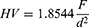Back to Journals » Orthopedic Research and Reviews » Volume 13
The Impact of Free Radical Stabilization Techniques on in vivo Mechanical Changes in Highly Cross-Linked Polyethylene Acetabular Liners
Authors Decker M , Price A , Khalili A, Klassen R, Walzak MJ , Teeter M, McCalden R, Lanting B
Received 5 April 2021
Accepted for publication 22 July 2021
Published 17 August 2021 Volume 2021:13 Pages 113—122
DOI https://doi.org/10.2147/ORR.S309210
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Clark Hung
Michael Decker,1 Amber Price,1 Aria Khalili,2 Robert Klassen,2 Mary Jane Walzak,3 Matthew Teeter,4– 7 Richard McCalden,8 Brent Lanting8
1Department of Orthopaedic Surgery, The University of New Mexico Health Sciences Center, University of New Mexico, Albuquerque, NM, USA; 2Department of Mechanical and Materials Engineering, Western University, London, Ontario, Canada; 3Surface Science Western, University of Western Ontario, London, Ontario, Canada; 4Department of Medical Biophysics, Schulich School of Medicine and Dentistry; 5Imaging Research Laboratories, Robarts Research Institute, Schulich School of Medicine and Dentistry; 6Surgical Innovation Program, Lawson Health Research Institute; 7Division of Orthopaedic Surgery, Schulich School of Medicine and Dentistry, Western University and London Health Sciences Centre, London, Ontario, Canada; 8Division of Orthopaedic Surgery, London Health Sciences Centre, University Hospital, London, Ontario, Canada
Correspondence: Amber Price
Department of Orthopaedics, The University of New Mexico, Albuquerque, NM, 87131-0001, USA
Tel +1 (505) 272-6472
Fax +1 505 272-809
Email [email protected]
Introduction: Numerous thermal free radical stabilization techniques are used in the production of highly cross-linked polyethylene (HXLPE) to improve oxidative stability. Little knowledge exists on the effects of in vivo time on the mechanical properties of HXLPE. The purpose of this study was to determine if free radical stabilization of HXLPE impacts mechanical properties as well as oxidative stability of acetabular liner rims after extended in vivo time.
Methods: Retrieved and control remelted, single annealed and sequentially annealed HXLPE liner rims were tested for mechanical properties. Oxidation was measured with FTIR spectroscopy and crystalline phase composition measured with Raman spectroscopy.
Results: No correlation was found between in vivo, ex vivo time and hardness for annealed groups. A statistically significant difference in hardness was identified between free radical stabilization groups. No correlation between maximum rim oxidation and in vivo time was found. Detectable levels of rim oxidation were present in 100% of single annealed, 75% of sequentially annealed, and 25% of remelted retrieved liners. Single and sequentially annealed liners demonstrated oxidation and increased crystallinity. Rim mechanical properties change in vivo for implant types. With in vivo time, retrieved remelted HXLPE demonstrated decreased mechanical properties, whereas retrieved single and sequentially annealed HXLPE properties remained stable. All liner cohorts demonstrated evidence of rim oxidation. Subsequent changes in crystallinity were only observed in oxidized annealed liners.
Conclusion: HXLPE acetabular liner rims show evidence of in vivo mechanical property degradation, notably in remelted HXLPE, which may be a risk factor in rim fracture and catastrophic implant failure.
Keywords: hip, arthroplasty, free radical, polyethylene, mechanical properties
Introduction
The success of primary total hip arthroplasty (THA) in reducing pain and improving function in patients with end-stage arthritis has been consistently demonstrated. However, revision surgery has been known to carry significant patient morbidity and financial costs. Thus, improving implant longevity remains critical to improved patient care and overall cost reduction. The introduction of highly crossed-linked polyethylene (HXLPE) has resulted in a significant reduction in wear and wear-related complications in THA.1–7 A variety of thermal free radical stabilization techniques, including remelting and various forms of annealing, are routinely used in the production of HXLPE in order to improve oxidative stability. Remelting effectively removes free radicals, however this results in decreased crystallinity and subsequently decreased mechanical properties. Annealing leaves residual-free radicals while maintaining crystallinity and mechanical properties. Furthermore, certain manufacturers utilize irradiation for implant sterilization, which can reintroduce or increase free radicals in the finished implant, depending on the sterilization environment and thermal stabilization technique. The perception at the onset of development of HXLPE was that remelted liners were expected to be oxidatively stable but more likely to have mechanical degradation. The opposite was true for annealed liners. They were perceived to be mechanically stable but more prone to oxidation. These mechanical property changes have resulted in clinically significant mechanical failures of first generation HXLPE THA liners, specifically at the implant rim.8–17 There remains a lack of knowledge regarding the effects of extended in vivo time on the mechanical and physical properties of HXLPE acetabular liners, specifically at the implant rim, and whether this might contribute to failures in this region of the implant.18–25 The purpose of this study was to determine the extent of mechanical and physical property changes of HXLPE THA liner rims after in vivo exposure. Single annealed, sequentially annealed and remelted liners were assessed. First, mechanical properties of retrieved liner rims were assessed using microindentation hardness for each type of oxidative stability technique after extended in vivo time. Liner rims were then assessed for evidence of oxidation and microstructural changes that could explain identified differences found in mechanical testing. The extent and location of oxidation at the implant rim was assessed by Fourier Transform Infrared Spectroscopy (FTIR), and crystallinity of the implant rim was assessed by Raman Spectroscopy. These factors were compared to the Vickers hardness represented by microindentation testing. It was hypothesized that the liners stabilized with thermal annealing would demonstrate increased oxidation resulting in an increase in relative crystallinity and thus reduced mechanical properties when compared to those that are remelted. In addition, it was predicted that the amount of oxidation would correlate with the extent of time in vivo and that microindentation testing would demonstrate a larger increase in hardness for annealed liners compared to remelted liners. It was anticipated that the results of the biomechanical testing would demonstrate a positive correlation between the oxidation index, crystallinity, and hardness at the rim with extended in vivo times. These changes, in theory, would predispose the liners to an increased rate of mechanical failure.
Methods
Sample Selection
A review of the implant retrieval laboratory (IRL) database was performed to obtain a list of available HXLPE acetabular liners with in vivo times greater than 4.5 years. This in vivo time was chosen given the lack on data on in vivo time above four years in the current literature. Institutional review board approval was obtained for access to the retrieved implants and associated patient data. This study had been approved by the Western University Research Ethics Board (IRB 00000940). Patient consent had been obtained. All participants were informed as to the purpose of this study, in accordance 3 with the Declaration of Helsinki. All authors contributed to data analysis, drafting, or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. All participants were informed as to the purpose of this study, in accordance 3 with the Declaration of Helsinki. All retrieved institutional implants underwent a sanitation and storage protocol including cleansing in a 10% bleach solution, fixation in 10% formalin solution, and storage wrapped in gauze in a closed cardboard box stored in a clean, dry and well-ventilated storage room at ambient temperature in room air. Outside institution implants were processed and frozen within 30 days of extraction. Implants were placed in 10% formalin solution for 2–14 days, rinsed in water for 30 minutes, hand scrubbed with mild soap, dried and stored in a −86°C freezer to prevent further oxidation. Inclusion criteria included implants with in vivo time greater than 4.5 years, implants having undergone thermal-free radical stabilization during manufacturing, the ability to identify the specific HXLPE material of the implant, and an implant rim without significant damage from removal with a suitable testing surface. Liners were divided into three groups based on free radical stabilization technique: remelted, single annealed, or sequential annealed. Never-implanted control liners from the same manufacturers were tested to assess for changes from baseline properties after time in vivo. Control liners were obtained directly from implant manufacturers and maintained in air impermeable post-manufacturing packaging until the time of sample preparation and testing. For oxidation and phase composition testing, a subset of retrieved liners was selected with an ex vivo time less than one year to minimize the potential effect of shelf oxidation. A summary of the included liners can be found in Table 1.
 |
Table 1 Summary of Included Liner Manufacturers and Stabilization Methods |
Microindentation Testing
Microindentation hardness testing was performed along the rim surface of each acetabular liner according to ASTM E384 using a Micromet II Vickers microhardness tester (Buehler Ltd, Lake Bluff, IL). A square-based diamond indenter was used to apply a load of 0.0254 kgf into the flat surface of the rim for a 10 second dwell time. The diagonal lengths (d1 and d2) of the resultant indentation were measured using the micro-ruler on the machine’s microscope, measured at 40x magnification. Each rim was tested with 10 to 16 indentations. The Vickers hardness (HV) for each sample was calculated using the following equation:
Where d is the mean diagonal length of the indentation in mm (d=(d1+d2)/2). A mean Vickers hardness was calculated for each sample.
Oxidation and Phase Composition Testing
For those liners undergoing oxidation and phase composition testing, each liner had a vertical cross section removed that included the implant rim. Thin slices (~200 microns thick) were removed parallel to the cross-sectioned surface, extending from the bearing side to the backside of the implant at both the central portion of the articular surface and the rim regions. Each slice was then boiled in hexane at a temperature of 69°C for six hours to extract absorbed esterified fatty acids, and subsequently air dried. The vertical sections from each region of the implant were then assessed for oxidation using a Bruker Hyperion 2000 Fourier transform infrared (FTIR) microscope (Bruker Daltonics Inc, Billerica, MA) attached to a Tensor II spectrometer. Oxidation index (OI) values were calculated according to ASTM F2102 by integrating the area of the peaks arising from the carbonyl groups from 1680 to 1775 cm−1 and rationing that area to the area of the peak arising from the polyethylene, at approximately 1368 cm−1. In order to characterize oxidation as a function of depth, line scans were collected using a 200 µm square window at 200 µm intervals from the bearing side to the backside of the implant at the central articular surface and from the top down 3mm into the bulk at the rim.
Raman spectroscopy was used to assess for changes in the crystalline phase fraction of polyethylene as it relates to oxidative changes. A Renishaw InVia Raman spectrometer (Renishaw Plc, Gloucestershire, UK) equipped with a 514 nm laser, delivering approximately 8 mw of power at the surface of the sample, was used in confocal mode for the analysis. The cross section of the rim section was mapped near the top surface, at the depth of maximum oxidation as detected with FTIR, and in the bulk of the material (~3mm depth) using a 20X objective. If no detectable oxidation (OI < 0.1) was noted by FTIR, the sample was mapped 1 mm from the top surface, carried out in a 50×50 µm area, collecting 121 data points which were averaged, and baseline corrected. After restricting the peak position and full width at half maximum to reasonable ranges, a spectral deconvolution was performed using an automatic curve fitting routine in the Renishaw Wire 4.1 software package. Using previously described calculation methods26,27 for determining the phase fraction of polyethylene (source), the fraction of the amorphous (∝a), crystalline (∝c), and intermediate (∝i) phases of UHMWPE is determined.
Results
Microindentation Testing
A total of 55 retrieved and 13 control liner rims were evaluated. Retrieved samples included 23 remelted, 16 single annealed, and 16 sequentially annealed liners. Average patient age at the time of the revision surgery was 69 years and 60% of patients were male. Indications for revision surgery included infection (22.6%), aseptic loosening (18.9%), instability (18.9%), periprosthetic fracture (15.1%), revision of a recalled implant (9.4%), recalcitrant pain (7.5%), implant malposition (5.7%), and trunnionosis (1.9%). In vivo and ex vivo times for remelted, single annealed and sequentially annealed times are presented in Table 2. There was no statistically significant difference between the groups for in vivo time (p=0.184) and ex vivo time (p=0.484). No correlation was found between in vivo and ex vivo time and hardness for the single annealed and sequentially annealed group. For remelted liners, a statistically significant correlation (p=0.11) between ex vivo time and hardness were found, which may be associated with ex vivo mechanical property degradation. To control for this finding, analysis of covariance (ANCOVA) with ex vivo time as a covariate was used to assess for differences in hardness and the retrieved liners base on thermal treatment. Statistical significance (p < 0.0005, η2 = 0.322) in hardness was identified between the free radical stabilization groups. Post hoc analysis revealed that remelted samples had a statistically significant lower hardness compared to single annealed (p=0.001) and sequentially annealed (p < 0.0005) samples, and no difference found between the single and sequentially annealed samples. A post hoc power analysis was performed at a significant level of 5%, demonstrating a power of 99.6%. Compared to control liners, remelted retrieved liners showed an increase in hardness by 0.40kgf/mm2 (95% CI 0.12–0.68, p = 0.007). No hardness difference was found between control and retrieval samples in the single or sequentially annealed groups as seen in Figure 1.
 |
Table 2 Correlation Between Vickers Hardness (HV) and in vivo and ex vivo Time |
Oxidation and Phase Composition Testing
A subset of 16 retrieved and 5 control liner rims were selected based on the above noted criteria (ie less than 1 year ex-vivo). Retrieved samples included 8 remelted, 4 single annealed, and 4 sequentially annealed liners. Average patient age at the time of the revision surgery was 69.1 years and 68.8% of patients were male. Indications for revision surgery were infection (31.3%), periprosthetic fracture (18.8%), recalcitrant pain (18.8%), aseptic loosening (6.2%), instability (6.2%), revision of recalled implant (6.2%), implant malposition (6.2%), and trunnionosis (6.2%). No statistical difference was found between groups for in vivo time (p = 0.295) and ex vivo time (p = 0.539). Rim oxidation as a product of depth with detectable oxidation index (OI > 0.1) was analyzed as seen in Figure 2. When found, rim oxidation was always in the subsurface region (Figure 3). Detectable rim oxidation was demonstrated in only 25% (2/8) of the remelted liners, with an average OIMax of 0.32. One in vivo liner of 7.93 years demonstrated significant oxidation (OIMax = 1.89). No correlation between maximum rim oxidation and in vivo time was found. In contrast, detectable levels of rim oxidation were present in 75% (3/4) of the retrieved sequentially annealed liners, with an average OIMax of 1.24. Control sequentially annealed liners demonstrated low but detectable rim oxidation (OIMax=0.10). There was no correlation between maximum rim oxidation and in vivo time for sequentially annealed liners. Lastly, all (4/4) of the single annealed liners demonstrated significant rim oxidation (OIMax > 0.1), with average OIMax of 3.50. Control single annealed liners demonstrated detectable but low rim oxidation (OIMax = 0.2). There was a positive correlation between the maximum rim oxidation and in vivo time (ρ=0.90, p=0.037).
In general, remelted liners were composed of a lower percentage of crystalline phase than single of sequentially annealed liner rims as seen in Table 3. No difference in the percentage of crystalline phase was seen in remelted liners when comparing the region of maximum oxidation to the bulk material. The crystalline phase percentage in the remelted liners was not different when comparing the oxidized samples (2/8) to the unoxidized samples (6/8). In contrast, in the single annealed retrieved liners, the average crystalline phase percentage in the region of maximum oxidation was 12% higher than the bulk and the control. Similarly, in the sequentially annealed retrieved liners, the average crystallinity in the region of maximum oxidation was 5% higher than the material bulk and 11% higher than control (Table 4).
 |
Table 3 Oxidation and Crystalline Phase Percentage Data Comparing Samples with and without Detectable Levels of Oxidation for Thermalization Stabilization Groups |
 |
Table 4 Oxidation, and Crystalline Phase Percentage Data from Retrieved versus Controlled Liner Rims for Each Thermal Stabilization Group |
Discussion
The purpose of this study was to determine if free radical stabilization of HXLPE impacts the mechanical and physical properties as well as the oxidative stability of acetabular liner rims after extended in vivo time.
Hardness
The results of microindentation testing revealed that both retrieved and control remelted liners display a lower hardness compared to both annealed cohorts. It has been shown that remelted liners demonstrate a lower crystallinity27 than annealed or conventional UHMWPE, and this is associated with lower yield strength, ultimate strength, and fatigue resistance.27–30 The results of our study are consistent with these findings. Importantly, our study did show that retrieved remelted HXLPE liners demonstrate an increase in hardness after in vivo exposure. This was not true for the single and sequentially annealed liners. It has been demonstrated that hardness correlates with the extent of oxidation31,32 thus, an increase in the hardness would be expected for implants with a predilection towards oxidation and prolonged in vivo exposure. The findings of no difference in hardness for annealed liners was surprising as a higher prevalence of rim oxidation has been reported for annealed liners,8,13–17 as has evidence of rim damage for annealed liners after in vivo time.14,17,33 Though remelted liners are subject to oxidative changes in vivo,8–12 rim oxidation is generally much lower in retrieved remelted liners when compared to single and sequentially annealed liners.8 This in turn would normally result in a decreased hardness. MacDonald et al evaluated 80 retrieved annealed HXLPE liners with 160 retrieved remelted HXLPE liners. The average oxidation index (OI) for annealed liners at the rim was 3.7 ± 3.1. For remelted liners, the average OI at the rim was 0.1 ± 0.1. Thus, oxidation and subsequent hardness would be anticipated to be greater for the annealed liners rather than the remelted liners. Prior studies have demonstrated that UHMWPE with oxidation indices greater than one can alter the mechanical behavior of the poly and indices greater than three result in complete mechanical integrity loss.14,34 We postulate that the increased hardness in the retrieved remelted HXLPE liners compared to controls may be evidence of compromised mechanical properties. It has been demonstrated that the mechanical properties of remelted HXLPE become compromised at much lower oxidation levels when compared to conventional UHMWPE.35,36 Fung et al also demonstrated that the critical oxidation levels for numerous mechanical properties was less than one in remelted HXLPE.37
Oxidation and Crystallinity
Oxidative changes have previously been identified in remelted, single annealed, sequentially annealed HXLPE acetabular liners, however these changes and their relation to certain mechanical and microstructural properties after in vivo time have not been well described.
In this study, rim oxidation was found to be both higher and more prevalent in retrieved single annealed liners. Sequentially annealed liners also demonstrated a high prevalence of rim oxidation. This was found to be less than single annealed liners but higher than remelted liners. Remelted liners showed both low levels and low prevalence of rim oxidation. Single annealed liner rims demonstrated a high level of crystallinity at the subsurface region where oxidation was found to be highest when compared to unoxidized rim and control implant rim. Previous literature has demonstrated an increased crystallinity associated with OIMax > 0.1.13 Our results corroborated this, with the average crystallinity of all retrievals in the region of maximum oxidation was 12.1% higher and 15.5% higher than the control liner subsurface and bulk, respectively. Sequentially annealed liners demonstrated lower levels of oxidation compared to single annealed liners. Prior retrieval analyses comparing single and sequentially annealed liners have demonstrated similar results.33,38 For both the single and sequentially annealed liners, when the OIMax > 1, there was a sharp increase in the crystallinity. The changes in the crystallinity seen are most likely secondary to oxidation from the presence of residual free radicals in the annealed polyethylene. This would explain the significantly higher oxidation and crystallinity seen in the single annealed liners as they have higher levels of residual free radicals after cross-linking. Despite increased levels of oxidation for both single and annealed liners, our results did not show a demonstrable change in hardness in these cohorts. This is in contradiction of prior studies that demonstrated that increased hardness was correlated with increased oxidation levels. Prior studies of retrieved single annealed liners have shown a relationship between oxidation and decreased mechanical properties at the implant articular surface,8,16 however this has not been found in sequentially annealed liners.33 The differences seen between the above studies and our results may be secondary to the method of mechanical testing or the difference in location of the testing on the implant, as the in vivo environments of the rim and articular surface are markedly different. Remelted liner rims demonstrated no appreciable difference in crystallinity between in vivo and control samples. Even when remelted liner rims demonstrated an OIMax >0.1, crystallinity was essentially unchanged. However, mechanical testing in the first phase of the study demonstrated an increase in hardness of ~14% in remelted liners after extended in vivo time when compared to controls. Oral et al demonstrated that HXLPE ultimate tensile strength and crosslink density decreased rapidly while elastic modulus increased in OI levels as low as 0.1.35,36 They proposed that oxidation of tie chain molecules found in the amorphous region and short chain recrystallization were responsible. Fung et al found that OI < 0.1 was the critical oxidation index for several mechanical properties in remelted liners.37 Although a significant increase in hardness was found in the first portion of the study, oxidation and crystallinity do not seem to be the driving forces after in vivo exposure. It has been previously demonstrated that remelted HXLPE demonstrates inferior mechanical behavior when compared to single and sequentially annealed liners, especially at the implant rim.18–25,27–29 As such, continued investigation into the source of this degradation in mechanical behavior is necessary.
This study demonstrates several strengths. To our knowledge, this is the first study to directly evaluate the impact of in vivo exposure on oxidation, microstructural and mechanical properties of HXLPE acetabular liner rims. This study included samples with greater than average in vivo times than most current studies. Ex vivo time was controlled for, reducing the potential impact of shelf time on results. The limitations of this study included small sample sizes for each cohort that may have limited the power of the study. Furthermore, the method of mechanical testing (ie microindentation) is relatively uncommon and therefore it may be difficult to compare to our results to those utilizing other mechanical testing methods. Lastly, remelted liners were obtained from multiple manufacturers with different radiation doses which has been previously shown to influence oxidative and mechanical properties.35,37
Conclusion
In conclusion, this study demonstrated that extended in vivo exposure leads to rim oxidation and increased crystallinity in single annealed and sequentially annealed liners without detectable changes in mechanical properties, whereas remelted liners showed no changes in oxidation or crystallinity while showing a significant change in mechanical properties. With extended in vivo time, retrieved remelted HXLPE liner rims demonstrated decreased mechanical properties whereas retrieved single and sequentially annealed HXLPE liner rims demonstrated no significant change in mechanical behavior. All liner cohorts demonstrated some evidence of oxidation at the rim however was more prevalent in the annealed liners. Oxidation was associated with an increase in crystallinity in the annealed cohorts but not the remelted cohorts. The significance of these findings with respect to the performance of HXLPE into the second decade and beyond remains unknown. Further investigation will be required to identify the source of these mechanical property changes.
Acknowledgments
Thomas Turgeon, BSc MD MPH FRCSC for contribution of samples for use in this study Concordia Joint Replacement Group, Winnipeg, Manitoba, Canada; Department of Surgery, University of Manitoba, Winnipeg, Manitoba, Canada.
Funding
This work was supported by grant funds received from the Lawson Internal Research Fund Grant Number IRF-28-17.
Disclosure
Dr Brent Lanting reports personal fees and research support, from Stryker, DePuy J&J, Smith and Nephew, IntelliJoint; also received academic funding during the conduct of the study from Zimmer-Biomet, DePuy J&J, Smith and Nephew and Stryker outside the submitted work. Dr Richard McCalden reports personal fees from Smith & Nephew and institutional research support from Smith & Nephew, DePuy J&J, and Stryker, during the conduct of the study. Matthew Teeter has stock in IdealFit Spacer and Solo Spine. Brent Lanting has stock in IdealFit Spacer. The authors report no other conflicts of interest in this work. We disclose that this manuscript was based on a thesis: Decker, Michael M., “The Impact of Free Radical Stabilization Techniques on In Vivo Property Changes in Highly Cross-Linked Polyethylene Acetabular Liners” (2018). Electronic Thesis and Dissertation Repository. 5769.
References
1. Jacobs CA, Christensen CP, Greenwald AS, McKellop H. Clinical performance of highly cross-linked polyethylenes in total hip arthroplasty. J Bone Joint Surg Am. 2007;89(12):2779–2786. doi:10.2106/JBJS.G.00043
2. Lachiewicz PF, Soileau ES. Highly cross-linked polyethylene provides decreased osteolysis and reoperation at minimum 10-year follow-up. J Arthroplasty. 2016;31(9):1959–1962. doi:10.1016/j.arth.2016.02.038
3. Kurtz SM, Gawel HA, Patel JD. History and systematic review of wear and osteolysis outcomes for first-generation highly crosslinked polyethylene. Clin Orthop Relat Res. 2011;469(8):2262–2277. doi:10.1007/s11999-011-1872-4
4. Glyn-Jones S, Thomas GER, Garfjeld-Roberts P, et al. The John Charnley award: highly crosslinked polyethylene in total hip arthroplasty decreases long-term wear: a double-blind randomized trial. Clin Orthop Relat Res. 2015;473(2):432–438. doi:10.1007/s11999-014-3735-2
5. Paxton EW, Inacio MCS, Namba RS, et al. Metal-on-conventional polyethylene total hip arthroplasty bearing surfaces have a higher risk of revision than metal-on-highly crosslinked polyethylene: results from a US registry. Clin Orthop Relat Res. 2015;473(3):1011–1021. doi:10.1007/s11999-014-4105-9
6. Paxton E, Cafri G, Havelin L, et al. Risk of revision following total hip arthroplasty: metal-on-conventional polyethylene compared with metal-on-highly cross-linked polyethylene bearing surfaces: international results from six registries. J Bone Joint Surg Am. 2014;96(Suppl 1):19–24. doi:10.2106/JBJS.N.00460
7. Hanna SA, Somerville L, McCalden RW, et al. Highly cross-linked polyethylene decreases the rate of revision of total hip arthroplasty compared with conventional polyethylene at 13 years’ follow-up. Bone Joint J. 2016;98-B(1):28–32. doi:10.1302/0301-620X.98B1.36527
8. MacDonald D, Sakona A, Ianuzzi A, et al. Do first-generation highly crosslinked polyethylenes oxidize in vivo? Clin Orthop Relat Res. 2011;469(8):2278–2285. doi:10.1007/s11999-010-1728-3
9. Currier BH, Van Citters DW, Currier JH, Collier JP. In vivo oxidation in remelted highly cross-linked retrievals. J Bone Joint Surg Am. 2010;92(14):2409–2418. doi:10.2106/JBJS.I.01006
10. Miura Y, Hasegawa M, Sudo A, et al. In-vivo degradation of middle-term highly cross-linked and remelted polyethylene cups: modification induced by creep, wear and oxidation. J Mech Behav Biomed Mater. 2015;51:13–24. doi:10.1016/j.jmbbm.2015.06.028
11. Muratoglu OK, Wannomae KK, Rowell SL, et al. Ex vivo stability loss of irradiated and melted ultra-high molecular weight polyethylene. J Bone Joint Surg Am. 2010;92(17):2809–2816. doi:10.2106/JBJS.I.01017
12. Rowell SL, Reyes CR, Malchau H, Muratoglu OK. In vivo oxidative stability changes of highly cross-linked polyethylene bearings: an ex vivo investigation. J Arthroplasty. 2015;30(10):1828–1834. doi:10.1016/j.arth.2015.05.006
13. Wannomae KK, Bhattacharyya S, Freiberg A, et al. In vivo oxidation of retrieved cross-linked ultra–high-molecular-weight polyethylene acetabular components with residual free radicals. J Arthroplasty. 2006;21(7):1005–1011. doi:10.1016/j.arth.2005.07.019
14. Currier BH, Currier JH, Mayor MB, Lyford KA, Collier JP, Van Citters DW. Evaluation of oxidation and fatigue damage of retrieved crossfire polyethylene acetabular cups. J Bone Joint Surg Am. 2007;89(9):2023. doi:10.2106/00004623-200709000-00019
15. Kurtz SM, Hozack W, Turner J, et al. Mechanical properties of retrieved highly cross-linked crossfire liners after short-term implantation. J Arthroplasty. 2005;20(7):840–849. doi:10.1016/j.arth.2005.07.015
16. Kurtz SM, Hozack WJ, Purtill JJ, et al. 2006 OTTO AUFRANC AWARD PAPER: significance of in vivo degradation for polyethylene in total hip arthroplasty. Clin Orthop Relat Res. 2006;453:47–57. doi:10.1097/01.blo.0000246547.18187.0b
17. Kurtz SM, Austin MS, Azzam K, et al. Mechanical properties, oxidation, and clinical performance of retrieved highly cross-linked crossfire liners after intermediate-term implantation. J Arthroplasty. 2010;25(4):614–623.e2. doi:10.1016/j.arth.2009.04.022
18. Moore KD, Beck PR, Petersen DW, et al. Early failure of a cross-linked polyethylene acetabular liner: a case report. J Bone Joint Surg Am. 2008;90(11):2499–2504. doi:10.2106/JBJS.G.01304
19. Tower SS, Currier JH, Currier BH, Lyford KA, Van Citters DW, Mayor MB. Rim cracking of the cross-linked longevity polyethylene acetabular liner after total hip arthroplasty. J Bone Joint Surg Am. 2007;89(10):2212. doi:10.2106/00004623-200710000-00016
20. Duffy GP, Wannomae KK, Rowell SL, Muratoglu OK. Fracture of a cross-linked polyethylene liner due to impingement. J Arthroplasty. 2009;24(1):
21. Blumenfeld TJ, McKellop HA, Schmalzried TP, Billi F. Fracture of a cross-linked polyethylene liner. J Arthroplasty. 2011;26(4):
22. Hara D, Nakashima Y, Yamamoto T, et al. Late failure of annealed highly cross-linked polyethylene acetabular liner. J Mech Behav Biomed Mater. 2013;28:206–212. doi:10.1016/j.jmbbm.2013.08.003
23. Ast MP, John TK, Labbisiere A, et al. Fractures of a single design of highly cross-linked polyethylene acetabular liners: an analysis of voluntary reports to the United States Food and Drug Administration. J Arthroplasty. 2014;29(6):1231–1235. doi:10.1016/j.arth.2013.12.022
24. Furmanski J, Anderson M, Bal S, et al. Clinical fracture of cross-linked UHMWPE acetabular liners. Biomaterials. 2009;30(29):5572–5582. doi:10.1016/j.biomaterials.2009.07.013
25. Waewsawangwong W, Goodman SB. Unexpected failure of highly cross-linked polyethylene acetabular liner. J Arthroplasty. 2012;27(2):
26. Strobl GR, Hagedorn W. Raman spectroscopic method for determining the crystallinity of polyethylene. J Polym Sci Polym Phys Ed. 1978;16(7):1181–1193. doi:10.1002/pol.1978.180160704
27. Atwood SA, Van Citters DW, Patten EW, et al. Tradeoffs amongst fatigue, wear, and oxidation resistance of cross-linked ultra-high molecular weight polyethylene. J Mech Behav Biomed Mater. 2011;4(7):1033–1045. doi:10.1016/j.jmbbm.2011.03.012
28. Bracco P, Bellare A, Bistolfi A, Affatato S. Ultra-high molecular weight polyethylene: influence of the chemical, physical and mechanical properties on the wear behavior. A review. Materials. 2017;10(7):791. doi:10.3390/ma10070791
29. Muratoglu OK, Bragdon CR. Highly cross-linked and melted UHMWPE. In: UHMWPE Biomaterials Handbook. Elsevier; 2016:264–273.
30. Muratoglu OK, Bragdon CR, O’Connor DO, et al. A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties. J Arthroplasty. 2001;16(2):149–160. doi:10.1054/arth.2001.20540
31. Wernlé JD, Gilbert JL. Micromechanics of shelf-aged and retrieved UHMWPE tibial inserts: indentation testing, oxidative profiling, and thickness effects. J Biomed Mater Res. 2005;75B(1):113–121. doi:10.1002/jbm.b.30285
32. Gilbert JL, Merkhan I. Rate effects on the microindentation-based mechanical properties of oxidized, crosslinked, and highly crystalline ultrahigh-molecular-weight polyethylene. J Biomed Mater Res. 2004;71A(3):549–558. doi:10.1002/jbm.a.30196
33. Kurtz SM, MacDonald DW, Mont MA, et al. Retrieval analysis of sequentially annealed highly crosslinked polyethylene used in total hip arthroplasty. Clin Orthop Relat Res. 2015;473(3):962–971. doi:10.1007/s11999-014-4113-9
34. Kurtz SM, Oral E. In vivo oxidation of UHMWPE. In: UHMWPE Biomaterials Handbook. Elsevier; 2016:488–505.
35. Oral E, Neils AL, Doshi BN, et al. Effects of simulated oxidation on the in vitro wear and mechanical properties of irradiated and melted highly crosslinked UHMWPE. J Biomed Mater Res. 2016;104(2):316–322. doi:10.1002/jbm.b.33368
36. Oral E, Ghali BW, Neils A, Muratoglu OK. A new mechanism of oxidation in ultrahigh molecular weight polyethylene caused by squalene absorption. J Biomed Mater Res. 2012;100B(3):742–751. doi:10.1002/jbm.b.32507
37. Fung M, Bowsher JG, Van Citters DW. Variation of mechanical properties and oxidation with radiation dose and source in highly crosslinked remelted UHMWPE. J Mech Behav Biomed Mater. 2018;82:112–119. doi:10.1016/j.jmbbm.2018.03.005
38. Reinitz SD, Currier BH, Van Citters DW, et al. Oxidation and other property changes of retrieved sequentially annealed UHMWPE acetabular and tibial bearings: in vivo chemical stability of SXL UHMWPE bearings. J Biomed Mater Res. 2015;103(3):578–586. doi:10.1002/jbm.b.33240
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.