Back to Journals » OncoTargets and Therapy » Volume 12
The hERG1 Potassium Channel Behaves As Prognostic Factor In Gastric Dysplasia Endoscopic Samples
Authors Lastraioli E , Romoli MR, Iorio J, Lottini T, Chiudinelli M , Bencivenga M, Vindigni C , Tomezzoli A, De Manzoni G, Compagnoni B, Manzi I, Messerini L, Saragoni L, Arcangeli A
Received 6 August 2019
Accepted for publication 16 October 2019
Published 7 November 2019 Volume 2019:12 Pages 9377—9384
DOI https://doi.org/10.2147/OTT.S226257
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Elena Lastraioli,1 Maria Raffaella Romoli,1 Jessica Iorio,1 Tiziano Lottini,1 Mariella Chiudinelli,2 Maria Bencivenga,3 Carla Vindigni,4 Anna Tomezzoli,5 Giovanni De Manzoni,3 Bruno Compagnoni,6 Ilaria Manzi,7 Luca Messerini,1 Luca Saragoni,8 Annarosa Arcangeli1
On behalf of Gruppo Italiano di Ricerca Cancro Gastrico (GIRCG)
1Department of Experimental and Clinical Medicine, Section of Internal Medicine, University Of Florence, Florence 50134, Italy; 2Pathology Division, Esine Hospital, ASST della Valcamonica, Esine, BS 25040, Italy; 3Surgery Division, University of Verona, Verona 37134, Italy; 4Pathology Division, Azienda Ospedaliero-Universitaria Senese, Siena 53100, Italy; 5Pathology Division, Borgo Trento Hospital, Verona 37134, Italy; 6Surgery Division, Esine Hospital, ASST della Valcamonica, Esine, BS 25040, Italy; 7Gastroenterology and Endoscopy Unit, Morgagni-Pierantoni Hospital, Forlì 47121, Italy; 8Pathology Division, Morgagni-Pierantoni Hospital, Forlì 47121, Italy
Correspondence: Elena Lastraioli
Department of Experimental and Clinical Medicine, Section of Internal Medicine, University of Florence, Viale GB Morgagni, 50, Florence 50134, Italy
Tel +39 055 2751319
Fax +39 055 2751281
Email [email protected]
Purpose: Gastric cancer (GC) is still a relevant health issue worldwide. The identification of prognostic factors for progression of gastric dysplasia (GD), the main pre-cancerous lesion of the intestinal-type GC, is hence mandatory.
Patients and methods: A cohort of 83 GD endoscopic samples belonging to Italian subjects was collected. hERG1 expression was evaluated by immunohistochemistry and scored 0–3, depending on the percentage of stained cells. Expression data were analysed in conjunction with clinico-pathological and survival data.
Results: hERG1 turned out to be expressed in 67.47% (56 out of 83) of the GD samples. hERG1 expression was higher in high-grade GD compared to low-grade GD (29 out of 39, 74.36% vs 27 out of 44, 61.36%), although the statistical significance was not reached (P=0.246). No association emerged between hERG1 expression and clinical features of the patients (age, gender, localization, H. pylori infection, gastritis and intestinal metaplasia). In a subset of cases for which sequential samples of gastric lesions (from GD to Early Gastric Cancer and Advanced Gastric Cancer) were available, hERG1 expression was maintained in all the steps of gastric carcinogenesis from GD onwards. A general trend to increased expression in advanced lesions was observed. hERG1 score had a statistically significant impact on both Progression-Free Survival (P=0.018) and Overall Survival (P=0.031). In particular, patients displaying a high hERG1 score have a shorter survival.
Conclusion: hERG1 is aberrantly expressed in human GD samples and has an impact on both PFS and OS, hence representing a novel prognostic marker for progression of GD towards GC of the intestinal histotype. Once properly validated, hERG1 detection could be included in the clinical practice, during endoscopic surveillance protocols, for the management of GD at higher risk of progression, as already proposed for Barrett’s oesophagus.
Keywords: Kv11.1, immunohistochemistry, endoscopic surveillance in gastric dysplasia, prognosis, intestinal type gastric adenocarcinoma
Introduction
Gastric cancer (GC) is still a leading cause of death nowadays, and according to the Globocan estimates in 2018, more than 1 million people were diagnosed with GC, with nearly 800,000 deaths associated to such disease (source: Globocan 2018, https://gco.iarc.fr/). In Europe, it has been estimated that in 2018, roughly 143,000 people developed GC, with a high mortality rate (100,000 people) (source: Globocan 2018, https://gco.iarc.fr/). Several risk factors for GC have been identified over years and pre-neoplastic lesions have been described. In 2012, the European Society of Gastrointestinal Endoscopy issued guidelines to be followed for the management of gastric precancerous lesions. According to these guidelines, surveillance and treatment are recommended for subjects with chronic gastritis, atrophy, intestinal metaplasia and dysplasia; for these conditions, a screening of the general population is not necessary.1 Gastric dysplasia (GD) is defined as a pre-neoplastic lesion for intestinal-type GC and it is associated to a higher risk, especially in case of high-grade lesions. GD arises due to abnormalities accumulated by the cells of the gastric lining mucosa. GD might be classified into two types: low-grade (LGD) and high-grade dysplasia (HGD) according to the level of disorganization, nuclear and architectural atypia.2,3 In LGD, cells grow slowly, lesions can regress and the risk of cancer progression is low; on the other hand, in HGD, the abnormal cells have several atypical features, grow quickly and the risk of progressing to GC is high: 0–23% for LGD and 60–85% for HGD.1 Due to the intra- and inter-observer differences, the assessment of the grade of GD might be highly subjective, and although recently the molecular profile of gastric pre-neoplastic lesions has been partially elucidated, no markers useful to discriminate between hyperplasia and LGD or between HGD and early tumours are currently available.4
Moreover, the management and treatment of the two types of GD are different due to their diverse associated risk to progress to GC; therefore, the identification of progression markers is strongly needed.
Among novel potential biomarkers, ion channels and transporters have been described to be altered in several tumours and regulate different cellular processes (reviewed in Ref. 5).
hERG1 (also named Kv11.1 or KCNH2), a potassium channel encoded by KCNH2 gene, is not expressed by normal non-excitable tissues as well as hyperplastic lesions of colon and endometrium.6,7 On the other hand, hERG1 is expressed in pre-neoplastic lesions of the oesophagus (Barrett’s oesophagus, BO),8 and more recently, we showed that its expression increases during progression from BO to adenocarcinoma.9 More strikingly, a statistically significant association between hERG1 and risk of progression to adenocarcinoma was found.9
The aim of the present paper was to evaluate hERG1 expression in gastric dysplasia and to search for association with clinico-pathological features and follow-up.
Materials And Methods
Tissue Collection
Eighty-three gastric dysplasia samples were retrieved from the archives of different Italian institutions (Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy; Pathology Division, Esine Hospital, ASST della Valcamonica, Italy; Pathology Division, Azienda Ospedaliero-Universitaria Senese, Siena, Italy; Pathology Division, Borgo Trento Hospital, Verona, Italy; Pathology Division, Morgagni-Pierantoni Hospital, Forlì, Italy). The study was approved by the local ethical committee following current guidelines about retrospective observational studies in biological samples, and for each patient, a written informed consent was obtained.
Diagnosis and histological grading were assessed using standard criteria by experienced pathologists in each institution (LM, MC, CV, AT and LS).
For 76 patients, detailed follow-up information were available. Moreover, for 7 patients whose lesions progressed towards malignancy, slides of the gastric adenocarcinoma were also evaluated.
Immunohistochemistry (IHC)
IHC was performed as previously reported6 using an anti-hERG1 Monoclonal antibody (MCK Therapeutics, Florence, Italy). Briefly, sections were dewaxed, dehydrated and incubated with 1% H2O2 solution in PBS to block endogenous peroxidases’ activity. Antigen retrieval was performed with Proteinase K (5 μg/mL) for 5 mins at 37°C and sections were then treated with a blocking solution (Ultra V Block, LabVision; Fremont CA, USA). Samples were incubated overnight at 4°C and the following day immunostaining was carried out with a commercially available kit (PicTure Max kit, Invitrogen; Carlsbad CA, USA).
Scoring Assessment
hERG1 expression was estimated as the percentage of positive cells. Samples were classified into four groups according to the percentage of positive cells: 0% (addressed as “0”), 1–25% (addressed as “Score 1”), 26–49% (addressed as “Score 2”) and >50% (addressed as “Score 3”) as previously reported.10 Slides were analysed field by field from top left to bottom right, under 40x magnification by two independent investigators (EL and MRR).
Statistical Methods
Data were analysed using the statistical softwares Stata 9.1 (StataCorp, College Station, TX, USA) and SAS 9.4 (SAS Institute Inc, Cary, NC, USA). Presence of association between hERG1 expression and demographic, clinical and biological characteristics was evaluated by Fisher’s exact test or Chi-square test, as appropriate. In any case, a two-sided P value ≤ 0.05 was considered significant.
hERG1 expression was investigated for its impact on Progression-Free Survival (PFS) and Overall Survival (OS). PFS was defined as the time between diagnosis and progression of the disease or last follow-up, OS was defined as the time between diagnosis and death, whatever the cause. Observation time of patients alive at the last follow-up was censored. PFS and OS were estimated by Kaplan–Meier method and hazard ratios (with the corresponding 95% CI) were calculated by Cox regression model.
Results
hERG1 Channel Is Expressed In Gastric Dysplasia Samples
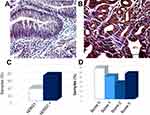
hERG1 potassium channels were found to be expressed in GD samples with variable intensity of staining and percentage of positive cells (Figure 1). In the representative pictures reported in Figure 1, some samples showed a quite intense staining (Figure 1B) while in other samples (Figure 1A), the protein was present at lower intensity.
Overall, hERG1 channels turned out to be expressed in a high percentage of GD samples (67.47%, 56 out of 83) (Figure 1C). When subdividing the samples according to hERG1 score (described in Materials and Methods), a variable distribution was observed (Figure 1D).
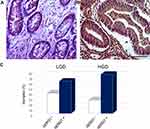
When GD samples were analysed according to the grade of dysplasia, it emerged that hERG1 was expressed in a higher percentage of HGD with respect to LGD (29 out of 39, 74.36% vs 27 out of 44, 61.36%), although no statistically significant difference emerged neither considering positive vs negative samples or subdividing the samples according to hERG1 scoring (P=0.246 and P=0.650, respectively) (Figure 2).
Association With Clinico-Pathological Features
A statistical analysis was performed to search for eventual association between hERG1 expression, demographic parameters and clinico-pathological features.
No association emerged when analysing age, gender, localization and grade of dysplasia according to hERG1 expression.
In a subset of patients for whom additional data were available, we also evaluated eventual associations between hERG1 expression and H. pylori infection, gastritis, intestinal metaplasia: no statistically significant association emerged from such analysis. The same analyses were also performed considering hERG1 scoring and similar results were obtained (not shown).
All the data obtained through statistical analysis are summarised in Table 1.
 |
Table 1 hERG1 Scoring Association With General Parameters And Clinico-Pathological Features |
Association With Progression And Survival
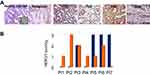
We analysed 7 patients for which sequential lesions were available. In the pictures shown in Figure 3A, slides belonging to a representative patient are reported.
hERG1 expression was absent in gastric healthy mucosa, with the exception of oxyntic mucosa (see inset), confirming what already published by our group;10 in metaplasia, a certain degree of hERG1 positivity was observed, confirming what already published in Barrett’s oesophagus.8,9 The expression of the channel is maintained throughout all the steps of gastric carcinogenesis from LGD and HGD to Early Gastric Cancer (EGC) and Advanced Gastric Cancer (AGC). In Figure 3B, a graph bar summarizing the results of the evaluation of hERG1 scoring in 7 patients for whom samples of the sequential lesions were available. As it can be observed, in 3/7 patients hERG1 scoring was higher in cancers than in GD, for one patient the scoring was equal in both lesions and for 3/7 patients hERG1 scoring was higher in GD than in cancer.
The median follow-up of the patients was 24 months and it emerged that hERG1 scoring had an impact on both PFS and OS. In particular, when analysing PFS, it emerged that patients with higher hERG1 expression have a shorter PFS both considering hERG1 scoring (Figure 4A) and hERG1 positive vs negative samples (Figure 4B) (P=0.018 and P=0.003, respectively). When analysing OS, statistically significant association emerged both considering hERG1 scoring (P=0.031) (Figure 4C) and subdividing the samples in only two classes using as a cut-off 25% of positive cells (P=0.006) (Figure 4D). In particular, patients displaying a high hERG1 scoring (≥25%, corresponding to Score 2 and 3) have shorter survival in terms of both PFS and OS (Figure 4) and are therefore at higher risk (Table 2).
 |
Table 2 Results Of The Univariate PFS And OS Analyses. |
These results are in accordance with that obtained analysing progression (identified by the clinicians during follow up visits) according to hERG1 scoring (P=0.017, Table 3), although in this case, no statistically significant results were obtained when considering just two categories (Negative vs Positive samples, P=0.327).
 |
Table 3 Results Of Chi-Square Test, Evaluating Follow-Up According To hERG1 Scoring |
Discussion
Gastric dysplasia represents the main pre-neoplastic lesion of the human stomach, and the estimation of risk to progression for this group of patients is still a great challenge.
In this manuscript, we provide evidence that hERG1 channels are overexpressed in human GD samples and are associated to an increased risk. It is well known that hERG1 is expressed both in GC cell lines11,12 and primary tumours.10,13,14
Mounting evidence has been gathered concerning hERG1 channels (and potassium channels in general) expression and role in solid tumours comprising GC (reviewed in Refs.15 and 16), but little has been reported in preneoplastic lesions.
We showed long ago that hERG1 channels are expressed in Barrett’s oesophagus,8 and more recently, we demonstrated that they represent novel biomarkers of oesophageal tumour progression.9 A similar scenario seems to apply to GC progression, and in the present paper, we provided evidence that i) hERG1 are expressed in GD samples; ii) hERG1 channels are expressed in a higher percentage of HGD with respect to LGD; iii) hERG1 channels are associated to the disease progression; iv) hERG1 expression is maintained during all the phases of the cancerogenetic process starting from intestinal metaplasia; and v) high hERG1 expression is associated to poorer progression-free and overall survival.
This last point is in accordance with our observation obtained in other tumours such as oesophageal adenocarcinoma,9 pancreatic ductal adenocarcinomas,17 and clear cell renal carcinomas.18 As in oesophageal adenocarcinomas, the identification of at-risk patients is a quite challenging issue, but it is mandatory since GC prognosis is still very poor.
Conclusion
Overall, the detection of hERG1 expression in gastric dysplastic lesions could represent a novel prognostic marker of progression towards gastric adenocarcinoma of the intestinal histotype.
Moreover, hERG1 detection could be also exploited for diagnostic purposes in clinical practice for high-risk GD management, such as endoscopic surveillance protocols, as proposed for oesophageal tumours.9 Nevertheless, it is important to stress out that these results represent a pilot study and confirmation on bigger cohorts of patients is warranted.
Acknowledgment
The authors thank Dr Emanuela Scarpi (Biostatistics and Clinical Trials Unit, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola (FC), Italy) for help and suggestions about statistical analysis.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy. 2012;44(1):74–94. doi:10.1055/s-0031-1291491
2. Kelly PJ, Lauwers GY. Clinical guidelines: consensus for the management of patients with gastric polyps. Nat Rev Gastroenterol Hepatol. 2011;8(1):7–8. doi:10.1038/nrgastro.2010.187
3. Sung JK. Diagnosis and management of gastric dysplasia. Korean J Intern Med. 2016;31(2):201–209. doi:10.3904/kjim.2016.021
4. Fassan M, Baffa R, Kiss A. Advanced precancerous lesions within the GI tract: the molecular background. Best Pract Res Clin Gastroenterol. 2013;27(2):159–169. doi:10.1016/j.bpg.2013.03.009
5. Lastraioli E, Iorio J, Arcangeli A. Ion channel expression as promising cancer biomarker. Biochim Biophys Acta. 2015;1848(10Pt B):2685–2702. doi:10.1016/j.bbamem.2014.12.016
6. Lastraioli E, Bencini L, Bianchini E, et al. hERG1 channels and Glut-1 as independent prognostic indicators of worse outcome in stage I and II colorectal cancer: a pilot study. Transl Oncol. 2012;5(2):105–112. doi:10.1593/tlo.11250
7. Cherubini A, Taddei GL, Crociani O, et al. HERG potassium channels are more frequently expressed in human endometrial cancer as compared to non-cancerous endometrium. Br J Cancer. 2000;83(12):1722–1729. doi:10.1054/bjoc.2000.1497
8. Lastraioli E, Taddei A, Messerini L, et al. hERG1 channels in human esophagus: evidence for their aberrant expression in the malignant progression of Barrett’s esophagus. J Cell Physiol. 2006;209(2):398–404. doi:10.1002/(ISSN)1097-4652
9. Lastraioli E, Lottini T, Iorio J, et al. hERG1 behaves as biomarker of progression to adenocarcinoma in Barrett’s esophagus and can be exploited for a novel endoscopic surveillance. Oncotarget. 2016;7(37):59535–59547. doi:10.18632/oncotarget.11149
10. Crociani O, Lastraioli E, Boni L, et al. hERG1 channels regulate VEGF-A secretion in human gastric cancer: clinicopathological correlations and therapeutical implications. Clin Cancer Res. 2014;20(6):1502–1512. doi:10.1158/1078-0432.CCR-13-2633
11. Lastraioli E, Gasperi Campani F, Taddei A et al. hERG1 channels are overexpressed in human gastric cancer and their activity regulates cell proliferation: a novel prognostic and therapeutic target?
12. Shao XD, Wu KC, Hao ZM, Hong L, Zhang J, Fan DM. The potent inhibitory effects of cisapride, a specific blocker for human ether-a-go-go-related gene (HERG) channel, on gastric cancer cells. Cancer Biol Ther. 2005;4(3):295–301. doi:10.4161/cbt.4.3.1500
13. Shao XD, Wu KC, Guo XZ, Xie MJ, Zhang J, Fan DM. Expression and significance of HERG protein in gastric cancer. Cancer Biol Ther. 2008;7(1):45–50. doi:10.4161/cbt.7.1.5126
14. Ding XW, Yang WB, Gao S, et al. Prognostic significance of hERG1 expression in gastric cancer. Dig Dis Sci. 2010;55(4):1004–1010. doi:10.1007/s10620-009-0834-0
15. Lastraioli E, Lottini T, Bencini L, Bernini M, Arcangeli A. hERG1 potassium channels: novel biomarkers in human solid cancers. Biomed Res Int. 2015;2015:896432. doi:10.1155/2015/896432
16. Comes N, Serrano-Albarrás A, Capera J, et al. Involvement of potassium channels in the progression of cancer to a more malignant phenotype. Biochim Biophys Acta. 2015;1848(10Pt B):2477–2492. doi:10.1016/j.bbamem.2014.12.008
17. Lastraioli E, Perrone G, Sette A, et al. hERG1 channels drive tumour malignancy and may serve as prognostic factor in pancreatic ductal adenocarcinoma. Br J Cancer. 2015;112(6):1076–1087. doi:10.1038/bjc.2015.28
18. Lastraioli E, Pillozzi S, Mari A, et al. hERG1 and CA IX expression are associated with disease recurrence in surgically resected clear cell renal carcinoma. Eur J Surg Oncol.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.