This study followed the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines.30
Participant Recruitment and Eligibility
Pain-free female and male adult nurses between 18 and 55 years of age were recruited from the greater Melbourne area (Victoria, Australia). This age range was selected to ensure a representative selection of nurses without a history of low back pain. Evidence shows that the first incidence of low back pain typically occurs before or during mid-life and the peak burden of disease is in the age bracket below 55 years.31 The recruitment process occurred within the months between October 2018 and August 2019. Due to time and cost restraints, and the impact of the COVID-19 pandemic and extensive government-imposed restrictions, this exploratory study ceased recruitment at the end of 2019. Primary recruitment strategies included word-of-mouth, social media (Facebook), and printed flyers distributed around hospitals and clinics. Screening against the eligibility criteria took place via phone by research personnel after participants expressed interest via the study-specific email address. The pain-free status of the participants was confirmed upon attendance to the initial testing session to reconfirm eligibility into the study. Exclusion criteria included the following: <18 or >55 years of age; traumatic spinal injury (eg, fracture); spinal surgery; low back pain history; scoliosis; prior acute spinal pain; any current spinal pain episode; treatment for low back pain (eg, allied health professional); other forms of chronic pain conditions (eg, fibromyalgia); any history of cardiovascular disease; unable to communicate in English; planning a pregnancy or currently pregnant; moving overseas or interstate; unable to abstain from smoking for 8 hours prior to testing; and prior or current elite athlete (defined as member of Australian Institute of Sport, State Institutes or Academies of Sport or the national squad of any sport). All participants provided signed and written informed consent prior to data collection.
Maximal Graded Aerobic Exercise Test
Participants undertook a maximal graded exercise test on an electronically braked cycle ergometer (Lode Excalibur Sport, Lode, GR) at Deakin University’s clinical exercise laboratories. The protocol was consistent with previous EIH studies by attaining high-intensity exercise (>75% VO2max) for approximately 10-minutes of test duration.1,2 Pressure pain thresholds (PPTs) were measured immediately prior to cycling to attain pre-exercise pain sensitivity. The progressive protocol commenced with a 7-minute warm-up at 50 watts and increased by 25 watts every 3 minutes until the mean respiratory exchange ratio (RER) was greater than 1.0 for an entire stage. Once mean RER surpassed 1.0, power output increased by 25 watts per minute until voluntary exhaustion (ie, cadence falls below 50 revolutions per minute). A 5-minute active cool-down was followed with a 10-minute quiet rest in which PPTs were obtained. A confirmation test was conducted after subsequent post-exercise PPTs32 and involved cycling at 110% peak power output achieved on the graded exercise test for up to 10 minutes or volitional fatigue.
Assessment of Pain Sensitivity
PPTs were quantified using a validated measure of pain sensitivity to pressure pain.33 A hand-held digital algometer (Commander Echo Console, J Tech Medical Industries, UT) with a stimulation area of 1-cm2 was used to experimentally induce and assess pain.34 PPTs were determined with the participants laying in prone on a plinth while the researcher applied pressure perpendicular to the test sites at a gradual rate of approximately 10 N/cm2/s.35 Participants were instructed to say “stop” once pressure first turned to pain.34 After a familiarisation trial, PPTs were determined bilaterally by taking two measures per test site with a 20-second interval between consecutive measures.36 Test sites at the upper quadrant (forearms), axial (low back), lower quadrant (calves) and body sites were chosen and defined as pain sensitivity remote, semi-remote and close to the exercising body parts.36 Test sites included the calves (gastrocnemius muscle; between the two heads one-third distal from the proximal muscle attachment), low back (lumbar paraspinal muscle; 4-cm lateral from mid-line), and forearms (extensor carpi radialis longus muscle; 3-cm posterior and distal from lateral epicondyle). These muscles were selected based on previous protocols.36–39 Measurements were applied in a non-randomised fashion whereby testing was completed at the forearm on the left-hand side, followed by the right-hand side. The same test order was completed at the low back and then the calves. The PPT per body site was calculated as the mean of the two measures per test site and by averaging the measures from the left and right test site. Assessment of global PPT was calculated as a pooled average of all sites. PPTs have an excellent intraclass correlation coefficient multiple bodily sites (wrist=0.81–0.97; leg=0.96–0.98; neck=0.92–0.98; back=0.94–0.99).40
Assessment of Psychosocial Factors
Self-reported questionnaires were implemented using Qualtrics survey software (Qualtrics, Provo, UT). Participants completed questionnaires using an iPad (Apple, Cupertino, CA) with the assistance of researchers for clarification purposes. All questionnaires were completed prior to the physical tests.
Pain Catastrophizing Scale
The Pain Catastrophizing Scale (PCS) is a valid measurement to assess pain-related catastrophic thinking in both non-clinical and clinical populations.41–43 This questionnaire is a 13-item self-reported measure with each question measured on a 5-point Likert scale, ranging from 0 (not at all) to 4 (all the time) for a maximum score of 52. Higher scores indicate more severe catastrophic thoughts about pain. A threshold of 30 points is considered to be clinically relevant.42 The overall score has been shown to have high internal consistency, with a Cronbach’s α of 0.87.43
Tampa Scale for Kinesiophobia
The Tampa Scale for Kinesiophobia (TSK) is a valid measurement to evaluate the fear of movement or reinjury44,45 and consists of 13 questions measured on a 4-point Likert scale ranging from 1 (strongly disagree) to 4 (strongly agree) for a maximum score of 52. Higher scores represent higher levels of kinesiophobia. Severity levels were determined as: sub-clinical (score = 13–22), mild (23–32), moderate (33–42), and severe (43–52)46. The TSK is valid in both non-clinical and clinical populations.44,45 The TSK-13 was utilised over the original TSK-17 due to improved psychometrics by removing the four reversed items.46 The TSK-13 has shown acceptable internal consistency, with a Cronbach’s α of 0.772.47
Depression Anxiety Stress Scale
The Depression Anxiety Stress Scale (DASS) is a 21-item instrument consisting of three subscales to measure stress, depression, and anxiety.48,49 This questionnaire has been validated in both non-clinical and clinical populations.48,50,51 Each item is measured on a 4-point Likert scale with scores of 0 (did not apply at all) to 3 (applied to me very much or most of the time),52 with a maximum score of 21 in each subscale. Subscale total scores are multiplied by two and categorised as follows: mild (depression = 10–13; anxiety = 8–9; stress = 15–18), moderate (depression = 14–20; anxiety = 10–14; stress = 19–25), severe and extremely severe (depression >20; anxiety >14; stress >25). This scale has high internal consistency, with a Cronbach’s α of 0.93–0.95.52,53
Stamps Index of Work Satisfaction
The Stamps Index of Work Satisfaction (IWS) is a valid tool to measure nursing satisfaction levels.54 The questionnaire is a 45-item self-reported form measured on a 7-point Likert scale.55 The questionnaire has a maximum total score of 270, where scores under the 25th percentile are considered extremely low work satisfaction, under the 50th percentile are low work satisfaction, greater than the 50th percentile are deemed moderate work satisfaction, and greater than the 75th percentile are high work satisfaction.56 The overall questionnaire has high internal consistency, with a Cronbach’s α ranging from 0.82 to 0.91.54
Multidimensional Scale of Perceived Social Support
The Multidimensional Scale of Perceived Social Support (MSPSS) is a valid questionnaire to measure social support.57,58 The questionnaire is a 12-item measure assessing three subscales: family, friends, significant others.57 The subscales are measured on a 7-point Likert scale, ranging from 1 (very strongly disagree) to 7 (very strongly agree), with a maximum total score of 28 for each subscale and 84 for the sum score. Scores were scaled by calculating the mean score for total and subscales by dividing by the number of items for each score. Higher values represent greater perceived social support. The questionnaire has high internal consistency, with a Cronbach’s α of 0.88.57
Statistical Analysis
Descriptive statistics were calculated for demographics, psychosocial variables, and medical history and presented as mean (SD) for continuous data and frequency (%) for categorical data.
Separate linear mixed-effects models were completed to evaluate changes in pain sensitivity within individuals prior to and following aerobic exercise. The model included time and PPT as fixed effects and participant as random effects.
To evaluate associations between psychosocial factors and EIH, correlation and regression analyses were performed. As TSK-13 data were missing for eight participants, missing values were imputed via random forest with the number of trees at 100 per random forest. No limitations were placed on the maximum number of iterations59 (R statistical package “missForest”, version 1.4). EIH was calculated by subtracting PPT post-exercise by PPT pre-exercise (PPTpost-exercise – PPTpre-exercise) as per standard protocol.60–62 Spearman rho correlation coefficients were calculated and classified as negligible (0.00–0.10), weak (0.10–0.39), moderate (0.40–0.69), strong (0.70–0.89), or very strong (0.90–1.00).63 Total scores for all psychosocial questionnaires were entered into separate multiple linear regression models as possible predictors for each EIH location.64 Assessment of multicollinearity was performed by evaluating the variance inflation factor for predictor variables. A variance inflation factor threshold of ≥10 was used to define excessive multicollinearity.65 A secondary exploratory analysis by best subset regression was performed to identify the best subset of psychosocial predictors for models with 1 through to 10 predictors for each EIH location. The model with lowest Bayesian information criterion (BIC) and highest adjusted r-squared values were chosen as the optimal unbiased model.66–68 Where two models were of similar adjusted r-squared the most simple model was selected. All analyses were performed in the “R” statistical environment (version 4.1.1, http://www.r-project.org). An alpha level of 0.05 was used to determine the statistical significance. This study was an exploratory pilot study on the association between EIH and psychosocial factors using existing data. Due to early cessation of participant recruitment a calculation of the design sensitivity was completed. The sample size of 38 participants would be sensitive to effects of r=0.44 with 80% power (alpha: 0.05, two-tailed) between each psychosocial variable and EIH. The design sensitivity power calculation was undertaken using G*Power (version 3.1.9.4, Dusseldorf, Germany).69
Results
Participants
In total, 38 pain-free adults with a mean age of 28 ± 6 years (range: 20–53 years) participated in the study with participant sample characteristics presented in Table 1. DASS scores showed most participants to have normal anxiety levels (73.6%), with mild (5.3%) and moderate (21.1%) levels of anxiety being less common. Similarly, DASS scores showed mild (n=4; 10.5%) to moderate (n=2; 5.2%) levels of symptoms of depression in a small subset of the sample, while the majority of the participants (84.3%) did not present with depressive symptoms. DASS scores were generally low with three participants showing mild stress (7.9%), one participant having moderate stress (2.6%), and one having severe stress (2.6%). Most PCS scores were considered normal (97.4%); only one participant (2.6%) considered to have clinically important levels of pain catastrophising. The TSK-13 scores showed 18 participants (47.4%) to have mild kinesiophobia levels, while the remaining 52.6% had no clinical kinesiophobia.
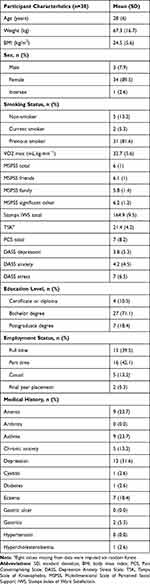 |
Table 1 Descriptive Sample Characteristics for All Participants |
Exercise-Induced Hypoalgesia
Table 2 presents the mean and standard deviation for pre and post exercise PPT for all regions. All sites except for the left forearm reached statistical significance (β=1.2 (2.06), p=0.564) (Table 2). The relative mean increase in PPT at the forearm, lumbar, calf, and globally was 6.0%, 10.1%, 13.9%, and 10.2%, respectively, when left and right sides were pooled.
 |
Table 2 Mean Pain Pressure Threshold Prior to and Following Exercise |
Correlations Between Exercise-Induced Hypoalgesia and Psychosocial Factors
Multiple imputations for missing values for TSK (n=8) was completed using random forest with resultant imputation estimated error (normalised root mean squared error) of 0.0144. There was evidence for a weak negative correlation between MSPSS (total) (rs = −0.380, p = 0.019), MPSS significant other subscale (rs = −0.357, p = 0.028), and MPSS friends subscale (rs =0.329, p = 0.044), with global EIH. There was also some evidence for a weak negative correlation between MSPSS significant other (rs = −0.333; P=0.041), MSPSS family (rs = −0.372; p =0.021) and MSPSS total (rs = −0.397; p = 0.014), with the pooled regional EIH located at the forearm. Negligible to weak, non-statistically significant correlations were observed for TSK and all EIH locations when analysed with (n=38) and without (n=30) imputed data for missing TSK values (see supplementary Tables S1 and S2, respectively).
Regression Analysis Between Exercise-Induced Hypoalgesia and Psychosocial Factors
Multiple linear regression was carried out to investigate the relationship between all psychosocial variables and EIH at each location (Supplementary Table S3). Due to multicollinearity when MSPSS total and MSPSS subscales were included in the model, the model was refined and the MSPSS total score only was included in the primary analysis. When all psychosocial variables were included in the model, none predicted remote (F (7, 30)= 0.8771; P=0.536, adjusted R2 of −0.024), local (F (7, 30)= 0.541; P=0.797, adjusted R2 of −0.095), semi-local (F (7, 30)= 1.10; P=0.390, adjusted R2 of 0.018), or global (F (7, 30)= 0.933; P=0.496, adjusted R2 of −0.013) EIH with any certainty.
Exploratory best subset multiple linear regression analysis was performed separately for each EIH location to determine which psychosocial variables predict EIH response. For local EIH at the calf (pooled), a two-predictor model comprising MSPSS friends subscale and PCS total score accounted for 24% of the variance (F (2, 35) = 6.84; P=0.003, adjusted R2 of 0.24) (Table 3). Semi-local EIH response may be explained by MSPSS total, PCS total, and DASS depression; however, the model did not reach statistical significance (F (3, 34) = 2.85; P=0.052, adjusted R2 of 0.13). A similar trend was observed with the best subset model for the remote site consisting of a single variable (F (1, 36) = 4.00; P=0.053, adjusted R2 of 0.08). The best subset regression analysis to explain global EIH for all pooled sites consisted of MSPSS friends and PCS total and may explains 17% of the variance (F (2, 35) = 4.78; P=0.015, adjusted R2 of 0.17).
 |
Table 3 Best Subset Multiple Linear Regression Between EIH and Psychosocial Predictor Variables |
Discussion
This study examined the effect of cycling exercise on EIH in pain-free nurses. Pain sensitivity decreased globally and at all individual body sites following exercise, indicating EIH. Social support and pain catastrophising presented as the most likely variables to explain up to one-quarter of the variance in EIH; however, a larger more diverse sample is required to confirm these findings.
Pain catastrophizing was found to have no correlation with EIH at any site; however, some evidence for pain catastrophizing predicting EIH for the fitted model selected from the best subset regression at the local site; and a trending significant contribution for global EIH was observed. These findings may infer higher pain catastrophizing reduces endogenous pain modulation as evidenced by reduced EIH. Previous evidence showed greater levels of pain catastrophizing influenced endogenous pain modulation and increased the risk of pain onset, chronic pain development, and higher pain intensity.21 This is important because nurses have higher rates of musculoskeletal injury.27 Three previous studies reported no correlation between pain catastrophising and EIH at the local muscle area following isometric hand grip exercise in pain-free adults.22,23,62 Our observation may partially be explained by low total pain catastrophising scores due to the recruitment of participants without a history of musculoskeletal injury or pain. Future studies in a more representative nursing population with a broader range of pain history and pain catastrophising are warranted.
Total MSPSS scores predicted EIH at the lumbar spine and trended toward significance at the forearm (p = 0.053); and MSPSS friends subscale predicted EIH at the lumbar spine and globally when all sites were pooled. Contrary to our expectations, the best subset linear mixed models showed that lower perceived social support predicted greater EIH following cycling. Although this is the first study to our knowledge to report the association between social support and EIH, it contrasts expectations because enhanced social support (eg, verbal support, social touch) was previously reported to reduce pain perception in pain-free and clinical pain populations.19 The effect of social support on pain sensitivity is complex, and there is evidence that, at least in the situational context, social hypoalgesia can occur (ie, exposure to a social interaction with feelings of disconnectedness and general negative affect can lead to an hypoalgesic response).70 Social disconnection alone may be sufficient to evoke a stress response and therefore hypoalgesia71,72 ; however, we did not assess a measure of situational stress. General perceptions of stress on EIH were evaluated with the DASS stress subscale, and while there was no correlation or predictive evidence of the stress subscale on EIH, this may be due to the responses reflecting perceptions of the past week and therefore not sensitive to situational stresses. Additionally, mental and physiological stress has been shown to be associated with shift work73 and therefore may also influence the results for some participants for measures of pain processing. However, this study did not control for the influence of recent shift work on pain sensitivity measures and remains a limitation of this study. More broadly, there is evidence that both social exclusion, inclusion, and social support can lead to hyperalgesia.74,75 Therefore, while we showed some evidence for lower social support to predict greater EIH in pain-free, mostly female nurses, further research of larger scale that includes a broad range of social support is required.
Kinesiophobia was not correlated with, nor predictive of EIH at any site. These findings support previous research in pain-free adults.23,60,62 This similarity in findings could be attributed to the shared sample characteristics of low kinesiophobia scores (TSK-17 = 31 points;60 TSK-11 = 19 points;62 Fear of Pain Questionnaire-III = 77.4 points23) in previous studies. Interestingly, the change in pressure pain intensity rating assessed every 30 seconds for 2 minutes using a Forgione-Barber pressure stimulator on the forefinger but not PPT was correlated with kinesiophobia in an earlier study.23 This suggests that kinesiophobia may moderate EIH when measured as a change in pressure pain tolerance rather than a change in PPT in pain-free adults. Larger scale research is required to confirm these findings in populations where kinesiophobia has been reported to be predictive of duration of sick leave.76
Symptoms of depression and anxiety were not found to have a correlation with EIH. Few studies have investigated the correlation between depressive symptoms and EIH in pain-free populations. One study23 reported total mood disturbance (Profile and Mood States questionnaire) predicted reduced EIH; however, the authors did not report depression subscales. Further, while the aforementioned study showed profile of mood states were predictive of EIH, our findings are consistent with previous studies to show that anxiety does not appear to be associated with EIH.22,77,78 Our sample presented with low levels of anxiety and may partially explain the lack of prediction capability for EIH. While anxiety has been shown to moderate pain sensitivity,79 it remains unclear whether anxiety affect endogenous pain modulation.
Global work satisfaction levels in the current study had no relationship with EIH. Our study was the first to explore the relationship between work satisfaction and EIH in a pain-free population. Previous research showed that both work and family environments may lead to similar environmental stresses (eg, cohesion, control) that were predictive of pain and psychological distress.80 Brellenthin et al23 investigated the correlation between the family environment and EIH following acute isometric exercise. Negative family environment scores (Family Environment Scale Questionnaire) were predictive of EIH for PPT. This is supported by observations that negative family environments (eg, lower cohesion and marital dissatisfaction) can influence the development and maintenance of chronic pain.81,82 Although Brellenthin et al did not explicitly assess work satisfaction, social environments that lead to poorer mental health status may contribute to the dysfunction of descending pain inhibitory pathways associated with EIH.12 Therefore, future studies should further investigate the association between work satisfaction and EIH. This will be particularly important in the nursing profession where job satisfaction and staff retention are low.83
A large effect size at global and all individual local sites was observed in this study and is consistent with several previous studies examining cycling.5,36,37,61,84–91 However, our study was the first to evaluate the association between psychosocial variables in a pain-free population following an effective aerobic EIH exposure. Two previous studies in this area did not achieve EIH,60,92 but also reported that no psychosocial variables predicted EIH. Research on multi-site hypoalgesic effects of cycling is conflicting, with some studies reporting EIH at remote locations,5,61,89 while other studies have not.36,86,90,93 These studies have utilised similar exercise protocols (approximately 70–75% VO2max; 10–30 minutes); however, in our study, participants also reached volitional fatigue. Training history related to the exercise intensity may explain some of the variability in EIH across studies more than the intensity itself. Exercise produces a physiological stress response which increases with higher exercise intensities, longer duration, and lower training history.94 Acute stress has been shown to have a hypoalgesic response via activation of opioid and endocannabinoid mechanisms,95 similar to that of EIH. The exposure to unaccustomed exercise resulting in acute situational stress may not fully explain these results because studies have shown that EIH can occur at various exercise intensities.3 Therefore, research is required to explore the effect of individual factors in addition to the absolute exercise prescription variables associated with exercise exposure on EIH.
A strength of this study is that the cycling protocol was appropriate and achieved EIH in this population. Two previous studies60,92 evaluating the effect of psychosocial factors on EIH did not achieve EIH with aerobic exercise protocols, and therefore this study is an important step towards understanding the effect of psychosocial factors on EIH in pain-free populations at high risk of musculoskeletal pain conditions. The use of valid questionnaires to measure psychosocial factors commonly associated with musculoskeletal pain conditions and the use of PPTs to measure local and remote pain sensitivity are the strengths of this study. This pilot study is limited by the cross-sectional design, and therefore threats to internal validity associated with learning, regression to the mean, contextual factors, and natural variation in response to multiple tests. The intention of the study was to recruit adults without pain, and while this is a strength of the current study, it does preclude the application of these findings more generally. The recruitment of relatively young, pain-free participants may have also led to a lack of diversity across the psychosocial health spectrum which can influence pain-processing pathways9,16 and may have contributed to the large effect size of EIH at all body regions.1 Therefore, the findings of this research should be considered in the context of the sample studied.
Conclusion
A maximal graded exercise cycling test led to EIH at local, regional, and global body regions in pain-free, predominantly female nurses. Regression analyses between psychosocial variables and EIH show that perceived social support and pain catastrophizing are the most likely variables to explain up to one-quarter of the variance in EIH; however, these results are not conclusive due to the small sample size. Based on this pilot study, replication in a larger, more representative sample of nurses is required to confirm whether psychosocial factors moderate EIH; and further, whether these measures may be used as early predictors of lost time injury in this profession.
Acknowledgments
The authors thank the participants for taking part in the study and their colleagues on the wider study.
Disclosure
Jessica Van Oosterwijck has a postdoctoral fellowship funded by the Research Foundation – Flanders (FWO; grant number 12L5616N). Scott Tagliaferri is supported by an Australian Government Research Training Program Scholarship. The authors report no conflicts of interest in this work.
References
1. Rice D, Nijs J, Kosek E, et al. Exercise-induced hypoalgesia in pain-free and chronic pain populations: state of the art and future directions. J Pain. 2019;20(11):1249–1266. doi:10.1016/j.jpain.2019.03.005
2. Naugle KM, Fillingim RB, Riley JL. A meta-analytic review of the hypoalgesic effects of exercise. Review article. J Pain. 2012;13(12):1139–1150. doi:10.1016/j.jpain.2012.09.006
3. Wewege MA, Jones MD. Exercise-induced hypoalgesia in healthy individuals and people with chronic musculoskeletal pain: a systematic review and meta-analysis. J Pain. 2021;22(1):21–31. doi:10.1016/j.jpain.2020.04.003
4. Koltyn KF, Umeda M. Contralateral attenuation of pain after short-duration submaximal isometric exercise. Article. J Pain. 2007;8(11):887–892. doi:10.1016/j.jpain.2007.06.003
5. Vaegter HB, Handberg G, Graven-Nielsen T. Similarities between exercise-induced hypoalgesia and conditioned pain modulation in humans. Article. Pain. 2014;155(1):158–167. doi:10.1016/j.pain.2013.09.023
6. Lima LV, Abner TSS, Sluka KA. Does exercise increase or decrease pain? Central mechanisms underlying these two phenomena. Article. J Physiol. 2017;595(13):4141–4150. doi:10.1113/JP273355
7. Koltyn KF, Brellenthin AG, Cook DB, Sehgal N, Hillard C. Mechanisms of exercise-induced hypoalgesia. J Pain. 2014;15(12):1294–1304. doi:10.1016/j.jpain.2014.09.006
8. Crombie KM, Brellenthin AG, Hillard CJ, Koltyn KF. Endocannabinoid and opioid system interactions in exercise-induced hypoalgesia. Pain Med. 2017;19(1):118–123. doi:10.1093/pm/pnx058
9. Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care. 2014;8(2):143–151. doi:10.1097/SPC.0000000000000055
10. Garland EL. Pain processing in the human nervous system: a selective review of nociceptive and biobehavioral pathways. Prim Care. 2012;39(3):561–571. doi:10.1016/j.pop.2012.06.013
11. Bannister K. Descending pain modulation: influence and impact. Review article. Curr Opin Physiol. 2019;11:62–66. doi:10.1016/j.cophys.2019.06.004
12. Adams LM, Turk DC. Central sensitization and the biopsychosocial approach to understanding pain. Article. J Appl Biobehav Res. 2018;23(2):e12125. doi:10.1111/jabr.12125
13. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133(4):581–624. doi:10.1037/0033-2909.133.4.581
14. Tracy LM. Psychosocial factors and their influence on the experience of pain. Pain Rep. 2017;2(4):e602. doi:10.1097/PR9.0000000000000602
15. Linton SJ, Shaw WS. Impact of psychological factors in the experience of pain. Phys Ther. 2011;91(5):700–711. doi:10.2522/ptj.20100330
16. Voscopoulos C, Lema M. When does acute pain become chronic? Br J Anaesth. 2010;105(Suppl1):i69–i85. doi:10.1093/bja/aeq323
17. Tagliaferri SD, Miller CT, Owen PJ, et al. Domains of chronic low back pain and assessing treatment effectiveness: a clinical perspective. Journal article. Pain Pract. 2020;20(2):211–225. doi:10.1111/papr.12846
18. Goodin BR, McGuire L, Allshouse M, et al. Associations between catastrophizing and endogenous pain-inhibitory processes: sex differences. Article. J Pain. 2009;10(2):180–190. doi:10.1016/j.jpain.2008.08.012
19. Che X, Cash R, Chung S, Fitzgerald PB, Fitzgibbon BM. Investigating the influence of social support on experimental pain and related physiological arousal: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;92:437–452. doi:10.1016/j.neubiorev.2018.07.005
20. Nahman-Averbuch H, Nir RR, Sprecher E, Yarnitsky D. Psychological factors and conditioned pain modulation: a meta-analysis. Clin J Pain. 2016;32(6):541–554. doi:10.1097/AJP.0000000000000296
21. Munneke W, Ickmans K, Voogt L. The association of psychosocial factors and exercise-induced hypoalgesia in healthy people and people with musculoskeletal pain: a systematic review. Pain Pract. 2020;20(6):676–694. doi:10.1111/papr.12894
22. Naugle KM, Naugle KE, Fillingim RB, Riley JL. Isometric exercise as a test of pain modulation: effects of experimental pain test, psychological variables, and sex. Pain Med. 2014;15(4):692–701. doi:10.1111/pme.12312
23. Brellenthin AG, Crombie KM, Cook DB, Sehgal N, Koltyn KF. Psychosocial influences on exercise-induced hypoalgesia. Article. Pain Med. 2017;18(3):538–550. doi:10.1093/pm/pnw275
24. Bernal D, Campos-Serna J, Tobias A, Vargas-Prada S, Benavides FG, Serra C. Work-related psychosocial risk factors and musculoskeletal disorders in hospital nurses and nursing aides: a systematic review and meta-analysis. Int J Nurs Stud. 2015;52(2):635–648. doi:10.1016/j.ijnurstu.2014.11.003
25. Pope AM, Snyder MA, Mood LH. Environmental Hazards for the Nurse as a Worker. Nursing Health, & Environment: Strengthening the Relationship to Improve the Public’s Health. National Academies Press (US); 1995.
26. Trinkoff AM, Geiger-Brown JM, Caruso CC, et al. Personal Safety for Nurses. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality; 2008.
27. Dressner MA, Kissinger SP. Occupational injuries and illnesses among registered nurses. Mon Labor Rev. 2018. doi:10.21916/mlr.2018.27
28. Harcombe H, McBride D, Derrett S, Gray A. Prevalence and impact of musculoskeletal disorders in New Zealand nurses, postal workers and office workers. Aust N Z J Public Health. 2009;33(5):437–441. doi:10.1111/j.1753-6405.2009.00425.x
29. Yang S, Chang MC. Chronic pain: structural and functional changes in brain structures and associated negative affective states. Int J Mol Sci. 2019;20(13):3130. doi:10.3390/ijms20133130
30. Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31–S34. doi:10.4103/sja.SJA_543_18
31. Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391(10137):2356–2367.
32. Poole DC, Jones AM. Measurement of the maximum oxygen uptake VO2max: VO2peak is no longer acceptable. J Appl Physiol. 2017;122(4):997–1002. doi:10.1152/japplphysiol.01063.2016
33. Mutlu EK, Ozdincler AR. Reliability and responsiveness of algometry for measuring pressure pain threshold in patients with knee osteoarthritis. J Phys Ther Sci. 2015;27(6):1961–1965. doi:10.1589/jpts.27.1961
34. Pelfort X, Torres-Claramunt R, Sanchez-Soler JF, et al. Pressure algometry is a useful tool to quantify pain in the medial part of the knee: an intra- and inter-reliability study in healthy subjects. Orthop Traumatol Surg Res. 2015;101(5):559–563. doi:10.1016/j.otsr.2015.03.016
35. Lacourt TE, Houtveen JH, van Doornen LJP. Experimental pressure-pain assessments: test-retest reliability, convergence and dimensionality. Scand J Pain. 2012;3(1):31–37. doi:10.1016/j.sjpain.2011.10.003
36. Gomolka S, Vaegter HB, Nijs J, et al. Assessing endogenous pain inhibition: test-retest reliability of exercise-induced hypoalgesia in local and remote body parts after aerobic cycling. Pain Med. 2019;20(11):2272–2282. doi:10.1093/pm/pnz131
37. Meeus M, Roussel NA, Truijen S, Nijs J. Reduced pressure pain thresholds in response to exercise in chronic fatigue syndrome but not in chronic low back pain: an experimental study. J Rehabil Med. 2010;42(9):884–890. doi:10.2340/16501977-0595
38. Graven-Nielsen T, Wodehouse T, Langford RM, Arendt-Nielsen L, Kidd BL. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum. 2012;64(9):2907–2916. doi:10.1002/art.34466
39. Gajsar H, Titze C, Hasenbring MI, Vaegter HB. Isometric back exercise has different effect on pressure pain thresholds in healthy men and women. Pain Med. 2016;18(5):917–923. doi:10.1093/pm/pnw176
40. Waller R, Straker L, O’Sullivan P, Sterling M, Smith A. Reliability of pressure pain threshold testing in healthy pain free young adults. Scand J Pain. 2015;9(1):38–41. doi:10.1016/j.sjpain.2015.05.004
41. Darnall BD, Sturgeon JA, Cook KF, et al. Development and validation of a daily Pain Catastrophizing Scale. J Pain. 2017;18(9):1139–1149. doi:10.1016/j.jpain.2017.05.003
42. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524–532. doi:10.1037//1040-3590.7.4.524
43. Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O’Neill E. Factor structure, reliability, and validity of the pain catastrophizing scale. J Behav Med. 1997;20(6):589–605. doi:10.1023/a:1025570508954
44. Vlaeyen JWS, Kole-Snijders AMJ, Boeren RGB, van Eek H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Article. Pain. 1995;62(3):363–372. doi:10.1016/0304-3959(94)00279-n
45. Goubert L, Crombez G, Van Damme S, Vlaeyen JW, Bijttebier P, Roelofs J. Confirmatory factor analysis of the Tampa Scale for Kinesiophobia: invariant two-factor model across low back pain patients and fibromyalgia patients. Clin J Pain. 2004;20(2):103–110. doi:10.1097/00002508-200403000-00007
46. Neblett R, Hartzell MM, Mayer TG, Bradford EM, Gatchel RJ. Establishing clinically meaningful severity levels for the Tampa Scale for Kinesiophobia (TSK-13). Article. Eur J Pain. 2016;20(5):701–710. doi:10.1002/ejp.795
47. Monticone M, Giorgi I, Baiardi P, Barbieri M, Rocca B, Bonezzi C. Development of the Italian version of the Tampa Scale of Kinesiophobia (TSK-I): cross-cultural adaptation, factor analysis, reliability, and validity. Spine. 2010;35(12):1241–1246. doi:10.1097/BRS.0b013e3181bfcbf6
48. Lee D. The convergent, discriminant, and nomological validity of the Depression Anxiety Stress Scales-21 (DASS-21). J Affect Disord. 2019;259:136–142. doi:10.1016/j.jad.2019.06.036
49. Ng F, Trauer T, Dodd S, Callaly T, Campbell S, Berk M. The validity of the 21-item version of the Depression Anxiety Stress Scales as a routine clinical outcome measure. Report author abstract. Acta Neuropsychiatr. 2007;19(5):304–310.
50. Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. 2005;44(Pt 2):227–239. doi:10.1348/014466505X29657
51. Wood BM, Nicholas MK, Blyth F, Asghari A, Gibson S. The utility of the short version of the Depression Anxiety Stress Scales (DASS-21) in elderly patients with persistent pain: does age make a difference? Pain Med. 2010;11(12):1780–1790. doi:10.1111/j.1526-4637.2010.01005.x
52. Lee EH, Moon SH, Cho MS, et al. The 21-item and 12-item versions of the Depression Anxiety Stress Scales: psychometric evaluation in a Korean population. Asian Nurs Res. 2019;13(1):30–37. doi:10.1016/j.anr.2018.11.006
53. Jiang LC, Yan YJ, Jin ZS, et al. The Depression Anxiety Stress Scale-21 in Chinese hospital workers: reliability, latent structure, and measurement invariance across genders. Front Psychol. 2020;11:247. doi:10.3389/fpsyg.2020.00247
54. Stamps PL. Nurses and work satisfaction: an index for measurement. AJN. 1998;98(3):16KK–16LL.
55. Ahmad N, Oranye NO, Danilov A. Rasch analysis of Stamps’s Index of Work Satisfaction in nursing population. Nurs Open. 2017;4(1):32–40. doi:10.1002/nop2.61
56. Oliveira EM, Barbosa RL, Andolhe R, Eiras FR, Padilha KG. Nursing practice environment and work satisfaction in critical units. Ambiente das praticas de enfermagem e satisfacao profissional em unidades criticas. Rev Bras Enferm. 2017;70(1):79–86. doi:10.1590/0034-7167-2016-0211
57. Zimet GD, Dahlem NW, Zimet SG, Farley GK. Multidimensional Scale of Perceived Social Support (MSPSS). J Pers Assess. 1988;51(1):30–41.
58. Osman A, Lamis DA, Freedenthal S, Gutierrez PM, McNaughton-Cassill M. The multidimensional scale of perceived social support: analyses of internal reliability, measurement invariance, and correlates across gender. Article. J Pers Assess. 2014;96(1):103–112. doi:10.1080/00223891.2013.838170
59. Stekhoven DJ, Bühlmann P. MissForest--non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28(1):112–118. doi:10.1093/bioinformatics/btr597
60. Smith A, Ritchie C, Pedler A, McCamley K, Roberts K, Sterling M. Exercise induced hypoalgesia is elicited by isometric, but not aerobic exercise in individuals with chronic whiplash associated disorders. Scand J Pain. 2017;15:14–21. doi:10.1016/j.sjpain.2016.11.007
61. Vaegter HB, Dorge DB, Schmidt KS, Jensen AH, Graven-Nielsen T. Test-retest reliability of exercise-induced hypoalgesia after aerobic exercise. Pain Med. 2018;19(11):2212–2222. doi:10.1093/pm/pny009
62. Ohlman T, Miller L, Naugle KE, Naugle KM. Physical activity levels predict exercise-induced hypoalgesia in older adults. Med Sci Sports Exerc. 2018;50(10):2101–2109. doi:10.1249/MSS.0000000000001661
63. Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126(5):1763–1768. doi:10.1213/ANE.0000000000002864
64. Schneider A, Hommel G, Blettner M. Linear regression analysis: part 14 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2010;107(44):776–782. doi:10.3238/arztebl.2010.0776
65. Salmerón R, García CB, García J. Variance inflation factor and condition number in multiple linear regression. J Stat Comput Simul. 2018;88(12):2365–2384. doi:10.1080/00949655.2018.1463376
66. Kadane JB, Lazar NA. Methods and criteria for model selection. J Am Stat Assoc. 2004;99(465):279–290.
67. Schwarz G. Estimating the dimension of a model. Ann Statist. 1978;6(2):461–464.
68. Lumley T, Miller A. Leaps: regression subset selection. R Package Version. 2009;2:2366.
69. Faul F, Erdfelder E, Lang A-G, Buchner A. G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191.
70. DeWall CN, Baumeister RF. Alone but feeling no pain: effects of social exclusion on physical pain tolerance and pain threshold, affective forecasting, and interpersonal empathy. J Pers Soc Psychol. 2006;91(1):1.
71. Butler RK, Finn DP. Stress-induced analgesia. Prog Neurobiol. 2009;88(3):184–202.
72. Borsook TK, MacDonald G. Mildly negative social encounters reduce physical pain sensitivity. PAIN®. 2010;151(2):372–377. doi:10.1016/j.pain.2010.07.022
73. Ferri P, Guadi M, Marcheselli L, Balduzzi S, Magnani D, Di Lorenzo R. The impact of shift work on the psychological and physical health of nurses in a general hospital: a comparison between rotating night shifts and day shifts. Risk Manag Healthc Policy. 2016;9:203–211. doi:10.2147/rmhp.S115326
74. Brown JL, Sheffield D, Leary MR, Robinson ME. Social support and experimental pain. Psychosom Med. 2003;65(2):276–283. doi:10.1097/01.psy.0000030388.62434.46
75. MacDonald G, Kingsbury R, Shaw S. Adding insult to injury: social pain theory and response to social exclusion. The social outcast: ostracism, social exclusion, rejection, and bullying. In: Sydney Symposium of Social Psychology Series. Psychology Press; 2005:77–90.
76. Dawson AP, Schluter PJ, Hodges PW, Stewart S, Turner C. Fear of movement, passive coping, manual handling, and severe or radiating pain increase the likelihood of sick leave due to low back pain. PAIN®. 2011;152(7):1517–1524.
77. Hoeger Bement MK, Dicapo J, Rasiarmos R, Hunter SK. Dose response of isometric contractions on pain perception in healthy adults. Med Sci Sports Exerc. 2008;40(11):1880–1889. doi:10.1249/MSS.0b013e31817eeecc
78. Lemley KJ, Drewek B, Hunter SK, Hoeger Bement MK. Pain relief after isometric exercise is not task-dependent in older men and women. Med Sci Sports Exerc. 2014;46(1):185–191. doi:10.1249/MSS.0b013e3182a05de8
79. Jones A, Zachariae R. Gender, anxiety, and experimental pain sensitivity: an overview. J Am Med Womens Assoc. 2002;57(2):91–94.
80. Feuerstein M, Sult S, Houle M. Environmental stressors and chronic low back pain: life events, family and work environment. Pain. 1985;22(3):295–307. doi:10.1016/0304-3959(85)90030-2
81. Palermo TM, Holley AL. The importance of the family environment in pediatric chronic pain. JAMA Pediatr. 2013;167(1):93–94. doi:10.1001/jamapediatrics.2013.428
82. Romano JM, Turner JA, Jensen MP. The family environment in chronic pain patients: comparison to controls and relationship to patient functioning. J Clin Psychol Med Settings. 1997;4(4):383–395. doi:10.1023/a:1026253418543
83. Van Hoof W, O’Sullivan K, O’Keeffe M, Verschueren S, O’Sullivan P, Dankaerts W. The efficacy of interventions for low back pain in nurses: a systematic review. Int J Nurs Stud. 2018;77:222–231. doi:10.1016/j.ijnurstu.2017.10.015
84. Koltyn KF, Garvin AW, Gardiner RL, Nelson TF. Perception of pain following aerobic exercise. Article. Med Sci Sports Exerc. 1996;28(11):1418–1421. doi:10.1097/00005768-199611000-00011
85. Van Oosterwijck J, Nijs J, Meeus M, et al. Pain inhibition and postexertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: an experimental study. J Intern Med. 2010;268(3):265–278. doi:10.1111/j.1365-2796.2010.02228.x
86. Van Oosterwijck J, Nijs J, Meeus M, Van Loo M, Paul L. Lack of endogenous pain inhibition during exercise in people with chronic whiplash associated disorders: an experimental study. J Pain. 2012;13(3):242–254. doi:10.1016/j.jpain.2011.11.006
87. Ickmans K, Malfliet A, De Kooning M, et al. Lack of gender and age differences in pain measurements following exercise in people with chronic whiplash-associated disorders. Article. Pain Physician. 2017;20(6):E829–E840.
88. Naugle KM, Naugle KE, Fillingim RB, Samuels B, Riley JL. Intensity thresholds for aerobic exercise-induced hypoalgesia. Med Sci Sports Exerc. 2014;46(4):817–825. doi:10.1249/MSS.0000000000000143
89. Vaegter HB, Handberg G, Jorgensen MN, Kinly A, Graven-Nielsen T. Aerobic exercise and cold pressor test induce hypoalgesia in active and inactive men and women. Pain Med. 2015;16(5):923–933. doi:10.1111/pme.12641
90. Micalos PS, Arendt-Nielsen L. Differential pain response at local and remote muscle sites following aerobic cycling exercise at mild and moderate intensity. Springerplus. 2016;5(1):91. doi:10.1186/s40064-016-1721-8
91. Naugle KM, Naugle KE, Riley JL. Reduced modulation of pain in older adults after isometric and aerobic exercise. J Pain. 2016;17(6):719–728. doi:10.1016/j.jpain.2016.02.013
92. Motl RW, O’Connor PJ, Boyd CM, Dishman RK. Low intensity pain reported during elicitation of the H-reflex: no effects of trait anxiety and high intensity cycling exercise. Brain Res. 2002;951(1):53–58. doi:10.1016/S0006-8993(02)03134-7
93. Vaegter HB, Handberg G, Graven-Nielsen T. Isometric exercises reduce temporal summation of pressure pain in humans. Eur J Pain. 2015;19(7):973–983. doi:10.1002/ejp.623
94. Hackney AC. Stress and the neuroendocrine system: the role of exercise as a stressor and modifier of stress. Expert Rev Endocrinol Metab. 2006;1(6):783–792. doi:10.1586/17446651.1.6.783
95. Hohmann AG, Suplita RL, Bolton NM, et al. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435(7045):1108–1112. doi:10.1038/nature03658
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
