Back to Journals » Journal of Pain Research » Volume 13
The Hypnotic Analgesia Suggestion Mitigated the Effect of the Transcranial Direct Current Stimulation on the Descending Pain Modulatory System: A Proof of Concept Study
Authors Beltran Serrano G, Pooch Rodrigues L, Schein B , Zortea M , Torres ILS , Fregni F, Caumo W
Received 13 March 2020
Accepted for publication 24 June 2020
Published 16 September 2020 Volume 2020:13 Pages 2297—2311
DOI https://doi.org/10.2147/JPR.S253747
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert B. Raffa
Gerardo Beltran Serrano,1– 3 Laura Pooch Rodrigues,2 Bruno Schein,1,2 Maxciel Zortea,1,2 Iraci Lucenada Silva Torres,1,4,5 Felipe Fregni,6 Wolnei Caumo1,2,5
1Post-Graduate Program in Medical Sciences, School of Medicine, Universidade Federal Do Rio Grande Do Sul (UFRGS), Porto Alegre, Brazil; 2Laboratory of Pain and Neuromodulation at Hospital De Clínicas De Porto Alegre (HCPA), Porto Alegre, Brazil; 3Psychology Department, Universidad Catolica De Cuenca, UCACUE, Cuenca, Ecuador; 4Department of Pharmacology, Institute of Health Sciences (ICBS), Universidade Federal Do Rio Grande Do Sul (UFRGS), Porto Alegre, Brazil; 5Pharmacology of Pain and Neuromodulation: Pre-Clinical Investigations Research Group, Universidade Federal Do Rio Grande Do Sul (UFRGS), Porto Alegre, Brazil; 6Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, USA
Correspondence: Wolnei Caumo Fax + (55) 51- 3359.8083
Email [email protected]
Objective: We evaluated whether active(a)-tDCS combined with hypnotic analgesia suggestion (HS) would be more effective than a single active(a)-tDCS, and/or sham-(s)-tDCS and s-tDCS/HS on the following outcomes: function of descending pain modulatory system (DPMS) during the conditioned pain modulation test (CPM-test) (primary outcome), heat pain threshold (HPT), heat pain tolerance (HPTo) and cold pressor test (CPT) (secondary outcomes). We also examined whether their effects are related to neuroplasticity state evaluated by serum brain-derived-neurotropic factor (BDNF).
Materials and Methods: Forty-eight females received one session of one of the four interventions (a-tDCS/HS, s-tDCS/HS, a-tDCS, and s-tDCS) in an incomplete randomized crossover sequence. The a-tDCS or s-tDCS was applied over the left dorsolateral prefrontal cortex (DLPFC) for 30 minutes at 2mA.
Results: A generalized linear model revealed a significant main effect for the intervention group (P < 0.032). The delta-(Δ) pain score on the Numerical Pain Scale (NPS0-10) during CPM-test in the a-tDCS/HS group was − 0.25 (0.43). The (Δ) pain score on NPS (0– 10) during CPM-test in the other three groups was a-tDCS=− 0.54 (0.41), HS − 0.01 (0.41) and s-tDCS/HS=− 0.19 (0.43). A-tDCS/HS intervention increased the CPT substantially compared to all other interventions. Also, higher baseline levels of BDNF were associated with a larger change in CPT and HPTo.
Conclusion: These findings indicate that the HS combined with a-tDCS mitigated the effect of the a-tDCS on the DPMS. The a-tDCS up-regulates the inhibition on DPMS, and the HS improved pain tolerance. And, together they enhanced the reaction time substantially upon the CPT.
Clinical Trial Registration: www.ClinicalTrials.gov, identifier NCT03744897.
Keywords: tDCS, hypnotic analgesia, conditioned pain modulation, pain perception
Introduction
Although we have witnessed a leap forward in comprehension in pain pathophysiology, a gap persists between pain research and pain management in clinical settings. The chronic pain is more prevalent in women (eg, migraine, fibromyalgia, irritable bowel syndrome, and temporomandibular disorders).1 Differences between sex on pain processing revealed that women, compared to men, showed higher medial prefrontal activation during the nociceptive stimulus.2 Women also showed higher connectivity between the periaqueductal gray (PAG) and the middle cingulate cortex.3 And in a recent study, we found the higher inhibitory function of the descending pain modulating system (DPMS) in females compared to males.4 The DPMS comprises midbrain and medullar sites to control over nociception bidirectionally. The periaqueductal gray (PAG) receives inputs from higher brain centers and projects down to the spinal dorsal horn neurons to inhibit (modulate) pain transmission information carried by pain fibers.5,6 Also, the rostroventromedial medulla (RVM) can both facilitate or inhibit nociceptive inputs and acts as a final relay in the control of descending pain facilitation.7 The dysfunction of the DPMS has been pointed out as a central mechanism of chronic pain,8 which can be evaluated by the conditioned pain modulation (CPM) paradigm. During CPM-test, the nociceptive heterotopic stimuli activate the descending inhibitory control (DNIC), and it produces a phenomenon where “pain-inhibits pain”.9,10
Although the pharmacological treatment has advanced, the response is heterogeneous among patients with the same diagnosis by several factors, such as the severity of disease and genetic, emotional, sex hormonal, and other factors related to the dysfunction of DPMS.8 The anodal-(a)-tDCS is a promising therapy for chronic pain because it may change the dysfunctional plasticity within pain circuits according to the specific polarity of the electrodes.11 The anodic current increases neuronal excitability.12 While the cathodic stimulation causes hyperpolarization, with a reduction in the excitability of the neuronal.11,13,14 According to earlier studies, the a-tDCS could modulate pain systems by altering excitability at the cortical level,15 in thalamic and sub-thalamic regions1,16 and it enhances the strength of the DPMS.17 However, studies indicate that the effect of transcranial direct current stimulation (tDCS) in healthy subjects is sex-dependent. The a-tDCS administered over the dorsolateral prefrontal cortex (DLPFC) produces a current flow to frontal regions higher in females,18 and another study found better performance in cognitive response to tDCS effects in women.19
The tDCS effect is related to the electrode’s places. Cumulative evidence has demonstrated that anodal(a)-tDCS montage applied over the primary motor cortex (M1) reduces pain levels.20 In contrast, its effect over the dorsolateral prefrontal cortex (DLPFC) with anodal at left and cathodal at right DLPFC can significantly improve pain perception.21 In recent n a recent study, using this montage in a home-based (HB) a-tDCS during twelve weeks for a total of 60 sessions, we observed significant improvement on the cardinal symptoms of fibromyalgia: pain level, psychological symptoms, sleep quality, and disability due to pain.22 Another study with healthy volunteers found that anodal applied on the left DLPFC modulated pain perception.23 Likewise, an earlier study showed that the same montage of a-tDCS upregulates reactions to positive emotional stimuli and the identification of positive emotions.24 Electrodes placed on the biparietal cortices produce bi hemispherical stimulation.25 This approach can be used purposefully to up regulate one region of the brain, while down-regulates another.26
According to recent study, we found that anodal stimulation over left DLPFC and cathodal over right DLPFC improved the inhibitory function of the DPMS, while hypnotic analgesia suggestion (HS) changes the pain perception.27 Although the mechanisms underpinning these effects are not entirely understood, these results are intriguing. And, they give us new insight into the mechanism involved in hypnotic suggestion analgesia, which likely changes the cortical pain processing, whereas a-tDCS induced either downregulation of the pain-facilitating pathways or upregulation of the inhibitory function of the DPMS.28 And, its effect was modulated positively by the serum brain-derived-neurotropic factor (BDNF).28
Hypnotic analgesia is a social interaction in which one person (the subject) responds to suggestions given by another person (the hypnotist) to produce creative experiences that involve changes in perception, memory, and voluntary control.29,30 According to a meta-analysis, which included both laboratory and clinical studies, the effect size (ES) of hypnotic analgesia was moderate (ES = 0.71).31 The influence of hypnotic analgesia to decrease the activity in supraspinal areas identified as components of the pain matrix, including the thalamus, sensory cortices, insula, anterior cingulate cortex (CCA), and the frontal area, has been demonstrated in previous studies.27 Also, earlier studies found that hypnotic analgesia dissociates sensory and affective components of the pain experience according to the suggestion32 and modulates the activity/connectivity of the pain matrix. In the same way, hypnotic analgesia reduced the unpleasantness of thermal pain perception and modulated the neural activity in the CCA by the changes in thermal pain perception as assessed by positron emission tomography (PET).33 Another study found that hypnotic analgesia effects in the somatosensory brain processing may be related to top-down somatosensory inhibition.32
Thus, we plan the current study to test our hypothesis under an experimental paradigm in which we can characterize the relationship between etiological components of pain (eg, nature, localization, intensity, frequency, and duration of the trigger necessary to evoke pain). We assumed that the HS effects on pain perception involve cortical pain processing. In contrast, the a-tDCS could down-regulate the pain-facilitating pathways, or it up-regulates the inhibitory function of the DPMS. Our purpose was to assess how the combined therapy (a-tDCS over left DLPFC and cathodal over right DLPFC combined with a hypnotic analgesia suggestion (HS)) would work in the pain processing based on measures to evaluate the pain perception (ie, pain threshold and pain tolerance) and the inhibitory function of DPMS by the CPM-test. We aimed to compare whether active(a)-tDCS combined with HS would be more effective than a single active(a)-tDCS, and/or sham-(s)-tDCS or s-tDCS/HS on the following outcomes: function of DPMS assessed by the change on Numerical Pain Scale (NPS 0–10) during the conditioned pain modulation (CPM-test) (primary outcome), heat pain threshold (HPT), heat pain tolerance (HPTo) and cold pressor test (CPT) (secondary outcomes). We also examined whether their effects are related to neuroplasticity state evaluated by serum brain-derived-neurotropic factor (BDNF).
Materials and Methods
Design Overview, Setting, and Participants
We conducted a randomized blinded crossover sham-controlled clinical study. The study was approved by the Research Ethics Committee (CEP) at the Hospital de Clínicas de Porto Alegre (HCPA) (Plataforma Brasil CAAE: 63,863,816,000,005,327 and CEP no: 16–0635) according to international ethical standards based on the Declaration of Helsinki. All participants were given written informed consent. The protocol was pre-registered at ClinicalTrials.gov under the number NCT03744897.
Subjects
The volunteers were healthy women ranging between 18 to 45 years old with more than 11 years of studies. They were recruited from the general population by advertisement postings in the universities, on the internet, and personal divulgation and invitation in public places in the Porto Alegre area.
We included 48 subjects with a score greater than or equal to 8/12 on the scale in the Waterloo-Stanford Group Scale of Hypnotic Susceptibility, Form C (WSGC).34 The participants also answered a structured demographic questionnaire assessing the following variables: current acute or chronic pain conditions, use of analgesics in the past week, rheumatologic disease, clinically significant or unstable medical or psychiatric disorder, history of alcohol or substance abuse in the past 6 months, neuropsychiatric comorbidity, and use of psychotropic drugs. They were excluded if presenting any of these variables or if hearing impairment or formal contraindication to transcranial direct-current stimulation (a-tDCS). Those subjects with scores higher than 12 on Beck Depression Inventory35 were also excluded, as were those with positive screening (>7) for minor psychiatric disorders (somatic symptoms, depressive moods, depressive thoughts, and decreased energy) on the World Health Organization (OMS) Self-Reporting Questionnaire (SRQ-20).
Experimental Protocol
Participants were randomized into four groups: (1) a-tDCS, (2) HS, (3) a-tDCS/HS and (4) s-tDCS/HS. It was an incomplete cross-over trial. The first group received single interventions in a cross-over manner (half received a sequence with HS first and later received a-tDCS; the other half received the opposite sequence). Another group received combined interventions (they were allocated to receive a sequence with an a-tDCS/HS or s-tDCS/HS, those receive a-tDCS/HS during the first trial received s-tDCS/HS in the second trial, or vice-versa). To avoid carry-over effects, we established a washout time of a minimum of seven days between the first and the second trial. Figure 1 presents the flowchart and progress through the study.
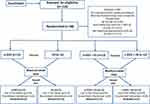 |
Figure 1 Flowchart of the study showing recruitment and progress through the study. |
Interventions
Transcranial Direct Current Stimulation
tDCS was applied using Starstim 8 equipment (Neuroelectrics, Barcelona, Spain). Cathodal and anodal electrodes featured an area of 25 cm2 each placed inside a round sponge soaked in saline solution. Electrodes were placed at spatial positions F3-(Anodal) and F4-(Cathodal) according to the international 10–20-system for electroencephalogram electrode placement,36 commonly considered surface locations above (mid-) left and right DLPFC, respectively.37 Stimulation was delivered at an intensity of 2 mA for 20 min, including a 30s ramp up to 2 mA at the start and a 30s ramp down to 0 mA at the end. In the sham group, current was initially ramped to 2 mA over 30 s and then immediately ramped down to 0 mA. In the final 30 s, the current ramped up to 2mA and back to 0 mA. During stimulation, participants were asked to relax their bodies while sitting and supported in a comfortable position.
Hypnotic Analgesia Suggestion Protocol Based on the Classical Approach
The techniques of hypnosis developed for this study are found on the classical approach developed by the American clinician and Ph.D. Mark P Jensen.38 To guarantee that the hypnotic analgesia suggestion would be conducted equally in all subjects, only one psychologist with more than ten years of experience in practicing hypnotherapy conducted all HS sessions. The standard hypnotic protocol begins with an induction to the subjects to focus their attention on a single stimulus and associate this with breathing and relaxation. Through the 8 final minutes of the induction, suggestions were used to reduce the pain of the participants and increase control over their own sensations.39 We used the protocol of HS previously published by Jansen,28 which follows standardized steps (see the hypnotic analgesia suggestion protocol in the Supplemental material (Appendix I)). The duration of experimental manipulation (induction + suggestions) is 20 min.
a-tDCS/Hypnotic Suggestion and s-tDCS/Hypnotic Suggestion
The a-tDCS/HS were performed concomitantly. When the a-tDCS stimulator was turned on, the therapist began the hypnosis induction as previously described. This session was conducted throughout the 20 min of the tDCS, ending with the final stimulation ramp, with the amperage reduced to zero. The s-tDCS/HS also had a protocol of 20 min duration. The hypnosis induction started with the initial 30s current ramp up to 2 mA, and then the current was immediately ramped down to 0 mA. In the final 30s, the current was ramped up to 2mA and back to 0 mA.
Instruments and Assessments
The tools used to evaluate the psychological state were validated in the Brazilian population and two trained psychologists performed the assessments. We used the refined version of the State-Trait Anxiety Inventory (STAI).40 State anxiety scores range from 13 to 52, and the trait anxiety scores from 12 to 36. A standardized questionnaire was applied to assess demographic data and medical comorbidities.
Outcomes
The primary outcome evaluated the change on NPS (0–10) by the delta (∆)-value (from post-intervention to pre-intervention) during the conditioned pain modulation test (CPM-test). Secondary outcomes assessed the pain perception by delta (∆)-value (from post-intervention to pre-intervention) in the HPT, HPTo and Cold Pressor Test (CPT).
Outcomes Assessment
In this study, we evaluated pain as a response to a nociceptive stimulus using the Quantitative Sensory Testing (QST), including the Conditioned Pain Modulation test (CPM-test) the Cold Pressor Test (CPT).
Sample Size
The sample size was estimated by the G*Power software, based on previous study with a similar methodology (Beltran 2019). The calculus indicated that would be necessary a sample size of 12 subjects to detect a 1.61 point difference in the numerical scale of pain [(NPS 0–10), average SD 2.45, effect size equal to 0.65], with a power of 0.80 and an α error of 0.05.
Randomization
The randomization to allocate each participant was generated by a computer program (Randomlogue) in a ratio of 1:1:1:1. They were allocated to receive the following interventions in incomplete crossover manner: a-tDCS/HS, s-tDCS/HS, HS or a-tDCS, as described above in the session 4.3 about the experimental protocol. Random codes were placed in brown envelopes sealed with the subject’s sequence number # 48 on the outside of the envelope. The allocation concealment was reached on account of no investigator involved in the assessments was aware of intervention allocations.
Blinding
To control possible biases, the following strategies were established: Participants were instructed on all aspects related to the interventions during the evaluations. Two independent evaluators who were not aware of the intervention received were trained to do the assessments. The randomization code was inside the brown envelopes prepared before starting the study. The envelopes were sealed, initialed, and numbered sequentially. These envelopes were opened only after the participant had given her informed consent to participate in the study. The subject`s name and number were immediately sent to those responsible for controlling the randomization process. The blinding was gauged at the end of each evaluation.
Statistical Analysis
To summarize the main socio-demographic features of the sample and to compare the baseline characteristics according to the allocation in the first trial was used the t-Test for independent samples. To test for normality, we used the Shapiro–Wilk test. To compare the mean of Δ-value between groups in univariate analysis was used the Kruskal–Wallis followed by Bonferroni correction to check for differences between groups.
It recognized that psychophysiological measures show individual reactivity to a stimulus of the same intensity. For example, one individual may be highly reactive into painful stimuli, whereas another shows limited changes receiving the same stimulus. Thus, to control for the inter-individual variability, we used the mean variation for delta (Δ)-values (post-intervention minus pre-intervention) of the following measures: change on NPS (0–10) during the CPM test, CPT, HPT, and HPTo.43
Generalized linear models (GLM) were used to analyze the main effect for intervention group (a-tDCS, HS, a-tDCS/HS, and s-tDCS/HS) on the following outcomes measures (dependent variables): change on the NPS (0–10) during the CPM-test, HPT, HPTo and CPT. According to literature, age can mediate changes in BDNF signaling associated with both excitatory and inhibitory synapses in the prefrontal cortex26 as well as the response on a conditioned stimulus. For these reasons, we included both as covariates in the GLM models.44 To perform the analyses, we used the software SPSS version 22.0 (SPSS, Chicago, IL, United States).
Results
Demographic and Characteristics of the Subjects
A total of 114 subjects were screened to participate. After applying the Waterloo-Stanford Group C (WSGC) Scale of Hypnotic Susceptibility, using a cutoff point (8/12) for hypnotic suggestibility, 64 subjects were selected for the hypnosis experiment. Two subjects were excluded because we identified the presence of minor psychiatric disorders. The final sample comprised 48 subjects, which were allocated in two groups: 24 received single interventions (HS and tDCS in cross-over) and 24 received combined interventions (a-tDCS/HS and s-tDCS/HS in cross-over). Participants were randomized and assigned for group and intervention sequence. This resulted in 96 evaluations (before and after intervention) for all outcomes. The sample characteristics of the subjects according to the sequence allocation in the first trial were comparable and are shown in Table 1. All subjects completed the protocol to which they had been randomized.
 |
Table 1 Demographic and Clinical Characteristic of the Sample. Data are Presented as Mean and Standard Deviation (SD) According to Group in the First Trial (n=24) |
Primary Outcome
Intervention Effect on the NPS (0-10) During the CPM-Test
The mean of interventions group before and after the intervention assessed by the Δ-value of means (post-intervention minus pre-intervention) in the NPS during the CPM-test (primary outcome) are presented in Table 2. The Δ-value means of the change on NPS (0–10) during CPM-test among groups were compared by Kruskal–Wallis followed by Bonferroni correction to check for differences between groups.
Change NPS (0-10) During the CPM Test – Multivariate Analysis
A generalized linear model revealed a significant main effect for interventions group (Wald χ2 = 17.50, Df = 3, P <0.001) when we compared the Δ-values of NPS during the CPM test (mean post-intervention minus mean pre-intervention). This result is presented in Table 3. It showed that all three groups were statistically different than s-tDCS. BDNF serum levels at baseline were conversely correlated with the magnitude of the intervention effect on the DPMS as assessed by the CPM-test. While the increase in age is positively correlated with the score on NPS (0–10) during CPM-test. One could realize that higher values in the change of NPS (0–10) during the CPM-test suggest activation of endogenous facilitation of pain, increasing the sensibility to painful stimuli, which indicates lower efficiency of DPMS. Thus, the increase in age is associated with lower efficiency of DPMS, and the higher levels of serum BDNF are associated with higher efficiency of DPMS.
 |
Table 3 Generalized Linear Model (GLM) to Assess the Intervention Effect Among Groups on Δ-Value (Post-Intervention Minus Pre-Intervention) of the Change on NPS (0–10) During the CPM-Test (n=48) |
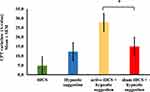
The mean Δ-value (SD) of NPS during the CPM-test in the a-tDCS compared to HS was −0.54 (0.41) vs −0.01 (0.41), respectively. This result indicates that the a-tDCS compared to HS enhanced the efficiency of DPMS. The mean Δ-value (SD) of NPS during the CPM-test in the a-tDCS compared to the a-tDCS/HS was −0.54 (0.41) and −0.25 (0.43), respectively. This finding showed that the combined intervention reduced the efficiency of DPMS. Whereas, the mean Δ-value (SD) of NPS during the CPM-test in the HS compared to the s-tDCS/HS was −0.01 (0.41) and 0.19 (0.43), respectively. This combined intervention decreases the efficiency of DPMS. Whereas the group of a-tDCS/HS compared to s-tDCS/HS was −0.25 (0.43) and −0.19 (0.43), respectively. The s-tDCS/HS reduced the efficiency of DPMS. The comparisons of means, according to the intervention group are presented in Figure 2.
Secondary Outcomes
HPT, HPTo, and CPT – Univariate Analysis
The effect of interventions group on the psychophysical measures assessed by the Δ-value (means post-intervention minus pre-intervention) in the HPT, HPTo, and CPT are presented in Table 4. The Δ-value means on the HPT, HPTo, and CPT among groups were compared by Kruskal–Wallis followed by Bonferroni correction to check for differences between groups.
Secondary Outcomes: HPT, HPTo, and CPT – Multivariate Analysis
Generalized linear models analyses of the main effects of the intervention on the Δ-value of the HPT, HPT, and CPT are presented in Table 5. GLM revealed a main effect of the interventions on the Δ-HPTo (Wald χ2 =8.936, Df = 3, P <0.030) and the Δ-CPT (Wald χ2 = 10.233, Df = 3, P <0.017). For the Δ-HPT, we did not observe a significant difference between the interventions group (Wald χ2 =6.299, Df = 3, P <0.098). The analysis showed that higher levels of BDNF in the baseline are positively correlated with a larger change in the Δ-value of CPT and in the HPTo. Age was not correlated with the change in these psychophysical measures.
The Δ-value (SD) of HPTo in the a-tDCS compared to the a-tDCS/HS was 0.12 (0.41) and 1.45 (0.42) respectively. The combined intervention increased the HPTo. Whereas, the Δ-value (SD) HPTo in the a-tDCS compared to s-tDCS/HS was 0.12 (0.41) and 1.40 (0.42), respectively. The combined intervention increased HPTo. Whereas in the HS compared to the s-tDCS/HS, the mean (SD) was 1.73 (0.41) and 1.40 (0.42), respectively. The s-tDCS/HS reduced the HPTo. The Δ-value mean (SD) of HPTo in the HS compared to the a-tDCS/HS was 1.73 (0.41) and 1.45 (0.42), respectively. The a-tDCS/HS reduced the HPTo. The comparisons of means, according to the intervention group are presented in Figure 3.
The Δ-value (SD) of CPT in the a-tDCS group compared to the a-tDCS/HS was 4.63 (4.41) and 24.20 (4.62), respectively. The combined intervention increased the CPT almost six times. Whereas, the Δ-value (SD) CPT in the a-tDCS compared to s-tDCS/HS was 4.63 (4.41) and 9.55 (4.64), respectively. The combined intervention increased the CPT. The Δ-value mean (SD) CPT in the HS compared to the a-tDCS/HS was 15.48 (4.44) and 24.20 (4.62), respectively. The a-tDCS/HS increased the CPT. The comparisons of means, according to the intervention group are presented in Figure 4.
Discussion
The main finding in this study was to reveal that the hypnotic suggestion, when combined with the a-tDCS, reduced the efficiency of DPMS. Although this result contrasts with our initial hypothesis that the HS combined with the a-tDCS would enhance the efficiency on the DPMS, this effect is incompatible with floor or ceiling effects or with “inverted U-shaped dose-effect curve,” since to justify an inverted U-shaped effect, it should increase up to a maximum and then decrease. Hence, this result suggests that their effects are dissociated and by distinct mechanisms. Although the neurobiological processes involved in the impact of a-tDCS to modulate the DPMS are not clear, and the evidence of its effect over DLPFC on pain are incipient, we can at least propose, that there is an improvement in the DPMS even when the a-tDCS is applied to a distinct area of primary motor cortex (M1). In the present study, the a-tDCS improved the efficiency of DPMS more than fourfold compared to s-tDCS/HS. This is a substantial size effect with a statistical significance and further clinical relevance, mainly because the dysfunction in the inhibitory DPMS is a central process in the chronic pain physiopathology.45 This finding suggests that a-tDCS induced downregulation of the pain-facilitation pathways (or an upregulation of the inhibitory function) of the DPMS. Given the results of a recent meta-analysis, the a-TDCS is effective in diverse chronic pain conditions, with more evidence when applied over M1, even though it suggests that it can be effective when it is used over DLPFC.46 This effect is plausible from the anatomical and neurophysiological perspective since the DLPFC is a critical structure for attention functions47 and modulates the inhibition of neuronal coupling along the ascending midbrain-thalamic-cingulate pathway through descending fibers from the prefrontal cortex.48 Thus, the stimulation over the DLPFC can activate the descending nociceptive inhibitory control system (DNIC), since the prefrontal cortex is involved in pain control, pain expectation, and the placebo effect. However, it is pertinent to emphasize that our results were obtained in healthy subjects who do not present neurobiological changes related to central sensitization. Thus, due to complex mechanisms of pain and the possible dependency of tDCS effects on neuroplasticity state, further studies are needed to test the combined effect of a-tDCS/HS in chronic pain conditions.
The HS effect of reducing pain perception is a finding supported by a recent study with a similar experimental paradigm.28 This result gives support for the idea that the hypnotic suggestion effect on pain processing is decoupled from the inhibitory function of the DPMS. Although the underlying mechanism is unclear, one can hypothesize that the reduction in the pain perception related to hypnotic suggestion mitigated the effect of heterotopic painful stimuli on the DPMS. It is plausible that the hypnotic suggestion competes with the painful heterotopic stimuli by the same descending pain inhibitory pathways as well. An alternative explanation is that the hypnotic suggestion shifted attention and may have altered the brain processing of pain. This hypothesis is in agreement with a previous study, which observed that the hypnotic suggestion changed the activity on the anterior cingulate and insular cortex.49 There are evidences suggesting a shift in the attentional focus can lead to reduction of pain and reduction of brain activation in pain-related areas, such as anterior cingulate and insular cortex and the thalamus.50 In the same way; hypnotic suggestion can have produced a “distraction effect.” This could explain how the hypnotic suggestion modulates pain perception at the supraspinal level and that emotions may modulate the inhibitory activity on the DPMS. Another explanation for this finding is that the a-tDCS may have changed the excitability in a large area of the prefrontal cortex with a consequent diminishing of the capacity for hypnotic response. This is plausible according to earlier studies when the transcranial magnetic stimulation (rTMS) in low frequency (1Hz) was applied over to the left DLPFC, and it reduced the hypnotic response beyond expectancy.51
According to our study, the hypnotic suggestion may modulate the pain reaction to HPTo and CPT differently. These results revealed no enhancement for HPTo for a-tDCS/HS compared to the interventions in which techniques were applied alone. However, an additive effect was observed for the a-tDCS/HS in the CPT, which increased more than two-fold compared to s-tDCS/HS, compared to HS, and a-tDCS. Earlier investigations found that a-tDCS is better than s-tDCS on acute pain perception in different conditions such as in experimental pain in healthy subjects,52 acute postoperative pain,17 and various symptoms related to chronic pain syndromes.53 However, a critical factor that may contribute to a difference in the a-tDCS effect on pain perception is the site of stimulation. Thus, a plausible explanation by the additive effect on the a-tDCS/HS in the CPT is that the anodal stimulation applied on the left DLPFC decreased the activity of the midbrain-medial thalamic pathway related to the emotional perception of pain.46 Another factor that may be corroborated for this effect is the high hypnotic susceptibility of the subjects in this study. This is plausible, given individuals with higher hypnotic susceptibility tend to demonstrate more significant responses to analgesia suggestions during experimental pain paradigms (eg, cold pressor tests, painful heat stimuli).54 Additionally, cold pain and heat pain sensations have been linked to different psychological and biological mechanisms.55 According to meta-analysis, different thermal stimuli (eg, heat or cold) both activate the anterior cingulate cortex (ACC) and insula.56 The cold noxious stimuli activated right subgenual ACC and the amygdala, while the noxious heat stimulus activates the left ACC and the right thalamus.56 Thus, the more substantial effect related to a-tDCS/HS upon the CPT may be linked to a more substantial effect in the unpleasant emotion sensation, which is linked to the amygdala activation by cold nociceptive stimulus compared to uncomfortable feeling related to heat stimulus during the HPTo.57
Additionally, the more substantial effect of a-tDCS/HS may be linked to effects on the inhibitory control, as found in studies with fibromyalgia patients who received a-tDCS over DLPFC coupled with a Go/No-go task to modulate attentional networks.21 The a-tDCS, combined with the cognitive task to induce inhibition, increased HPT, and HPTo compared to s-tDCS.21 Another study in healthy subjects showed that a-tDCS over DLPFC combined with a brief cognitive intervention increased HPT and HPTo.58 Accordingly, it has been suggested that the tDCS effect is state-dependent23 and can modulate prefrontal circuitry and also induce a priming effect. Thus, a-tDCS/HS may work together to enhance the capacity to tolerate and downregulate the emotional component of the pain experience. Thus, HS can maintain these gains and create a potential synergistic effect. In this study, we did not observe the impact of a-tDCS on HPT. Our result aligns with another study with healthy subjects who received a-tDCS over the left DLPFC, which did not produce an effect with a significant impact on the HPT.59
The current study also expands data in the literature that collaborates with our previous studies upon the critical role of BDNF on the DPMS.60 This result is in agreement with an earlier study in healthy females, which showed that higher levels of BDNF have related to reducing pain perception.61 At date, our findings, together with the literature, suggest that in healthy subjects, the BDNF may increase the diffuse inhibition activated by a robust heterotopic stimulus applied in remote areas of the body.62 Furthermore, this set of findings can give us the insight to comprehend the role of the BDNF as a factor to predict the a-tDCS effect on the function of DPMS. And, it can provide us some theoretical support to understand the neurobiological processes underpinning to the a-tDCS impact found in previous studies on chronic pain conditions (eg, osteoarthritis, fibromyalgia, hallux valgus, etc.).17,45,63 Additionally, we found that age is associated positively with the variations of Δ-value on NPS (0–10) during CPM-test. This indicates that the increase in age may be related to a decline in the efficacy of endogenous pain control mechanisms. Although we do not have a clear explanation for this result, the relationship between age and antidromic modulation of DPMS is a matter of intense debate. And, the literature related to this issue is mixed; then, more studies are needed to allow definitive conclusions.
This study has some methodological limitations that should be addressed. First, we included females in our sample since sex differences in response to pain have been found.64 We are conscious that the exclusion of men reduces external validity. However, we aimed to comprehend neurophysiological mechanisms. Thus, a homogeneous sample can minimize the risk of bias. Second, the absence of a group of subjects with a low hypnotizable propensity limits the scope of generalization of our results. Third, the effects of hypnosis after dehypnotization have been observed in earlier studies.65 However, it is not straightforward to quantify a residual potential impact and, if it exists, to identify how much of it has a real effect on the outcomes. From a pragmatic view, we would need counterbalancing with the impact of inter-individual variability if we had compared different individuals on physiological measures, as in the case of psychophysical tests. This approach was used because, in this case, the starting point of these measures is individualized, and the reactivity to the same stimulus can vary from one individual to another. Taking this into account, we conducted a crossover study to consider this effect, if it exists, as an inherent characteristic of this intervention type. Even though we cannot exclude some carryover effect, we believe that it is unlikely to change the conclusions because the aim is to explore the combined intervention (eg, tDCS/HSA) and in both arms of combined interventions, the tDCS device was used, which can offer active or sham stimulation, according to randomization. Regarding the t-DCS, previous studies have shown that one session induces some aftereffect that could persist for one hour if we applied for only a course. Thereby, we assumed that the seven-day washout period is sufficient to prevent cumulative effects.66 Fourth, we did not observe a carryover effect, which means that the results for each phase of the experiment do not reflect the impacts of any residual effects of therapy provided during previous periods of the trial.67 Despite these limitations, our findings were evaluated using psychophysical parameters, which are less prone to assessment bias than self-reported measures. Finally, heat and cold pain have specific physiological mechanisms and can be interpreted in a very particular way,55 which may limit deducing the same effects to other somatosensorial modalities, such as mechanical pain.
In summary, these results indicate that the HS combined with a-tDCS blunted the effect of the a-tDCS on the function of the DPMS. The a-tDCS up-regulates the inhibition on DPMS, and the HS improved pain tolerance. And, together they enhanced the reaction time substantially upon CPT.
Data Availability
The data that support the findings of this study are available from the corresponding author, WC, upon reasonable request.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data. All authors took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10(5):447–485. doi:10.1016/j.jpain.2008.12.001
2. Gupta A, Mayer EA, Fling C, et al. Sex-based differences in brain alterations across chronic pain conditions. J Neurosci Res. 2017;95(12):604–616. doi:10.1002/jnr.23856
3. Kong J, Tu P, Zyloney C, Su T. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav Brain Res. 2010;211(2):215–219. doi:10.1016/j.bbr.2010.03.042
4. Gasparin A, Zortea M, Dos Santos VS, et al. Brain-derived neurotrophic factor modulates the effect of sex on the descending pain modulatory system in healthy volunteers. Pain Med. 2020. doi:10.1093/pm/pnaa027
5. Bajic D, Proudfit HK. Projections of neurons in the periaqueductal gray to pontine and medullary catecholamine cell groups involved in the modulation of nociception. J Comp Neurol. 1999;405(3):359–379. doi:10.1002/(SICI)1096-9861(19990315)405:3<359::AID-CNE6>3.0.CO;2-W
6. Bruinstroop E, Cano G, Vanderhorst VGJM, et al. Spinal projections of the A5, A6 (locus coeruleus), and A7 noradrenergic cell groups in rats. J Comp Neurol. 2012;520(9):1985–2001. doi:10.1002/cne.23024
7. Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80(2):53–83. doi:10.1016/j.pneurobio.2006.08.001
8. Botelho LM, Morales-Quezada L, Rozisky JR, et al. A framework for understanding the relationship between descending pain modulation, motor corticospinal, and neuroplasticity regulation systems in chronic myofascial pain. Front Hum Neurosci. 2016;10:308. doi:10.3389/fnhum.2016.00308
9. Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain. 1979;6(3):283–304. doi:10.1016/0304-3959(79)90049-6
10. Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23(5):611–615. doi:10.1097/ACO.0b013e32833c348b
11. Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1:206–223. doi:10.1016/j.brs.2008.06.004
12. Stagg CJ, Best JG, Stephenson MC, et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci. 2009;29(16):5202–5206. doi:10.1523/JNEUROSCI.4432-08.2009
13. Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–639. doi:10.1109/ICC.1999.765531
14. Vaseghi B, Zoghi M, Jaberzadeh S. How does anodal transcranial direct current stimulation of the pain neuromatrix affect brain excitability and pain perception? A randomised, double-blind, sham-control study. Langguth Bed. PLoS One. 2015;10(3):e0118340. doi:10.1371/journal.pone.0118340
15. Krause B, Márquez-Ruiz J, Kadosh RC. The effect of transcranial direct current stimulation: a role for cortical excitation/inhibition balance? Front Hum Neurosci. 2013;7:1001–1004. doi:10.3389/fnhum.2013.00602
16. Moreno-Duarte I, Morse LR, Alam M, Bikson M, Zafonte R, Fregni F. Targeted therapies using electrical and magnetic neural stimulation for the treatment of chronic pain in spinal cord injury. Neuroimage. 2014;85:1003–1013. doi:10.1016/j.neuroimage.2013.05.097
17. Ribeiro H, Sesterhenn RB, Souza AD, et al. Preoperative transcranial direct current stimulation: exploration of a novel strategy to enhance neuroplasticity before surgery to control postoperative pain. A randomized sham-controlled study. Avenanti A ed. PLoS One. 2017;12(11):e0187013. doi:10.1371/journal.pone.0187013
18. Russell MJ, Goodman TA, Visse JM, et al. Sex and electrode configuration in transcranial electrical stimulation. Front Psychiatry. 2017. doi:10.3389/fpsyt.2017.00147
19. Martin AK, Huang J, Hunold A, Meinzer M. Sex mediates the effects of high-definition transcranial direct current stimulation on “mind-reading.”. Neuroscience. 2017;366:84–94. doi:10.1016/j.neuroscience.2017.10.005
20. da Graca-tarragó M, Deitos A, Patrícia Brietzke A, et al. Electrical intramuscular stimulation in osteoarthritis enhances the inhibitory systems in pain processing at cortical and cortical spinal system. Pain Med. 2015;3(1):
21. Silva AF, Zortea M, Carvalho S, et al. Anodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex modulates attention and pain in fibromyalgia: randomized clinical trial. Sci Rep. 2017;7(1):135. doi:10.1038/s41598-017-00185-w
22. Brietzke AP. Large treatment effect with extended home-based transcranial direct current stimulation over dorsolateral prefrontal cortex in fibromyalgia: a proof of concept sham-randomized clinical study. J Pain. 2020. 21:212–224
23. Mariano TY, Van’t Wout M, Jacobson BL, et al. Effects of Transcranial Direct Current Stimulation (tDCS) on pain distress tolerance: a preliminary study. Pain Med. 2015;16:1580–1588. doi:10.1111/pme.12798
24. Nitsche MA, Koschack J, Pohlers H, Hullemann S, Paulus W, Happe S. Effects of frontal transcranial direct current stimulation on emotional state and processing in healthy humans. Front Psychiatry. 2012;3:58. doi:10.3389/fpsyt.2012.00058
25. Benwell CSY, Learmonth G, Miniussi C, Harvey M, Thut G. Non-linear effects of transcranial direct current stimulation as a function of individual baseline performance: evidence from biparietal tDCS influence on lateralized attention bias. Cortex. 2015;69:152–165. doi:10.1016/j.cortex.2015.05.007
26. Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75(24):2176–2184. doi:10.1212/WNL.0b013e318202013a
27. Jiang H, White MP, Greicius MD, Waelde LC, Spiegel D. Brain activity and functional connectivity associated with hypnosis. Cereb Cortex. 2017. doi:10.1093/cercor/bhw220
28. Beltran Serrano G, Rodrigues LP, Schein B, et al. Comparison of hypnotic suggestion and transcranial direct-current stimulation effects on pain perception and the descending pain modulating system: a crossover randomized clinical trial. Front Neurosci. 2019;13:662. doi:10.3389/fnins.2019.00662
29. Kihlstrom J. Hypnosis. Elsevier; 2016.
30. Oakley DA, Halligan PW. Hypnotic suggestion: opportunities for cognitive neuroscience. Nat Rev Neurosci. 2013;14(8):565–576. doi:10.1038/nrn3538
31. Montgomery GH, Duhamel KN, Redd WH. A meta-analysis of hypnotically induced analgesia: how effective is hypnosis? Int J Clin Exp Hypn. 2000;48(2):138–153. doi:10.1080/00207140008410045
32. Zeev-Wolf M, Goldstein A, Bonne O, Abramowitz EG. Hypnotically induced somatosensory alterations: toward a neurophysiological understanding of hypnotic anaesthesia. Neuropsychologia. 2016;87:182–191. doi:10.1016/j.neuropsychologia.2016.05.020
33. Wik G, Fischer H, Bragée B, Finer B, Fredrikson M. Functional anatomy of hypnotic analgesia: a PET study of patients with fibromyalgia. Eur J Pain. 1999;3(1):7–12. doi:10.1016/S1090-3801(99)90183-0
34. Carvalho C, Kirsch I, Mazzoni G, Leal I. Portuguese norms for the Waterloo-Stanford Group C (WSGC) scale of hypnotic susceptibility. Int J Clin Exp Hypn. 2008;56(3):295–305. doi:10.1080/00207144.2012.675299
35. Warmenhoven F, van Rijswijk E, Engels Y, et al. The Beck Depression Inventory (BDI-II) and a single screening question as screening tools for depressive disorder in Dutch advanced cancer patients. Support Care Cancer. 2012;20(2):319–324. doi:10.1007/s00520-010-1082-8
36. Derbyshire SWG, Jones AKP, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73(3):431–445. doi:10.1016/S0304-3959(97)00138-3
37. Homan RW, Herman J, Purdy P. Cerebral location of international 10-20 system electrode placement. Electroencephalogr Clin Neurophysiol. 1987;66(4):376–382. doi:10.1016/0013-4694(87)90206-9
38. Jensen MP, Patterson DR. Hypnotic approaches for chronic pain management clinical implications of recent research findings. Am Psychol. 2014;69(2):167–177. doi:10.1037/a0035644
39. Patterson DR, Jensen MP. Hypnosis and clinical pain. Psychol Bull. 2003;129(4):495–521. doi:10.1037/0033-2909.129.4.495
40. Kaipper MB, Chachamovich E, Hidalgo MPL, da Silva Torres IL, Caumo W. Evaluation of the structure of Brazilian State-trait anxiety inventory using a rasch psychometric approach. J Psychosom Res. 2010;68(3):223–233. doi:10.1016/j.jpsychores.2009.09.013
41. Schestatsky P, Stefani LC, Sanches PR, et al. Validation of a Brazilian quantitative sensory testing (QST) device for the diagnosis of small fiber neuropathies. Arq Neuropsiquiatr. 2011;69:943–948. doi:10.1590/S0004-282X2011000700019
42. Von Baeyer CL, Piira T, Chambers CT, Trapanotto M, Zeltzer LK. Guidelines for the cold pressor task as an experimental pain stimulus for use with children. J Pain. 2005;6:218–227. doi:10.1016/j.jpain.2005.01.349
43. Randell EW, Yenice S. Delta checks in the clinical laboratory. Crit Rev Clin Lab Sci. 2019;56:75–97. doi:10.1080/10408363.2018.1540536
44. Lakshminarayanan MY, Horton R. The general linear model. Technometrics. 1988;30(1):130. doi:10.2307/1270349
45. Caumo W, Deitos A, Carvalho S, et al. Motor cortex excitability and BDNF levels in chronic musculoskeletal pain according to structural pathology. Front Hum Neurosci. 2016;10(2016JULY). doi:10.3389/fnhum.2016.00357
46. Zortea M, Ramalho L, Alves RL, et al. Transcranial direct current stimulation to improve the dysfunction of descending pain modulatory system related to opioids in chronic non-cancer pain: an integrative review of neurobiology and meta-analysis. Front Neurosci. 2019:13. doi:10.3389/fnins.2019.01218.
47. Fuster JM. The Prefrontal Cortex. Elsevier. Epub ahead of print 2008. doi:10.1016/B978-0-12-373644-4.X0001-1
48. Lorenz J, Cross DJ, Minoshima S, Morrow TJ, Paulson PE, Casey KL. A unique representation of heat allodynia in the human brain. Neuron. 2002;35:383–393. doi:10.1016/S0896-6273(02)00767-5
49. Jensen MP, Day MA, Miró J. Neuromodulatory treatments for chronic pain: efficacy and mechanisms. Nat Rev Neurol. 2014;10(3):167–178. doi:10.1038/nrneurol.2014.12
50. Valet M, Sprenger T, Boecker H, et al. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain – an fMRI analysis. Pain. 2004;109(3):399–408. doi:10.1016/j.pain.2004.02.033
51. Dienes Z, Hutton S. Understanding hypnosis metacognitively: RTMS applied to left DLPFC increases hypnotic suggestibility. Cortex. 2013;49:386–392. doi:10.1016/j.cortex.2012.07.009
52. Braulio G, Passos SC, Leite F, et al. Effects of transcranial direct current stimulation block remifentanil-induced hyperalgesia: a randomized, double-blind clinical trial. Front Pharmacol. 2018;9. doi:10.3389/fphar.2018.00094
53. da Graca-tarragó M, Lech M, Angoleri LDM, et al. Intramuscular electrical stimulus potentiates motor cortex modulation effects on pain and descending inhibitory systems in knee osteoarthritis: a randomized, factorial, sham-controlled study. J Pain Res. 2019:209–221. doi:10.2147/JPR.S181019.
54. Thompson T, Terhune DB, Oram C, et al. The effectiveness of hypnosis for pain relief: a systematic review and meta-analysis of 85 controlled experimental trials. Neurosci Biobehav Rev. 2019;99:298–310. doi:10.1016/j.neubiorev.2019.02.013
55. Morin C, Bushnell CM. Temporal and qualitative properties of cold pain and heat pain: a psychophysical study. Pain. 1998;74(1):67–73. doi:10.1016/S0304-3959(97)00152-8
56. Duerden EG, Albanese MC. Localization of pain-related brain activation: a meta-analysis of neuroimaging data. Hum Brain Mapp. 2013. doi:10.1002/hbm.21416
57. Rainville P. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277(5328):968–971. doi:10.1126/science.277.5328.968
58. Powers A, Madan A, Hilbert M, et al. Effects of combining a brief cognitive intervention with transcranial direct current stimulation on pain tolerance: a randomized controlled pilot study. Pain Med. 2018;19:677–685. doi:10.1093/pm/pnx098
59. Mylius V, Jung M, Menzler K, et al. Effects of transcranial direct current stimulation on pain perception and working memory. Eur J Pain. 2012;16(7):974–982. doi:10.1002/j.1532-2149.2011.00105.x
60. Stefani LC, Torres ILDS, De SICC, Rozisky JR, Fregni F, Caumo W. BDNF as an effect modifier for gender effects on pain thresholds in healthy subjects. Neurosci Lett. 2012;514:62–66. doi:10.1016/j.neulet.2012.02.057
61. Cardinal TM, Antunes LC, Brietzke AP, et al. Differential neuroplastic changes in fibromyalgia and depression indexed by up-regulation of motor cortex inhibition and disinhibition of the descending pain system: an exploratory study. Front Hum Neurosci. 2019;13. doi:10.3389/fnhum.2019.00138
62. Almeida TF, Roizenblatt S, Tufik S. Afferent pain pathways: a neuroanatomical review. Brain Res. 2004;1000(12):40–56. doi:10.1016/j.brainres.2003.10.073
63. da Graca-tarragó M, Deitos A, Patrícia Brietzke A, et al. Electrical intramuscular stimulation in osteoarthritis enhances the inhibitory systems in pain processing at cortical and cortical spinal system. Pain Med. 2016;17(5):877–891. doi:10.1111/pme.12930
64. Sorge RE, Totsch SK. Sex differences in pain. J Neurosci Res. 2017;95(6):1271–1281. doi:10.1002/jnr.23841
65. Colby F. An analogue study of the initial carryover effects of meditation, hypnosis, and relaxation using naive college students. Biofeedback Self Regul. 1991;16:157–165. doi:10.1007/BF01000190
66. Fregni F, Nitsche MA, Loo CK, et al. Regulatory considerations for the clinical and research use of transcranial direct current stimulation (tDCS): review and recommendations from an expert panel. Clin Res Regul Aff. 2015;32(1):22–35. doi:10.3109/10601333.2015.980944
67. Freeman PR. The performance of the two-stage analysis of two-treatment, two-period crossover trials. Stat Med. 1989;8(12):1421–1432. doi:10.1002/sim.4780081202
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.