Back to Journals » Journal of Inflammation Research » Volume 13
The IL-33/sST2 Axis in Thromboangiitis Obliterans
Authors Sharebiani H, Mohareri M, Mirhosseini A, Fazeli B
Received 25 March 2020
Accepted for publication 8 July 2020
Published 16 July 2020 Volume 2020:13 Pages 317—323
DOI https://doi.org/10.2147/JIR.S253980
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Hiva Sharebiani,1 Mehran Mohareri,1 Ali Mirhosseini,1 Bahare Fazeli1,2
1Immunology Research Center, Inflammation and Inflammatory Diseases Division, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran; 2Vascular Independent Research and Education, European Foundation, Milan, Italy
Correspondence: Bahare Fazeli
Immunology Research Center, Inflammation and Inflammatory Diseases Division, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
Tel +985138002379
Fax +985138414499
WhatsApp ID +98 9151108249 Email [email protected]
Background: Until recently, it remains unknown whether thromboangiitis obliterans (TAO) is a type of systemic vasculitis. A high level of IL-33 and its soluble decoy receptor sST2 in the acute phase of systemic vasculitis has been demonstrated.
Methods: The serum level of IL-33 and sST2 in 50 TAO patients, 20 age- and smoking habit-matched controls and 19 age-matched non-smoker controls was evaluated.
Results: The mean level of IL-33 in TAO, smokers and non-smokers was 370.2± 61.7ng/mL,132.14± 2.6ng/mL and 11.3± 0.38ng/mL, respectively. The IL-33 was significantly higher in the TAO than in either control groups (p < 0.001). The IL-33 in the acute phase of TAO was significantly higher than in the patients in the quiescent phase of the disease (p = 0.019). Also, IL-33 in the patients with gangrene was significantly higher than in the patients with non-healing ulcers (p = 0.021). The sST2 in the TAO patients was 49.3± 5.58ng/mL, and in smoker and non-smoker controls, it was 45.3± 6.3ng/mL and 4.11± 0.17ng/mL, respectively. No significant difference was found between the patients and smoker control groups (p = 0.87). The mean ratio of IL-33/sST2 was 27.89± 10.44 in the TAO group and, in smokers and non-smokers, it was 2.85± 0.48 and 2.84± 0.14, respectively. A significantly high level of IL-33/sST2 ratio was observed in TAO patients in both the active and quiescent phases of the disease in comparison to both control groups (p< 0.001).
Conclusion: The regulation pattern of IL-33/sST2 was different in TAO in comparison to autoimmune vasculitis.
Keywords: interleukin 33, soluble ST2, Buerger’s disease, thromboangiitis obliterans, vasculitis
Introduction
The aetiology and underlying mechanism of thromboangiitis obliterans (TAO) remain unknown.1 It also remains unknown whether TAO is a type of systemic vasculitis or a peripheral vascular disease.1 In addition, until recently, no marker for evaluating the severity of TAO had been suggested.
Interleukin-33 (IL-33), a member of the IL-1 cytokine family, is emerging in terms of its recognition as a regulator of immune responses and inflammatory vascular diseases.2 Notably, endothelial cells are one of the main targets of IL-33 in regulating inflammation.3–5
Soluble ST2 (sST2) is a decoy receptor for IL-33. It is produced by fibroblasts underneath the vascular endothelium.5 It appears that sST2 is only released into circulation upon disruption of the endothelial layer, in which case sST2 would be indicative of the extent of tissue injury.5 A high level of sST2 and its correlation with disease severity in patients with vasculitis, such as systemic lupus erythematosus, advanced systemic sclerosis, Wegener granulomatosis and Behcet’s disease, have been reported.6,7
In this study, IL-33 and sST2 were evaluated in TAO patients as molecules involved in inflammation, cell damage, fibrosis and angiogenesis.
Methods
Patients with a clinical diagnosis of TAO, according to Shionoya’s criteria (including disease onset before the age of 50, history of tobacco use, infrapopliteal arterial occlusion, either upper limb involvement or phlebitis migrans, and absence of atherosclerotic risk factors other than smoking)8 with imaging confirmation were enrolled in the study from February 2016 through August 2017. All patients and controls signed a written informed consent form. The study was conducted in accordance with the Declaration of Helsinki and it was approved by the National Ethics Committee for Clinical Research of the Health Ministry (IR.MUMS.MEDICAL.REC.1398.640).
Patients who experienced pain at rest, painful ischemic ulcers, gangrene or progressive claudication were included in a study group designated as being in the acute phase of TAO. The quiescent phase group comprised patients with a prior history of hospital admissions who were called and invited to participate in the study after undergoing a physical exam. Both groups of patients were matched by age and smoking habit, according to the influence of age and smoking on the serum levels of IL-33 and sST2. Additionally, two control groups of healthy smokers and non-smokers were included who were matched according to age, gender and smoking habit.
IL-33 and sST2 were evaluated by the enzyme-linked immunosorbent assay (ELISA) method (ZellBio GmbH, Ulm, Germany). The Statistical Package for the Social Sciences (SPSS) version 11.5 (SPSS Inc., Chicago, IL, USA) was used for the analysis of the patient data. The descriptive data are presented as mean ± standard error. According to the distribution of the data, independent t-test, ANOVA and mann whitney u-test were used.
Results
A total of 50 serum samples from patients who had been clinically diagnosed with TAO in addition to 20 healthy smokers and 19 non-smoker controls were selected for the current study. All TAO patients were active smokers. Of the 50 TAO patients, 22 were in the acute phase of the disease, whilst 28 patients were in the quiescent phase. All patients and controls were Caucasian men and current smokers at the time of blood sampling.
The chief complaints amongst the TAO patients in the acute phase were burning pain at rest (9.2%), toe or limb gangrene with rest pain (54.5%) and non-healing ulcer with pain (36.3%).
The mean ages of the patients in the acute group, the quiescent phase group and the smoker and non-smoker control groups were 40.6 ± 1.1, 43 ± 1.8, 40.3 ± 0.5 and 41.6 ± 1.4 years, respectively. No significant differences were observed relative to age amongst the studied groups (P = 0.34). The mean number of cigarettes smoked per day in the acute phase group was 17.7 ± 1.2 versus 18.9 ± 3.3 in the quiescent phase group and 18.8 ± 0.79 in the heathy smokers control group. No significant differences in terms of cigarette consumption were found amongst the groups (P = 0.85).
The mean level of IL-33 in the TAO patients was 370.2 ± 61.7ng/mL. In the smoker control group, it was 132.14 ± 2.6ng/mL, and in the non-smoker control group, it was 11.3 ± 0.38ng/mL (Figure 1A). However, the IL-33 serum level was significantly higher in the TAO patients than in either control group (p < 0.001). Also, the serum level of IL-33 was significantly higher in the smoker controls than in the non-smokers (p < 0.001).
The mean level of IL-33 in the acute phase patients was 583 ± 136ng/mL, whilst in the quiescent phase group, it was 242 ± 23ng/mL (Figure 1B). Interestingly, IL-33 in the acute phase group was significantly higher than in the patients in the quiescent phase of the disease (p = 0.019). At the same time, the mean serum level of IL-33 in the patients with gangrene was significantly higher than in the patients with non-healing ulcers (p = 0.021) (Figure 1C).
The mean serum level of sST2 in the TAO patients was 49.3 ± 5.58ng/mL. In the smoker controls, it was 45.3 ± 6.3ng/mL, and in the non-smoker controls, it was 4.11 ± 0.17ng/Ml (Figure 2A). No significant difference was found between the patients and smoker control groups (p = 0.87). However, sST2 was significantly higher in the TAO patients and the smokers in comparison with the non-smoker controls (p < 0.001).
The sST2 levels in the patients in both the acute and quiescent phases were 46.7 ± 9.8ng/mL and 51.8 ± 5.8ng/mL, respectively (Figure 2B). No significant difference in the serum level of ST2 in the patients in the acute phase of the disease in comparison with the patients in the quiescent phase was found (p = 0.21). In addition, the level of sST2 in the patients with gangrene was 46.1 ± 15.3ng/mL, whilst in the patients with chronic ulcer, it was 58.2 ± 5.08ng/Ml (Figure 2C). No significant difference was found between the serum level of ST2 according to clinical manifestation (p = 0.58). Notably, no correlation between IL-33 and sST2 was found (p = 0.17).
The mean ratio of IL-33/sST2 was 27.89 ± 10.44 in the TAO group. The mean ratios of IL-33/sST2 in the smoker and non-smoker control groups were 2.85 ± 0.48 and 2.84 ± 0.14, respectively (Figure 3). No significant difference between the ratios of the two control groups was found (p = 0.9). However, the ratio was significantly higher in the TAO patients in comparison to both control groups (p < 0.001).
 |
Figure 3 Ratio of Il-33/sST2 in TAO, smoker and non-smoker groups. The numbers inside the figure are associated to the patients and controls’ code. |
Notably, according to previous studies regarding the evaluation of IL-17, toll-like receptor 4 (TLR4), neopterin and high sensitivity C-reactive protein (hsCRP) on the same TAO samples,9,10 there was a significant negative correlation between IL-33 and IL-17 (p = 0.041, CC = −0.42). Also, the IL-33 level was 459 ± 125.5ng/mL in CRP-negative patients and 222.6 ± 11.26ng/mL in CRP-positive patients (p = 0.017, Z = −2.38). However, there was a significant positive correlation between IL-33 and TLR4 (p < 0.001, CC = 0.6). Also, the serum level of IL-33 was 909.3 ± 490.7ng/mL in neopterin-negative patients and 320.3 ± 64.2ng/mL in neopterin-positive patients, which was significantly higher in neopterin-negative patients during the active phase of the disease (p = 0.024).
Discussion
This study demonstrated the significant elevation of serum IL-33 levels without an increase in the serum levels of sST2 in TAO patients, particularly those in the acute phase of the disease, in comparison to healthy smokers. IL-33 is predominantly expressed by stromal cells, including endothelial cells.3 Smoking and oxidative stress will lead to both over-expression of IL-33 and vascular endothelium damage, which could lead to the release of IL-33.11,12 The IL-33 can activate the ST2 receptor complex on a variety of immune cells or it can be neutralised by binding to sST2.13
Notably, smoking not only influences the level of IL-33 but also increases the sST2 level.14 In our study, smoking habit was matched between the patients and smoker controls, and both groups had significantly higher levels of sST2 than non-smokers. Although the level of IL-33 was significantly higher in the TAO patients in comparison to the smokers, no difference was found between the sST2 levels of the TAO patients and the smokers. In the healthy smokers and non-smokers, the mean of the IL-33/sST2 ratio was almost equal. This represents a balance between IL-33 and sST2 in the healthy control groups.
Also, IL-33 can induce angiogenesis by stimulating endothelial nitric oxide production.15 Therefore, a high level of IL-33 might be a defensive mechanism of TAO patients due to vascular obliteration. However, ineffective angiogenesis has been indicated in TAO.16 Owing to the reports of a low level of nitric oxide in TAO patients due to polymorphism in the promoter region of endothelial nitric oxide synthesis, a high level of IL-33 may not induce angiogenesis.17,18
Moreover, in autoimmune diseases, such as systemic lupus erythematosus (SLE), the level of IL-33 in the acute phase is high, but there is no difference in this level between quiescent phase patients and control groups.7 However, in the current study, the level of IL-33 was significantly higher in both the acute and quiescent phases of TAO in comparison to the controls. Unlike with IL-33, there was no significant difference between the levels of sST2 in the quiescent phase group and the control group, perhaps due to the preserved architecture of the vessels in TAO19 in comparison to other types of vasculitis, since ST2 can be released into circulation upon disruption of the endothelial layer. This result demonstrates a different regulation pattern of IL-33 and sST2 between TAO and autoimmune diseases like SLE. Thus, this difference can be considered a differential marker that can help to distinguish between these two diseases. In addition, the existence of considerable levels of IL-33, even in the quiescent phase, implies the presence of a pathogenesis mechanism, or an interaction between host and a trigger more likely an infection. Notably, footprints of infectious pathogens, including Rickettsia and oral bacteria, have been reported.20–22
It has also been demonstrated that IL-33 participates in balancing IL-10 and IL-17 and consequently leads to T helper 2 (Th2) differentiation.23 In this study, the levels of IL-33 had a significant negative correlation with IL-17 in the active phase of the disease. Also, IL-33 was significantly lower in neopterin-positive patients. Neopterin is a marker of T helper 1 (Th1) type cellular immunity.24 However, in the quiescent phase of the disease, IL-33 had no correlation with IL-17 or neopterin levels. Moreover, it has been demonstrated through the interaction of TLR4 and IL-33/ST2 signalling that Th2 response can be developed.25,26 Notably, a significant positive correlation between IL-33 and TLR4 in the TAO patients was found in this study. In addition, a single nucleotide polymorphism (SNP) in MyD88 as a key factor of TLR4 signalling pathway has been reported in TAO patients. This polymorphism along with smoking could induce the expression of IL-33 by vascular endothelium and consequently shift the immune response from Th1 to Th2 type.27
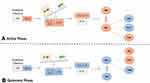
Therefore, it appears that the high level of IL-33 in the active phase of TAO leads to the immune response to Th2 type, which might not be a favourable response if any intercellular pathogen plays a role in TAO pathogenesis (Figure 4). However, Th2-type immune response is protective against atherosclerosis.28 Therefore, the absence of atherosclerotic plaques in the angiography of TAO patients might be due to the high level of IL-33 in these patients.
In the quiescent phase of TAO, the mean levels of IL-33 were significantly higher in CRP-negative patients than in the CRP-positive patients. Therefore, it would appear that IL-33 plays an anti-inflammatory role in TAO patients. This anti-inflammatory effect of IL-33 could be harmful if TAO is an infectious disease. On the other hand, it could be beneficial if TAO is an autoimmune disease. Owing to the fact that the level of IL-33 was significantly higher in the patients with gangrene and that TAO patients with high IL-33 had poor outcomes, it appears that the main underlying mechanism of TAO is an infection, not autoimmunity.
Conclusion
In conclusion, a significantly high level of IL-33/sST2 ratio was observed in TAO patients in both the active and quiescent phases of the disease in comparison to both the smoker and non-smoker control groups. According to the literature search, the regulation pattern of IL-33/sST2 was different in TAO in comparison to autoimmune vasculitis, such as SLE. Also, our findings demonstrated that IL-33 had an anti-inflammatory effect in TAO patients. Because the higher level of IL-33 was detected in the patients with gangrene and tissue loss, TAO appears more likely an infectious disease as opposed to an autoimmune disease. Also, a high level of IL-33 can lead to Th2-type immune response in TAO patients, which does not appear to be a good response toward intracellular pathogens, such as Rickettsia. Therefore, the active phase of TAO might be due to changing the type of immune response from mainly Th1 to Th2 toward an intracellular pathogen. Further studies to evaluate this hypothesis are highly recommended.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fazeli B, Rezaee SA. A review on thromboangiitis obliterans pathophysiology: thrombosis and angiitis, which is to blame? Vascular. 2011;19(3):141–153. doi:10.1258/vasc.2010.ra0045
2. Miller AM. Role of IL-33 in inflammation and disease. J Inflamm. 2011;8(1):22. doi:10.1186/1476-9255-8-22
3. Demyanets S, Konya V, Kastl SP, et al. Interleukin-33 induces expression of adhesion molecules and inflammatory activation in human endothelial cells and in human Atherosclerotic Plaques. Arterioscler Thromb Vasc Biol. 2011;31(9):2080–2089. doi:10.1161/ATVBAHA.111.231431
4. Pollheimer J, Bodin J, Sundnes O, et al. Interleukin-33 drives a proinflammatory endothelial activation that selectively targets nonquiescent cells. Arterioscler Thromb Vasc Biol. 2013;33(2):e4755. doi:10.1161/ATVBAHA.112.253427
5. Demyanets S, Kaun C, Pentz R, et al. Components of the interleukin-33/ST2 system are differentially expressed and regulated in human cardiac cells and in cells of the cardiac vasculature. J Mol Cell Cardiol. 2013;60:16–26. doi:10.1016/j.yjmcc.2013.03.020
6. Kuroiwa K, Arai T, Okazaki H, Minota S, Tominaga S. Identification of human ST2 protein in the sera of patients with autoimmune diseases. Biochem Biophys Res Commun. 2001;284:1104–1108. doi:10.1006/bbrc.2001.5090
7. Pei C, Barbour M, Fairlie-clarke KJ, Mu R. Emerging role of interleukin-33 in autoimmune diseases. Immunology. 2014;141(1):9–17. doi:10.1111/imm.12174
8. Shionoya S. Diagnostic criteria of Buerger’s disease. Int J Cardiol. 1998;66(Suppl 1):
9. Keramat S, Sadeghian MH, Keramati MR, Fazeli B. Assessment of T helper 17-associated cytokines in thromboangiitis obliterans. J Inflamm Res. 2019;12:251–258. doi:10.2147/JIR.S218105
10. Mohareri M, Mirhosseini A, Mehraban S, Fazeli B. Thromboangiitis obliterans episode: autoimmune flare-up or reinfection? Vasc Health Risk Manag. 2018;14:247–251. doi:10.2147/VHRM.S172047
11. Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3(10):e3331. doi:10.1371/journal.pone.0003331
12. Aizawa H, Koarai A, Shishikura Y, et al. Oxidative stress enhances the expression of IL-33 in human airway epithelial cells. Respir Res. 2018;19(1):116. doi:10.1186/s12931-018-0817-9
13. Pace E, Di C, Sca V, et al. Cigarette smoke alters IL-33 expression and release in airway epithelial cells. Biochim Biophys Acta. 2014;1842(9):1630–1637. doi:10.1016/j.bbadis.2014.06.009
14. Lin Y, Zhang R, Hou L, Wang K, Ye Z. Distribution and clinical association of plasma soluble ST2 during the development of type 2 diabetes. Diabetes Res Clin Pract. 2016;118:140–145. doi:10.1016/j.diabres.2016.06.006
15. Choi YS, Choi HJ, Min JK, et al. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood. 2009;114:3117–3126. doi:10.1182/blood-2009-02-203372
16. Hewing B, Stangl V, Stangl K, et al. Circulating angiogenic factors in patients with thromboangiitis obliterans. PLoS One. 2012;7(4):e34717. doi:10.1371/journal.pone.0034717
17. Aliee A, Zahedi Avval F, Taheri H, et al. The status of nitric oxide and its backup, heme oxygenase 1 in thromboangiitis obliterans. Rep Biochem Mol Biol. 2018;6(2):197–202.
18. Masoudian M, Fazeli B, Sharebiani H, et al. Association of the five gene related endothelial cell dysfunction polymorphisms with Buerger’s disease development. Int Angiol. 2016;35(2):205–211.
19. Kobayashi M, Sugimoto M, Komori K. Endarteritis obliterans in the pathogenesis of Buerger’s disease from the pathological and immunohistochemical points of view. Circ J. 2014;78(12):2819–2826. doi:10.1253/circj.CJ-14-0656
20. Fazeli B, Ravari H, Ghazvini K. Rickettsia infection could be the missing piece of the Buerger’s disease puzzle. Int Angiol. 2017;36(5):410–416. doi:10.23736/S0392-9590.17.03420-4
21. Bartolo M, Antignani PL, Todini AR, Ricci G. Buerger’s disease: etiologic role of the rickettsiae? J Mal Vasc. 1987;12(1):82–84.
22. Iwai T, Matsui Y, Homma K, et al. Oral-bacterial-induced arterial and venous thrombus in rats: pathological and immunological studies. Clin Exp Dent Res. 2019;5(5):497–504. doi:10.1002/cre2.215
23. Morrow KN, Coopersmith CM, Ford ML, Ford ML. IL-17, IL-27, and IL-33: a novel axis linked to immunological dysfunction during sepsis. Front Immunol. 2019;10:1982. doi:10.3389/fimmu.2019.01982
24. Pingle SK, Tumane RG, Jawade AA. Neopterin: biomarker of cell-mediated immunity and potent usage as biomarker in silicosis and other occupational diseases. Indian J Occup Environ Med. 2008;12(3):107–111. doi:10.4103/0019-5278.44690
25. Ball DH, Al-Riyami L, Harnett W, Harnett MM. IL-33/ST2 signalling and crosstalk with FcεRI and TLR4 is targeted by the parasitic worm product, ES-62. Sci Rep. 2018;8(1):4497. doi:10.1038/s41598-018-22716-9
26. Chang J, Xia Y, Wasserloos K, et al. Cyclic stretch induced IL-33 production through HMGB1/TLR-4 signaling pathway in murine respiratory epithelial cells. PLoS One. 2017;12(9):e0184770. doi:10.1371/journal.pone.0184770
27. Sun XL, Law BY, de Seabra Rodrigues Dias IR, Mok SWF, He YZ, Wong VK. Pathogenesis of thromboangiitis obliterans: gene polymorphism and immunoregulation of human vascular endothelial cells. Atherosclerosis. 2017;265:258–265. doi:10.1016/j.atherosclerosis.2017.08.009
28. Mallat Z, Taleb S, Ait-Oufella H, Tedgui A. The role of adaptive T cell immunity in atherosclerosis. J Lipid Res. 2009;50(Suppl):S3649. doi:10.1194/jlr.R800092-JLR200
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.