Back to Journals » Patient Preference and Adherence » Volume 10
The impact of patient support programs on adherence, clinical, humanistic, and economic patient outcomes: a targeted systematic review
Authors Gangjuli A, Clewell J, Shillington A
Received 24 November 2015
Accepted for publication 23 February 2016
Published 28 April 2016 Volume 2016:10 Pages 711—725
DOI https://doi.org/10.2147/PPA.S101175
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Arijit Ganguli,1 Jerry Clewell,2 Alicia C Shillington3
1Department of Health Economics and Outcomes Research, 2Department of Medical Affairs, AbbVie, North Chicago, IL, USA; 3EPI-Q Inc., Oak Brook, IL, USA
Background: Patient support programs (PSPs), including medication management and counseling, have the potential to improve care in chronic disease states with complex therapies. Little is known about the program’s effects on improving clinical, adherence, humanistic, and cost outcomes.
Purpose: To conduct a targeted review describing medical conditions in which PSPs have been implemented; support delivery components (eg, face-to-face, phone, mail, and internet); and outcomes associated with implementation.
Data sources: MEDLINE – 10 years through March 2015 with supplemental handsearching of reference lists.
Study selection: English-language trials and observational studies of PSPs providing at minimum, counseling for medication management, measurement of ≥1 clinical outcome, and a 3-month follow-up period during which outcomes were measured.
Data extraction: Program characteristics and related clinical, adherence, humanistic, and cost outcomes were abstracted. Study quality and the overall strength of evidence were reviewed using standard criteria.
Data synthesis: Of 2,239 citations, 64 studies met inclusion criteria. All targeted chronic disease processes and the majority (48 [75%]) of programs offered in-clinic, face-to-face support. All but 9 (14.1%) were overseen by allied health care professionals (eg, nurses, pharmacists, paraprofessionals). Forty-one (64.1%) reported at least one significantly positive clinical outcome. The most frequent clinical outcome impacted was adherence, where 27 of 41 (66%) reported a positive outcome. Of 42 studies measuring humanistic outcomes (eg, quality of life, functional status), 27 (64%) reported significantly positive outcomes. Only 15 (23.4%) programs reported cost or utilization-related outcomes, and, of these, 12 reported positive impacts.
Conclusion: The preponderance of evidence suggests a positive impact of PSPs on adherence, clinical and humanistic outcomes. Although less often measured, health care utilization and costs are also reduced following PSP implementation. Further research is needed to better quantify which support programs, delivery methods, and components offer the greatest value for any particular medical condition.
Keywords: patient support services, patient assistance programs, medication management, specialty pharmacy, mediation adherence
Introduction
Chronic disease in the United States (US) accounts for a large proportion of health care expenditures. In the past 5 years, chronic disease has been responsible for over 75% of all health care-related costs,1,2 and it is projected by 2020, that an additional 16 million US patients will be diagnosed with a chronic condition.3 Chronic disease frequently requires multiple long-term medications and/or complex therapies. Particularly in the elderly, patients with chronic illnesses require long-term treatment to prevent disease progression, complications, and disability.4 Patients with chronic illness often exhibit lower than recommended adherence to medications. In the US, approximately 50% of chronically treated patients do not adhere to their prescription medications, and many lack understanding of the importance of adherence and self-care.5–7 Poor adherence to medication is significant and can lead to increased complications of disease, reduced quality of life, and increased overall health care costs related to complications.1
Self-management support programs are designed to provide patient education to support self-management behavior. These programs have demonstrated improved outcomes in a wide variety of diseases8–12 through individual and group support13 and multidisciplinary health care team coaching.14 Patient support programs (PSPs) are enhanced self-management support programs that include interventions such as individualized medication counseling, training, support, and virtual reminders to improve medication-taking behavior. The underlying objective is to help patients better manage their disease and complex medication regimens, improve medication adherence, and reduce complications and related costs.
Despite the growing availability of PSPs, evidence on outcomes is not well understood. Specifically, there is insufficient understanding of PSPs’ impact on clinical, adherence, humanistic, and economic outcomes. The objective of this targeted review is to answer the following questions: 1) in which disease processes have PSPs been implemented and published; 2) what components of support are encompassed within programs; and 3) what outcomes are impacted and measured related to PSPs (ie, adherence, clinical, humanistic, economic/utilization)?
Methods
The literature was systematically searched for studies describing PSPs implemented for chronic disease therapy reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement for reviews. PSPs were defined as interventions provided to patients with chronic disease requiring chronic and or complex medication therapy to manage symptoms and/or to control disease progression. Specifically, we targeted programs that included a medication counseling or management component incorporated into the interventions. Databases searched were PubMed/Medline and Web of Science using the terms (“patient support program” or “patient assistance program” or “medication management” or “disease therapy management” or “medication” or “drug therapy”) and (“counseling” or “telemedicine” or “telehealth” or “health communication” or “health promotion” or “follow-up” or “reminder” or “reinforcement” or “supportive care”) and (“face-to-face” or “in-person” or “home” or “internet” or “phone” or “telephone”). The search timeframe was 10 years, spanning from March 10, 2005 through March 10, 2015. Initial search results were deduplicated; titles and abstracts were screened independently for relevance by two reviewers with a third acting as adjudicator for discrepancies.
Included studies met the following criteria: 1) the intervention described included active medication counseling consisting of at least two live contacts for a specific chronic disease; 2) the study population consisted of adult patients; 3) the publication reported at least one clinical outcome that allowed a comparison between those receiving the intervention and a control group (derived from either randomized or nonrandomized controlled trials (non-RCTs), as well as pre- and post-implementation study designs); and 4) the follow-up period was at least 3 months. Studies evaluating programs that included interventions limited to medication refill reminders and publications not available in full-text or not in English were excluded. Self-described pilot studies and those with stated limitations of inadequate power or sample size were also excluded. Full-text articles were reviewed against these criteria. Reference lists of included studies and relevant review articles were handsearched for additional manuscripts meeting inclusion criteria.
Data abstraction
Data were abstracted from full text manuscripts by two individuals reviewing each manuscript, with a third acting as adjudicator for discrepancies. Abstracted data included disease states in which programs were implemented with related treatments and medications; components of implemented support interventions, including method of delivery (eg, face-to-face either in-clinic, in-pharmacy, or in home, by phone, via the Internet); implementing organization (eg, provider, payer, or other [eg, pharmacy benefit manager {PBM}]); background of the staff delivering support (eg, pharmacist, nurse, physician); funding source (eg, public/governmental, for-profit entities including insurers, PBM, pharmaceutical industry); and outcomes measured resulting from interventions (eg, clinical, adherence, humanistic, and economic). Evidence quality was examined in two ways. PSP evaluation studies using a randomized or cluster-RCT methodology were deemed the highest quality. Quasi-experimental, prospective observational cohort studies including single arm pre- and poststudies were defined as medium quality, and retrospective cohort studies as lower quality. Quality was also assessed using a checklist for identification of bias risk adapted from the Cochrane Collaboration.15 This included selection bias (systematic differences between baseline characteristics of the groups that are compared), attrition bias (systematic differences between groups in withdrawals from a study), performance bias (systematic differences between groups in the care that is provided, or in exposure to factors other than the interventions of interest), and reporting bias (systematic differences between reported and unreported findings). Each classification was marked as having a high, unclear, or low risk of bias. Studies were also evaluated for other sources of bias and were reported in a separate category from the classification biases. It was only determined as high risk if a bias was present (low risk for no presence of a bias).
Analysis
The data were analyzed and abstracted descriptively to understand the types of programs and related outcomes. The program-related clinical, adherence, humanistic, and health care cost outcomes were characterized as either positive – results indicate statistically significant for all primary and secondary end points, mixed – results indicate both met and failed end points, negative – no significant differences in any measured end point, and unclear – results not adequately described to determine program impact.
Results
Program composition
Of the 2,239 records reviewed, 64 were included in this review (Figure 1). Of programs’ geographic distribution, 22 (34.3%) were implemented in the US, six (9.4%) in sub-Saharan Africa, five (7.8%) in the UK, three each (4.7%) in Canada, Germany, People’s Republic of China, Spain, Taiwan, the Netherlands, and the Middle East, and two (3.1%) in India and Italy. Australia, Malaysia, Poland, Portugal, Thailand, and the Dominican Republic contributed one study each.
  | Figure 1 PRISMA diagram. |
The most frequently targeted disease states for PSPs were for type 2 diabetes mellitus with 12 (18.8%) programs cited, followed by 11 (17.2%) for human immunodeficiency virus (HIV), with most programs evaluated via RCTs (Table 1). The vast majority (59 [92%]) of programs were developed and implemented by health care providers (92.2%), with the remainder created by insurers or specialty pharmacy providers, and one European Union governmental entity (Trans-European Network). Twenty-seven (42%) of the programs were specifically focused on recruiting and supporting patients receiving a specific drug or class of drugs for their disease (eg, highly active retroviral therapy in HIV, long-acting β-agonists in asthma, immunosuppressants/immunomodulatory in posttransplantation, anti-tumor necrosis factors in rheumatoid arthritis [RA]). The remainder were disease-focused with nonspecific medication counseling across all therapeutic classes prescribed for that condition (eg, congestive heart failure, metabolic syndrome). Fifty-four of the 64 included studies reported a source of funding. Seventeen studies (31%) were funded by the pharmaceutical industry or a PBM, 17 studies (31%) were funded by the government, and 20 studies (37%) were privately funded.
In terms of program components, the majority (48 [75%]) of programs offered in-clinic service including face-to-face support with a health care provider. Thirty-five (54.7%) incorporated phone support, and 9 (14.0%) provided in-home support. Ten (15.6%) incorporated mailed or emailed reminders and information. Six programs (18.2%) included only phone support. Three (4.7%) included in-pharmacy consultations. Programs were administered by a variety of disciplines, with 29 (46.7%) overseen by pharmacists, 20 (31%) managed by nurses, 9 (13.8%) by physicians, and 8 (12.5%) by paraprofessionals such as health educators, trained counselors, community health workers, and patient advocates, with the remainder delivered using multidisciplinary teams (Table 2).
Overall program outcomes
All included studies measured at least one clinical end point in program evaluation. Of these, 43 (67.2%) also measured a humanistic outcome, 41 (64.1%) measured adherence, and 15 (23.4%) measured an economic/utilization outcome, including health care utilization such as prevention of hospitalization and costs to provide care. Most programs were evaluated against standard care (Table 2).
Among all programs assessed, the 41 (64.1%) that measured clinical outcomes reported at least one positive response related to the program studied. Of the 41 measuring one or more adherence outcome, 27 (65.9%) reported at least one significantly positive adherence end point. Of 42 studies measuring one or more humanistic outcomes, 27 (64.3%) reported at least one significantly positive result. Relatively few programs reported an economic outcome (n=15). Of these, 12 reported at least one significantly positive economic end point (Figure 2).
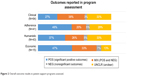  | Figure 2 Overall outcome results in patient support programs assessed. |
Overall assessment of quality and risk of bias
Of the 64 studies, 46 (71.9%) used the highest quality randomized or cluster RCT design, followed by 16 (25%) that used lower quality retrospective and observational designs. Most studies adequately addressed reporting bias – 48 (75%), attrition bias – 37 (57.8%), and selection bias – 35 (54.7%). The most frequently identified bias was performance bias – 20 (31.2%) – which was primarily related to the lack of blinding of participants, personnel, and outcome assessors. This is likely an underestimate of performance bias, however, as 31 (48.4%) of the studies assessed contained inadequate information related to study procedures.
Discussion
In the decades following the passage of the Medicare Modernization Act and the rise of managed care, health professionals, payers, and policymakers have sought to lower costs and improve care quality associated with chronic illness. Medication-focused PSPs have taken a variety of forms, evolving with disease management and medication therapy management, among others. This targeted review is the first to attempt to describe the structure, methods, and outcomes reported in the literature for PSPs. Of the 64 studies, the majority of the interventions were conducted by a health care professional (HCP) in various clinical settings. This included outpatient clinics, primary care practices, inpatient hospital settings, and services conducted at the patient’s home by nurses, pharmacists, physicians, and other health care team members. Interventions included verbal counseling sessions, scheduled follow-up telephone calls, and discharge training sessions. Other indirect patient services included text messaging, refill reminder calls, and written educational materials provided regularly to the patient.
Adherence measures were found to be the most positively impacted through the use of PSPs, followed by humanistic outcomes (eg, patient reported outcomes, quality of life, functional status). PSPs that operated in a clinic (with or without additional phone services) were identified as the most common service in this targeted review. Although clinical outcomes were evaluated most frequently compared to the other measures, there was less evidence supporting the positive impact of this outcome. Minimal evidence was reported for studies focusing on cost, particularly PSPs’ impact on total medical costs, where the majority of health care dollars are spent. Where hospital utilization was assessed, a trend toward reduction in utilization was observed, suggesting that PSPs may provide a benefit in intervening prior to hospitalization becoming necessary.
The evaluation for adherence varied across disciplines and the type of interventions, including face-to-face encounters, group teaching, regular refill reminders, and mailed communications. The method of delivery of these services was heterogeneous, and evidence suggests that PSPs can lead to a positive impact toward patient medication adherence. It can also be suggested that increased patient education combined with regular interventions contribute toward improved patient adherence. Positive benefits were realized for both adherence and humanistic outcomes resulting from face-to-face interventions during a patient encounter, in addition to educational materials supplementing the patient’s understanding. Similarly, existing literature, including that of Warsi et al,10 found that interventions in providing patient and provider reminders, patient education, and financial incentives improved the quality of care for patients with chronic disease. They also showed that two or more interventions were more likely to be successful than a single intervention. Many studies in our targeted review reported two or more interventions for positive adherence and humanistic outcomes, demonstrating a possible increased benefit compared to a single intervention.
Our review was not without limitations. Due to the high volume of initial hits, we limited our search strategy to two databases, PubMed/Medline and Web of Science. A broader search in additional databases may have yielded additional citations. When examining the results of all outcomes in light of study quality, single-armed cohort studies, in which patients served as their own controls at baseline, produced more positive results across the outcomes measured. It is not clear if this methodology overoptimistically portrays results, as RCTs with standard care as a comparator led to more mixed and negative results. Accordingly, there is a need for further evidence surrounding the clinical and financial benefits of PSPs.
Implications to clinical practice and industry
Our analysis found that support programs are heterogeneous with regard to medical conditions served, therapeutic drug classes included, methods of delivery, and funding source. The range of study designs included in this analysis (eg, randomized-controlled trials, cohort, nonrandomized) allows for some generalization to real-world situations and application in a variety of settings. The findings are relevant to PSP developers and HCPs interested in improving the care of chronic, debilitating, and costly disease. They also reveal meaningful gaps in the empiric evidence supporting the use of PSPs.
Unexpected was such a large proportion of PSPs being sponsored by entities, including PBMs and the pharmaceutical industry. While never intended to directly provide health care or replace the role of HCPs, the growth of non-HCP-sponsored programs suggests a genuine need to support the medical professional’s advice beyond time-constrained office visits. Our findings suggest that non-HCP entities may play an increasingly important role in developing and implementing these programs. PSPs supported by these stakeholders target a wide audience through large health plans. For example, Stockl et al16 invited patients with multiple sclerosis (MS) to participate in an enhanced disease therapy management program offered through a PBM, to improve adherence and maximize quality of life. Participants received clinician telephone consultations, care plan mailings, and educational material mailings based on a predefined schedule for up to 6 months post enrollment. An initial phone consultation typically lasted 40–60 minutes, and follow-up consultations lasted 20–30 minutes. During each consultation, the clinician assessed patient knowledge and health concerns and provided education on core topics. Each clinician developed a personalized care plan that summarized the telephone consultation and sent it to the patient and the prescriber of the injectable medications. Patients also received monthly educational mailings specific to MS for 6 months. Patients participating in the program had significantly higher injectable MS medication adherence compared with community pharmacy patients. In addition to increased adherence and persistence with injectable MS medications, a clinical benefit of lower MS relapse was also observed.
In a similar program, also nested in a PBM,17 patients with an injectable RA medication were enrolled into a therapy management program. The primary goal was to facilitate improved adherence to injectable RA medications, and with participation, patients reported significant improvements in physical functioning and work productivity. These two examples illustrate the potential benefits of multifaceted PSPs on medication use as well as clinical and humanistic outcomes.
Given the rising cost of complex diseases such as arthritis, MS, and oncology, the implementation of PSPs should be considered to maximize health outcomes and value in patient-focused care. The site or origin of service is a factor to consider when evaluating program effectiveness. Existing literatures have explored the impact of pharmaceutical services provided in the ambulatory and community settings. Singhal et al’s18 systematic review focused on pharmacist-provided support and revealed evidence that “pharmaceutical services in community and ambulatory care settings make a positive impact on patient outcomes”. Interventions included patient counseling performed by the pharmacist, weekly refill reminders, and scheduled patient follow-up visits that positively impacted clinical, humanistic, and economic outcomes.
Patients and HCPs have not universally embraced services offered through PSPs. Reasons for this are beyond the scope of this targeted review. It is, however, noteworthy that a preponderance of the published evidence corroborates the utility of PSPs for common chronic illnesses to the extent that PSP sponsors can demonstrate improved outcomes from their programs, and HCPs and their patients stand to benefit from participation.
Applicability of findings
The rapid growth in the development and availability of specialty pharmaceuticals combined with fundamental changes in health care delivery are helping to drive new models of care where efficiencies and outcomes are taken into serious account. Conditions that often required hospitalization, treatment administration by a HCP, or very close monitoring can now be treated with medications through retail and specialty pharmacies. By transferring responsibility for obtaining and administering complex and costly medications to patients in the community setting, patient behavior becomes a major influence on the effectiveness and costs of care. Therefore, at least in theory, efforts aimed at improving otherwise unfavorable behaviors regarding medication use should enhance effectiveness, mitigate waste and inefficiency, and improve both treatment satisfaction and outcomes. PSPs intend to achieve such results within discrete populations of greatest perceived need. While still limited in evidentiary strength, the published evidence suggests that the majority of sophisticated, “high-touch” PSPs are having the intended effect.
Limitations of evidence
A systematic review, by its nature, is subject to synthesize information from existing literature and can consequently lead to probable publication bias. Due to the inclusion criteria of this study, articles evaluated were published in English, likely to be cited more frequently, and be presented as a positive study. The majority of the trials included in this review were less methodologically robust as even RCTs relied on heterogeneous control arms in the form of “usual care”. Literature evaluated included quasi-experimental, prospective observational cohort studies, retrospective cohort studies, and RCTs.
Although this review identified evidence for clinical and economic outcomes for PSPs, the constraints for populations, interventions, and settings identified in this systematic review may limit its applicability. Many studies evaluated in this review provided insufficient detail to understand the quality of the interventions. For instance, patient self-reporting was implemented in a number of studies, but this approach can limit the accuracy and validity of the results presented. While the preponderance of data are positive or neutral in outcome, a minority of studies report negative findings, particularly in the economic category of outcomes. It remains unknown if this truly reflects the success of PSPs or underpublication of negative findings.
Suggestions for future research
This review is meant to describe the current state of PSPs from a broad public health perspective. Further comparative analysis within the most common medical conditions may illuminate the specific interventions, methods of delivery, and origin of program components that are most beneficial for a given disease state. Additionally, methodologic rigor in study design is heterogeneous, which highlights a need for greater use of valid comparison groups, standardization of outcomes measured, and greater use of end points that quantify the economic benefits of PSPs. The underrepresentation of clinical, humanistic, and economic outcomes compared to medication adherence illustrates important gaps in this body of evidence. Additionally, there is a need for reporting of both negative and positive findings associated with specific programs so that developers may build upon the experience of others when constructing support programs.
Conclusion
Our review was the first to broadly evaluate the impact of PSPs on adherence, clinical, humanistic, and economic outcomes. The growing implementation of these programs in the pharmaceutical industry, specialty pharmacies, and life-science companies coexist with the need to further explore the utilization of these programs. Little is known about the costs associated with PSPs, and further research is needed to determine the effectiveness of different implementation strategies on adherence, clinical, humanistic, and economic outcomes in PSPs.
Acknowledgments
The authors acknowledge the valuable role of Dr Margaret Yung (EPI-Q Inc.) and Lillian Bellfi (University of Illinois-Chicago, College of Pharmacy) in screening citations, abstracting data, and editing the manuscript.
Disclosure
Arijit Ganguli and Jerry Clewell are employees (and shareholders) of AbbVie Inc. Alicia Shillington is an employee and shareholder of EPI-Q Inc. This systematic review and manuscript development was funded by AbbVie, Inc. The design, study conduct, and financial support for the study/trial were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the poster; all authors contributed to the development of the publication and maintained control over the final content. The authors report no other conflicts of interest in this work.
References
World Health Organization. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization; 2003. Available from: http://apps.who.int/iris/bitstream/10665/42682/1/9241545992.pdf. Accessed March 2, 2016. | ||
Milani RV, Lavie CJ. Health care 2020: reengineering health care delivery to combat chronic disease. Am J Med. 2015;128(4):337–343. | ||
Wu S, Green A. Projection of Chronic Illness Prevalence and Cost Inflation. Santa Monica, CA: RAND Corporation; 2000. | ||
Eney RD, Goldstein EO. Compliance of chronic asthmatics with oral administration of theophylline as measured by serum and salivary levels. Pediatrics. 1976;57(4):513–517. | ||
Haynes RB, McDonald H, Garg AX, Montague P. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev. 2002;(2):CD000011. | ||
Sackett DL, Haynes RB, Gibson ES, Taylor DW, Roberts RS, Johnson AL. Patient compliance with antihypertensive regimens. Patient Couns Health Educ. 1978;1(1):18–21. | ||
Jin J, Sklar GE, Min Sen Oh V, Chuen Li S. Factors affecting therapeutic compliance: a review from the patient’s perspective. Ther Clin Risk Manag. 2008;4(1):269–286. | ||
Norris SL, Engelgau MM, Venkat Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24(3):561–587. | ||
Weingarten SR, Henning JM, Badamgarav E, et al. Interventions used in disease management programmes for patients with chronic illness-which ones work? Meta-analysis of published reports. BMJ. 2002;325(7370):925. | ||
Warsi A, Wang PS, LaValley MP, Avorn J, Solomon DH. Self-management education programs in chronic disease: a systematic review and methodological critique of the literature. Arch Intern Med. 2004;164(15):1641–1649. | ||
Gonseth J, Guallar-Castillón P, Banegas JR, Rodríguez-Artalejo F. The effectiveness of disease management programmes in reducing hospital re-admission in older patients with heart failure: a systematic review and meta-analysis of published reports. Eur Heart J. 2004;25(18):1570–1595. | ||
McAlister FA, Lawson FM, Teo KK, Armstrong PW. A systematic review of randomized trials of disease management programs in heart failure. Am J Med. 2001;110(5):378–384. | ||
Patienteducation.stanford.edu [homepage on the Internet]. Palo Alto, CA: Stanford Patient Education Research Center; c2015 [updated 2015; cited August 13, 2015]. Available from: http://patienteducation.stanford.edu/programs/cdsmp.html. Accessed March 2, 2016. | ||
Bennett HD, Coleman EA, Parry C, Bodenheimer T, Chen EH. Health coaching for patients with chronic illness. Fam Pract Manag. 2010;17(5):24–29. | ||
Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. Available from: http://www.cochrane-handbook.org. Accessed March 2, 2016. | ||
Stockl KM, Shin JS, Gong S, Harada AS, Solow BK, Lew HC. Improving patient self-management of multiple sclerosis through a disease therapy management program. Am J Manag Care. 2010;16(2):139–144. | ||
Stockl KM, Shin JS, Lew HC, et al. Outcomes of a rheumatoid arthritis disease therapy management program focusing on medication adherence. J Manag Care Pharm. 2010;16(8):593–604. | ||
Singhal PK, Raisch DW, Gupchup GV. The impact of pharmaceutical services in community and ambulatory care settings: evidence and recommendations for future research. Ann Pharmacother. 1999;33(12):1336–1355. | ||
Abdelhamid E, Awad A, Gismallah A. Evaluation of a hospital pharmacy-based pharmaceutical care services for asthma patients. Pharm Pract. 2008;6(1):25–32. | ||
Achieng L, Musangi H, Ong’uti S, et al. An observational cohort comparison of facilitators of retention in care and adherence to anti-eetroviral therapy at an HIV treatment center in Kenya. PLoS One. 2012;7(3):e32727. | ||
Aguado O, Morcillo C, Delàs J, et al. Long-term implications of a single home-based educational intervention in patients with heart failure. Heart Lung. 2010;39(6 Suppl):S14–S22. | ||
Al Hayek AA, Robert AA, Al Dawish MA, Zamzami MM, Sam AE, Alzaid AA. Impact of an education program on patient anxiety, depression, glycemic control, and adherence to self-care and medication in Type 2 diabetes. J Family Community Med. 2013;20(2):77–82. | ||
Ali M, Schifano F, Robinson P, et al. Impact of community pharmacy diabetes monitoring and education programme on diabetes management: a randomized controlled study. Diabet Med. 2012;29(9):e326–e333. | ||
Amado Guirado E, Pujol Ribera E, Pacheco Huergo V, Borras JM; ADIEHTA Group. Knowledge and adherence to antihypertensive therapy in primary care: results of a randomized trial. Gac Sanit. 2011;25(1):62–67. | ||
Antonicelli R, Testarmata P, Spazzafumo L, et al. Impact of telemonitoring at home on the management of elderly patients with congestive heart failure. J Telemed Telecare. 2008;14(6):300–305. | ||
Böhme S, Geiser C, Mühlenhoff T, Holtmann J, Renneberg B. Telephone counseling for patients with chronic heart failure: results of an evaluation study. Int J Behav Med. 2012;19(3):288–297. | ||
Cate H, Bhattacharya D, Clark A, Fordham R, Holland R, Broadway DC. Improving adherence to glaucoma medication: a randomised controlled trial of a patient-centred intervention (The Norwich Adherence Glaucoma Study). BMC Ophthalmol. 2014;14:32. | ||
Chiou PY, Kuo BI, Lee MB, Chen YM, Chuang P, Lin LC. A programme of symptom management for improving quality of life and drug adherence in AIDS/HIV patients. J Adv Nurs. 2006;55(2):169–179. | ||
Chung MH, Richardson BA, Tapia K, et al. A randomized controlled trial comparing the effects of counseling and alarm device on HAART adherence and virologic outcomes. PLoS Med. 2011;8(3):e1000422. | ||
Cleland JG, Louis AA, Rigby AS, Janssens U, Balk AH; TEN-HMS Investigators. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans-European Network-Home-Care Management System (TEN-HMS) study. J Am Coll Cardiol. 2005;45(10):1654–1664. | ||
Clifford RM, Davis WA, Batty KT, Davis TM; Fremantle Diabetes Study. Effect of a pharmaceutical care program on vascular risk factors in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care. 2005;28(4):771–776. | ||
Criswell TJ, Weber CA, Xu Y, Carter BL. Effect of self-efficacy and social support on adherence to antihypertensive drugs. Pharmacotherapy. 2010;30(5):432–441. | ||
Crowley MJ, Powers BJ, Olsen MK, et al. The Cholesterol, Hypertension, And Glucose Education (CHANGE) study: results from a randomized controlled trial in African Americans with diabetes. Am Heart J. 2013;166(1):179–186. | ||
de Bruin M, Hospers HJ, van Breukelen GJ, Kok G, Koevoets WM, Prins JM. Electronic monitoring-based counseling to enhance adherence among HIV-infected patients: a randomized controlled trial. Health Psychol. 2010;29(4):421–428. | ||
Del Sindaco D, Pulignano G, Minardi G, et al. Two-year outcome of a prospective, controlled study of a disease management programme for elderly patients with heart failure. J Cardiovasc Med. 2007;8(5):324–329. | ||
DiIorio C, McCarty F, Resnicow K, et al. Using motivational interviewing to promote adherence to antiretroviral medications: a randomized controlled study. AIDS Care. 2008;20(3):273–283. | ||
Erhun WO, Agbani EO, Bolaji EE. Positive benefits of a pharmacist-managed hypertension clinic in Nigeria. Public Health. 2005;119(9):792–798. | ||
Evans CD, Eurich DT, Taylor JG, Blackburn DF. The Collaborative Cardiovascular Risk Reduction in Primary Care (CCARP) study. Pharmacotherapy. 2010;30(8):766–775. | ||
Gabbay RA, Añel-Tiangco RM, Dellasega C, Mauger DT, Adelman A, Van Horn DH. Diabetes nurse case management and motivational interviewing for change (DYNAMIC): results of a 2-year randomized controlled pragmatic trial. J Diabetes. 2013;5(3):349–357. | ||
Grosset KA, Grosset DG. Effect of educational intervention on medication timing in Parkinson’s disease: a randomized controlled trial. BMC Neurol. 2007;7:20. | ||
Heisler M, Hofer TP, Schmittdiel JA, et al. Improving blood pressure control through a clinical pharmacist outreach program in patients with diabetes mellitus in 2 high-performing health systems: the adherence and intensification of medications cluster randomized, controlled pragmatic trial. Circulation. 2012;125(23):2863–2872. | ||
Hlubocky JM, Stuckey LJ, Schuman AD, Stevenson JG. Evaluation of a transplantation specialty pharmacy program. Am J Health Syst Pharm. 2012;69(4):340–347. | ||
Hohmann C, Klotz JM, Radziwill R, Jacobs AH, Kissel T. Pharmaceutical care for patients with ischemic stroke: improving the patients quality of life. Pharm World Sci. 2009;31(5):550–558. | ||
Holzemer WL, Bakken S, Portillo CJ, et al. Testing a nurse-tailored HIV medication adherence intervention. Nurs Res. 2006;55(3):189–197. | ||
Jacobs M, Sherry PS, Taylor LM, Amato M, Tataronis GR, Cushing G. Pharmacist Assisted Medication Program Enhancing the Regulation of Diabetes (PAMPERED) study. J Am Pharm Assoc. 2012;52(5):613–621. | ||
Jorstad HT, von Birgelen C, Alings AM, et al. Effect of a nurse-coordinated prevention programme on cardiovascular risk after an acute coronary syndrome: main results of the RESPONSE randomised trial. Heart. 2013;99(19):1421–1430. | ||
Kennedy CA, Beaton DE, Warmington K, Shupak R, Jones C, Hogg-Johnson S. Prescription for education: development, evaluation, and implementation of a successful interprofessional education program for adults with inflammatory arthritis. J Rheumatol. 2011;38(10):2247–2257. | ||
Koenig LJ, Pals SL, Bush T, Pratt Palmore M, Stratford D, Ellerbrock TV. Randomized controlled trial of an intervention to prevent adherence failure among HIV-infected patients initiating antiretroviral therapy. Health Psychol. 2008;27(2):159–169. | ||
Lai PS, Chua SS, Chan SP. Impact of pharmaceutical care on knowledge, quality of life and satisfaction of postmenopausal women with osteoporosis. Int J Clin Pharm. 2013;35(4):629–637. | ||
Liekweg A, Westfeld M, Braun M, et al. Pharmaceutical care for patients with breast and ovarian cancer. Support Care Cancer. 2012;20(11):2669–2677. | ||
Maduka O, Tobin-West CI. Adherence counseling and reminder text messages improve uptake of antiretroviral therapy in a tertiary hospital in Nigeria. Niger J Clin Pract. 2013;16(3):302–308. | ||
Márquez Contreras E, Vegazo García O, Martel Claros N, et al. Efficacy of telephone and mail intervention in patient compliance with antihypertensive drugs in hypertension. ETECUM-HTA study. Blood Press. 2005;14(3):151–158. | ||
McDermott MM, Reed G, Greenland P, et al. Activating peripheral arterial disease patients to reduce cholesterol: a randomized trial. Am J Med. 2011;124(6):557–565. | ||
Morgado M, Rolo S, Castelo-Branco M. Pharmacist intervention program to enhance hypertension control: a randomised controlled trial. Int J Clin Pharm. 2011;33(1):132–140. | ||
Mugusi F, Mugusi S, Bakari M, et al. Enhancing adherence to antiretroviral therapy at the HIV clinic in resource constrained countries; the Tanzanian experience. Trop Med Int Health. 2009;14(10):1226–1232. | ||
Nieuwkerk PT, Nierman MC, Vissers MN, et al. Intervention to improve adherence to lipid-lowering medication and lipid-levels in patients with an increased cardiovascular risk. Am J Cardiol. 2012;110(5):666–672. | ||
Ogedegbe G, Chaplin W, Schoenthaler A, et al. A practice-based trial of motivational interviewing and adherence in hypertensive African Americans. Am J Hypertens. 2008;21(10):1137–1143. | ||
Phumipamorn S, Pongwecharak J, Soorapan S, Pattharachayakul S. Effects of the pharmacist’s input on glycaemic control and cardiovascular risks in Muslim diabetes. Prim Care Diabetes. 2008;2(1):31–37. | ||
Ruan Y, Xing H, Wang X, et al. Virologic outcomes of first-line HAART and associated factors among Chinese patients with HIV in three sentinel antiretroviral treatment sites. Trop Med Int Health. 2010;15(11):1357–1363. | ||
Sadik A, Yousif M, Mcelnay JC. Pharmaceutical care of patients with heart failure. Br J Clin Pharmacol. 2005;60(2):183–193. | ||
Sauvageot J, Kirkpatrick MA, Spray JW. Pharmacist-implemented pharmaceutical manufacturers’ assistance programs: effects on health outcomes for seniors. Consult Pharm. 2008;23(10):809–812. | ||
Shanmugam S, Varughese J, Nair MA, et al. Pharmaceutical care for asthma patients: a developing country’s experience. J Res Pharm Pract. 2012;1(2):66–71. | ||
Sisk JE, Hebert PL, Horowitz CR, Mclaughlin MA, Wang JJ, Chassin MR. Effects of nurse management on the quality of heart failure care in minority communities: a randomized trial. Ann Intern Med. 2006;145(4):273–283. | ||
Skowron A, Polak S, Brandys J. The impact of pharmaceutical care on patients with hypertension and their pharmacists. Pharm Pract. 2011;9(2):110–115. | ||
Solomon DH, Iversen MD, Avorn J, et al. Osteoporosis telephonic intervention to improve medication regimen adherence: a large, pragmatic, randomized controlled trial. Arch Intern Med. 2012;172(6):477–483. | ||
Sriram S, Chack LE, Ramasamy R, Ghasemi A, Ravi TK, Sabzghabaee AM. Impact of pharmaceutical care on quality of life in patients with type 2 diabetes mellitus. J Res Med Sci. 2011;16(Suppl 1):S412–S418. | ||
Stone RA, Rao RH, Sevick MA, et al. Active care management supported by home telemonitoring in veterans with type 2 diabetes: the DiaTel randomized controlled trial. Diabetes Care. 2010;33(3):478–484. | ||
Stroup JS, Rivers SM, Abu-baker AM, Kane MP. Two-year changes in bone mineral density and T scores in patients treated at a pharmacist-run teriparatide clinic. Pharmacotherapy. 2007;27(6):779–788. | ||
Tan H, Yu J, Tabby D, Devries A, Singer J. Clinical and economic impact of a specialty care management program among patients with multiple sclerosis: a cohort study. Mult Scler. 2010;16(8):956–963. | ||
Thompson DR, Roebuck A, Stewart S. Effects of a nurse-led, clinic and home-based intervention on recurrent hospital use in chronic heart failure. Eur J Heart Fail. 2005;7(3):377–384. | ||
Triller DM, Hamilton RA. Effect of pharmaceutical care services on outcomes for home care patients with heart failure. Am J Health Syst Pharm. 2007;64(21):2244–2249. | ||
Tschida S, Aslam S, Khan TT, Sahli B, Shrank WH, Lal LS. Managing specialty medication services through a specialty pharmacy program: the case of oral renal transplant immunosuppressant medications. J Manag Care Pharm. 2013;19(1):26–41. | ||
Van Camp YP, Huybrechts SA, Van Rompaey B, Elseviers MM. Nurse-led education and counselling to enhance adherence to phosphate binders. J Clin Nurs. 2012;21(9–10):1304–1313. | ||
Villeneuve J, Genest J, Blais L, et al. A cluster randomized controlled trial to evaluate an ambulatory primary care management program for patients with dyslipidemia: the TEAM study. CMAJ. 2010;182(5):447–455. | ||
Wang J, Wu J, Yang J, et al. Effects of pharmaceutical care interventions on blood pressure and medication adherence of patients with primary hypertension in China. Clin Res Regul Aff. 2011;28(1):1–6. | ||
Wang KY, Chian CF, Lai HR, Tarn YH, Wu CP. Clinical pharmacist counseling improves outcomes for Taiwanese asthma patients. Pharm World Sci. 2010;32(6):721–729. | ||
Wei L, Yang X, Li J, et al. Effect of pharmaceutical care on medication adherence and hospital admission in patients with chronic obstructive pulmonary disease (COPD): a randomized controlled study. J Thorac Dis. 2014;6(6):656–662. | ||
Winter MC, Halpern M, Brozovich A, Neu N. Evaluation of an HIV adherence counseling program in La Romana, Dominican Republic. J Int Assoc Provid AIDS Care. 2014;13(4):361–365. | ||
Wu SF, Liang SY, Wang TJ, Chen MH, Jian YM, Cheng KC. A self-management intervention to improve quality of life and psychosocial impact for people with type 2 diabetes. J Clin Nurs. 2011;20(17–18):2655–2665. | ||
Zolfaghari M, Mousavifar SA, Pedram S, Haghani H. The impact of nurse short message services and telephone follow-ups on diabetic adherence: which one is more effective? J Clin Nurs. 2012;21(13–14):1922–1931. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




