Back to Journals » Patient Preference and Adherence » Volume 16
The Hemophilia Gene Therapy Patient Journey: Questions and Answers for Shared Decision-Making
Authors Wang M , Negrier C, Driessler F, Goodman C, Skinner MW
Received 24 December 2021
Accepted for publication 13 May 2022
Published 9 June 2022 Volume 2022:16 Pages 1439—1447
DOI https://doi.org/10.2147/PPA.S355627
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Michael Wang,1,* Claude Negrier,2,* Frank Driessler,3,* Clifford Goodman,4,* Mark W Skinner5,6,*
1University of Colorado Anschutz Medical Campus, Aurora, CO, USA; 2National Reference Center for Haemophilia, Louis Pradel Cardiology Hospital, University of Lyon, Lyon, France; 3Bayer, Basel, Switzerland; 4The Lewin Group, Falls Church, VA, USA; 5Institute for Policy Advancement Ltd, Washington, DC, USA; 6McMaster University, Hamilton, ON, Canada
*These authors contributed equally to this work
Correspondence: Frank Driessler, Bayer AG, Peter Merian Straße 84, Basel, CH-4052, Switzerland, Tel +41 79 422 32 18, Email [email protected]
Purpose: The anticipated emergence of hemophilia gene therapy will present people with hemophilia (PWH) and treating clinicians with increasingly complex treatment options. It will be critical that PWH and their families be empowered to participate fully in decision-making through transparent communication and the development of targeted educational resources.
Methods: The Council of Hemophilia Community (CHC) convened across a series of roundtable meetings to define the patient journey for hemophilia gene therapy, and to develop a question-and-answer style resource to guide discussion between healthcare professionals (HCPs) and their patients. Patient groups were also consulted during the development of this tool.
Results: The CHC defined 5 key stages in the hemophilia gene therapy patient journey: pre-gene therapy (information-seeking and decision-making), treatment initiation, short- and long-term post-gene therapy follow-up. PWH will have different questions and concerns at each stage of their journey, which should be discussed with their HCP to aid decision-making. The resulting patient journey infographic and Q&A resource (see Supplementary Materials) has been developed for HCPs and PWH to provide a novel and practical roadmap of key issues and considerations throughout all stages.
Conclusion: These resources support a collaborative, patient-centric, shared decision-making approach to inform treatment decision discussions between HCPs and PWH. The value of such discussions will be influenced by the language adopted; health literacy is a particularly important consideration, and these discussions should be accessible and tailored to PWH. HCPs and PWH can benefit from awareness of the common questions and uncertainties as they progress together along the patient journey. While the contents of this article are specific to hemophilia gene therapy, the concepts developed here could be adapted to aid patients in other disease states.
Keywords: hemophilia, patients, HCP guidance, health literacy, disease awareness, treatment landscape
Introduction
Limited literature is available that combines the concepts of the patient journey in hemophilia gene therapy with corresponding life stages. As clinical developments to address the unmet need in hemophilia advance there is also a great need to address the educational and clinical decision-making challenges that will follow market authorization. Herein, we provide a new, interactive, question and answer (Q&A) PDF resource (see Supplementary Materials) for shared decision-making along the patient journey.
The Unmet Need in Hemophilia
Hemophilia is a rare X-linked recessive genetic disease, with an estimated prevalence of 17.1 and 3.8 cases per 100,000 males worldwide for hemophilia A and B, respectively.1 The current standard of care for severe hemophilia, in countries where it is available, is prophylaxis with factor or non-factor replacement therapy, which aims to prevent bleeds and greatly improves patient outcomes and quality of life.2 However, people with hemophilia (PWH) remain at increased risk of bleeds (including joint and post-surgical) as well as hematomas (and ensuing synovitis and joint disease)3 and life-threatening intracerebral hemorrhage.4,5
As single-gene disorders, hemophilia A and B are promising candidates for gene therapy. Introduction of the missing F8 (hemophilia A) or F9 (hemophilia B) gene aims to deliver a potentially long-term, functionally curative treatment by restoring coagulation factor production to clinically effective levels. Simultaneous goals include ensuring no vector- or transgene-related toxicity and broad patient eligibility across age groups and in patients with inhibitors.6–8 Gene therapy can therefore offer a new therapeutic option within this treatment landscape, to fit the needs of each individual.
Different gene therapy products are likely to meet varied unmet needs of PWH. While several hemophilia gene therapy clinical trials are in progress, no product had been approved at the time of publication.8 Among the approaches in development, adeno-associated viral (AAV) gene transfer solutions are the most advanced in clinical trials, though these products have different constructs, serotypes, promoter/enhancer regions, and manufacturing methods that will affect patient eligibility and choice.8,9
Defining the Patient Journey
The gene therapy patient journey extends throughout all life stages for PWH–from birth/diagnosis through to later adulthood–with different checkpoints corresponding to patient life stages. The constantly evolving treatment landscape involves numerous decisions that require guidance and support from healthcare professionals (HCPs). The patient journey for PWH undergoing gene therapy in particular can raise common concerns and experiences, eg, understanding benefits, risks, adverse events, impact on quality of life, and eligibility.10 Therefore, full transparency and personalized information about the existing knowledge, therapeutic options, and unmet needs are essential to empower PWH to make informed decisions before, during, and after treatment.7,10–12
Patient Centricity and Shared Decision-Making in Clinical Practice
Current Gaps and Limitations
Patient engagement and personalized care are gaining attention in medication development, validation, and adoption, beyond the more traditional emphases on safety, efficacy, regulatory aspects, and communication with HCPs. PWH are typically instructed about their current and future treatments, rather than engaged in conversations and decision-making on treatment options.13 Often, healthcare materials are written at a technical level in what may be the patient’s second language.14 This has made it difficult for them to access, interpret, and apply health information to their decisions, contributing to inadequate health literacy. PWH and families are also exposed to a multitude of information sources via social media and traditional news channels, which often present incomplete, inaccurate, and contradictory content.15,16 Furthermore, in some countries, direct-to-patient marketing may present an additional confounder to patient comprehension, particularly in vulnerable groups.17 In addition to inadequate patient educational materials, a discordance often exists between the physician’s perception of their patients’ understanding and the patient’s actual comprehension.14,18 This communication failure leads to postponement of treatment decision-making, non-adherence, and poorer health outcomes.14
Appropriate, individualized communication is necessary to facilitate targeted treatment approaches and disease prevention.19 This issue extends beyond patient understanding. Particularly, with emerging therapies and novel mechanisms of action, it is inappropriate to assume that HCPs have adequate understanding of gene therapy.
Proposed Solutions
A complementary approach includes patient centricity, defined as engaging with patients to achieve the best experience and outcomes for the individual and their family.13 The process of shared decision-making can reduce the information and knowledge imbalance, and lead to more personalized treatment decisions, better medical outcomes, treatment adherence, improved quality of life, and decreased costs of treatment and absenteeism.20–22 The introduction of a new option, such as gene therapy, provides opportunities for shared decision-making to ensure patients understand their best, individualized option.22 This type of patient-centric decision-making is relevant to clinical practice and is particularly important for AAV gene therapy due to current limitations on re-treatment via that modality. Importantly, shared decision-making is distinct from informed consent, with a primary difference being the timing and extent of patient involvement.23 Shared decision-making is more than a one-size-fits-all description of the risks and benefits associated with each treatment option in order to meet a legal requirement; patients, and family members where appropriate, should be actively involved in an individually tailored, two-way discussion with their care provider, with patient preferences and concerns at the forefront.23
Appropriate Language in Patient Communication
A key element in a patient-centric approach is dialogue, which must be accessible to PWH and their families according to widely diverse levels of health and medical comprehension, as well as personal circumstances.13,18
PWH who are diagnosed and treated, and families, tend to be well informed about their condition and are committed to its management, including keeping abreast of treatment options.24 However, the evolving treatment landscape poses an array of questions, so it is imperative that PWH have access to health information that they can easily find, understand, and use to inform their decisions.
General recommendations to improve physician–patient communication include: avoiding assumptions of low or high patient health literacy, avoiding jargon and unnecessary detail, implementing the “teach-back” method (reiterating in your own words what was learned) to ensure full patient understanding, and preparing written materials, with only key points and graphics or other visuals where relevant.18 A recent review identified additional variables relevant to shared decision-making in hemophilia treatment, including bleeding phenotype, musculoskeletal status, treatment adherence, venous access, and lifestyle.3
When considering health literacy, it is also important to understand the issues facing PWH enrolling in clinical trials, and the clinical and technical terminologies involved. Physicians and the surrounding care team can play an important role in ensuring that patients achieve clinical trial literacy and are fully informed when making decisions about participation.25 This is also necessary when communicating with patients who have already started their gene therapy journey in clinical practice,11 who may still have outstanding questions or concerns.
The Q&A resource presented here will help to tailor communications and support a patient-centric shared decision-making process.
Aim
The Council of the Hemophilia Community (CHC) convened with the aim of filling an information gap through the development of a resource to support discussions between HCPs and PWH about gene therapy. Here, we summarize the results of focus group discussions conducted by the CHC, which aimed to define the patient journey in hemophilia gene therapy within the different life stages of PWH and create an accompanying Q&A resource for HCPs and PWH. The intention is to support shared decision-making in real-world practice by giving HCPs insight into likely patient questions regarding gene therapy and clinical trial participation.
Methods
Resource Development Strategy
The resources described here are based on expert discussions involving HCPs and patients. The CHC was established in 2019 as a multidisciplinary expert group, formed of independent advisors, HCPs, industry leads, and patient representatives with experience in medicine, research, advocacy, patient support, health economics, data, and healthcare access. The CHC collaborate on behalf of PWH to provide guidance for: 1) overcoming clinical and access challenges in the hemophilia environment; 2) perceived gaps in programs and initiatives; and 3) developing initiatives based on emerging insights.
Over the course of 3 roundtable meetings held between November 2020 and May 2021, the CHC first conducted a process of insight identification to understand the elements of the specific patient journey that could best guide the hemophilia community and facilitate treatment decision-making. The life cycle of PWH was aligned to patient journey stages, based on exchanges of opinion within the interdisciplinary working group. The CHC then identified and refined key questions based on those defined in an early patient questionnaire and by the National Hemophilia Foundation (NHF).26 The selection of these source materials was based on a consensus of expert opinion; no formal literature search was conducted.
Patient questions were then selected and assigned to the patient journey stages and refined by the CHC members during iterative CHC expert workstream discussions using a focus group format. Answers to the questions were refined separately by the authors following multiple rounds of review. The target audience for the developed questions and answers were hematologists and their patients.
Collaboration with Patients
This collaborative development and feedback process engaged all members of the CHC, patient groups, patient representatives, and nurses. In addition, both resources were reviewed by groups of European and US-based patients, caregivers, and parents of PWH (Bayer Global and European Patient Councils for Hemophilia) and the Bayer Hemophilia Employee Council, to seek their insight on the relevance and accessibility of the text, as well as their opinions on the need for any additional questions and answers. All members of the Bayer Global and European Patient Councils groups are PWH or a caregiver of a person with hemophilia. Members of the Bayer Hemophilia Council include Bayer employees with hemophilia. The resultant patient journey roadmap and Q&A resource were shared with the entire CHC for feedback. The authors met regularly to discuss this feedback, and to decide on the content and placement of key questions along the patient journey.
Results
The Patient Journey: Key Stages and Aims
As an initial step in developing the Q&A resource, the CHC undertook defining the patient journey through gene therapy for hemophilia. The CHC has categorized 5 pillars of the patient decision-making journey in the current treatment landscape:
- Pre-gene therapy information seeking
- Pre-gene therapy decision-making
- Treatment initiation
- Short-term post-gene therapy follow-up (≤1 year)
- Long-term post-gene therapy follow-up (>1 year)
The patient journey as mapped onto these 5 pillars is shown in Figure 1.
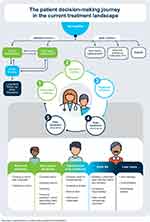 |
Figure 1 The patient decision-making journey. |
Key Themes of the Q&A Resource
The Q&A development process revealed several areas contributing to patient decision-making in hemophilia gene therapy. First, expectations of PWH need to be managed, as the potential benefits and risks of gene therapy are likely to vary among PWH. In addition, the need for a commitment to participating in a long-term registry21,27 should be communicated before gene therapy is initiated. Further explanations of eligibility and effects should be clarified, notably that any gene expression occurs only in somatic cells (not germline cells), hence any effect is not conveyed to future generations.21 The questions should also cover the current unknowns of treatment, including long-term durability, whether the achieved level of FVIII/FIX is sufficient to effectively prevent bleeds, and treatment options in the event that gene therapy does not achieve its therapeutic goals.
Summary of the Q&A Resource
The CHC agreed that questions from PWH concerning gene therapy tend to fall under the following broad categories: treatment regimen/adherence requirements, treatment predictability and variability, treatment durability, and the risk:benefit profile. The full, collated list of questions and answers is presented in the Supplementary Materials. The resource is organized from initial information-seeking to decision-making, treatment initiation and trial information, and short- and long-term follow-up. The reading level of the document is a grade 10–11 (high school) level, based on a Flesch-Kincaid readability test. It is fully referenced, as appropriate, for further context and can serve as a practical tool for the treating physician in shared decision-making. Information to facilitate discussion regarding participation in clinical trials is also included, from eligibility through follow-up. Some of the questions deal with complex queries, such as explaining what gene therapy is and how it works in the body, as well as efficacy and safety.
Additional online resources to support patients considering gene therapy are summarized at the end of the Supplementary Materials.
Discussion
Hemophilia Gene Therapy Awareness
The CHC-defined patient journey and Q&A resource reported here are designed to help to inform HCPs regarding the questions and considerations likely to be encountered by PWH at different life stages, and when discussing gene therapy options. Each patient’s journey will encounter the emergence of new therapeutic options, growing evidence and evolving individual preferences, goals, aspirations, and risk tolerance.
By identifying and providing key information to aid these discussions on gene therapy, this tool will help to address knowledge gaps of HCPs and PWH. A recent global survey sent to patient organizations reported that although most (68%) PWH express that they have a basic understanding of gene therapy, only a small proportion (6%) consider themselves to have an advanced understanding.28 A similar pattern was observed for HCPs, with 44% recording a basic or intermediate understanding, and only 12% reporting an advanced understanding.28 A different international survey of physicians and scientists echoed these results, with 40% of physicians directly in hemophilia care claiming limited ability to discuss gene therapy with their patients.29 As gene therapy emerges, it will be critical to meet the knowledge gaps of HCPs and PWH. Educating physicians, nurses, and the surrounding care teams who care for PWH will better enable them to meet the needs of their patients through constructive and informative discussions.29 Although the majority of PWH report positive opinions of gene therapy, including with respect to such considerations as annual bleeding rates, factor levels, long-term impacts, use of factor replacement, and treatment costs,30–32 many believe that they will not benefit from new products (eg, gene therapy and new factor/non-factor replacement therapies).31,32 Therefore, HCPs will need to educate PWH about all available treatment options as they engage them in shared decision-making.31
HCP-Patient Communication on Gene Therapy Advances
The resources will be shareable during in-person or virtual consultations and available for reference afterwards. In order to maximize the impact of this tool, HCPs should become familiar with appropriate language and educational materials to answer these questions. Results from a qualitative study across a variety of stakeholders suggest that consistency in the language and terminology used when discussing hemophilia can reduce miscommunication and facilitate conversations, promoting informed decision-making. In particular, the need for appropriate terminology to describe gene therapy has been previously highlighted and that it is preferable to discuss “what gene therapy is,” rather than what current treatments “are not.”24 As HCPs will likely become increasingly engaged in the use of gene therapy, their adoption and comfort with the preferred terminology will enhance their communications. The usefulness of the resource provided here should be evaluated by an assessment of publication metrics as well as focus group discussions to understand the uptake of the tool by the hemophilia community.
Lessons on communicating with patients may be learned from past and ongoing miscommunications between the scientific community and the public,33 eg, the harmful misconceptions around antimicrobial resistance or those that arose from the flawed and ultimately retracted report of an association of the measles, mumps and rubella vaccine with autism development.34,35 Poorly delivered or incorrect information, including in clinical trials, can result in a lack of confidence in treatment, unnecessary costs, and discontinuity of care. Transparency is an essential criterion for gaining trust.36 In particular, the need for adequate education of HCPs will be vital for facilitating these conversations, alongside the need to adjust the information and method of delivery to particular care contexts.
Strengths and Limitations
The Q&A resource provided here is a novel, readily available educational tool for HCPs and their patients. Its strengths include an easy-to-follow format, ready for use as a comprehensive shared decision-making resource in the clinic. Moreover, the development of the resource was heavily guided by its intended audience, therefore we hope it proves immediately useful.
The key limitation of the Q&A resource is its reading level (grade 10–11; high school), which is higher than the grade 5–8 level (10–14 years old) at which the average patient comfortably comprehends patient educational materials.37,38 However, this resource is intended for use by HCPs to tailor their delivery of the material appropriately to different patients (for example, by omitting or describing content based on patient knowledge and/or age) and thus the reading level is targeted higher to include relevant details. In addition, while the Q&A resource is intended to guide conversations with PWH, the patient journey starts in infancy and hence will also involve discussions with parents or other caregivers of pediatric patients, who may have additional specific questions. While gene therapy is not currently available to children, advice to parents of pediatric PWH should be provided to ensure full awareness of future considerations.39 The Q&A resource therefore functions as a starting point from which HCPs can individualize discussions. Other concerns, including those of reimbursement and financing, while important, will vary by region and are beyond the scope of this article and Q&A resource. Clinical evaluations to date have been conducted predominantly in high-income countries,40 and therefore, this communication tool is currently limited to patients in those regions. Finally, despite good levels of engagement across key stakeholder groups, the relatively small size and composition of the CHC group could limit the generalizability of these conclusions to the global hemophilia community.
Future Directions
Ideally, the resource would be updated and published as needed to provide the latest relevant information and address any feedback from the community. This work would benefit from even wider stakeholder engagement including surveying a larger, more diverse group of PWH. It would incorporate findings of clinical trials and other research (such as from registries) that would be of potential relevance to decision-making (eg, probability of expressing FVIII, levels of FVIII expression, duration of expression, probability of side effects) and shared and reviewed with patients as it becomes available.41–43
Furthermore, while this resource provides an educational and decision support tool to aid discussions between HCPs and patients, barriers to providing requisite biomedical and clinical education about gene therapy for HCPs (eg, time and travel costs) should also be identified and addressed.29
Conclusions and Key Takeaways
Gene therapy offers a potential alternative to lifelong infusions, with the promise of transformative changes in the lives of PWH7,44 and a major shift within the treatment paradigm.8 The emergence of gene therapy will also add to the complexity of treatment decisions faced by PWH and their families. HCPs will have a crucial role in providing the appropriate, personalized guidance and support to ensure that PWH fully understand the options available to them.
The educational and decision support resources described herein recognize that each patient’s decision journey will evolve throughout their lifetime with their individual preferences at different life stages, and with the emergence of new therapies and a growing evidence base.
The Q&A resource provides HCPs and PWH with timely, relevant information, facilitates discussions, and empowers PWH to engage in shared decision-making. As gene therapy products enter the market, the themes and questions mapped here should stimulate discussion and aid interactions among HCPs, PWH, and family members, to ensure that they are fully informed and realize the clinical potential of this treatment. While the issues discussed here pertain to hemophilia, they could also be applied to other hereditary diseases with multiple treatment options.
Acknowledgments
The authors would like to acknowledge all other members of the Council of the Hemophilia Community for their contributions to this manuscript, particularly in the development of the Q&A resource: Erik Berntorp, Alfonso Iorio, Christoph Königs, Keiji Nogami, Johannes Oldenburg, Brian O’Mahony, Mark Reding, and Dawn Rotellini. This independent group was funded by Bayer. They would also like to thank members of the Bayer Global and European Patient Councils for Hemophilia, as well as Sophie Babicz and Alex Mehuys, and the Bayer Hemophilia Employee Council (particularly Gregory LeCleir) for their insight into the Q&A resource. The authors would like to thank Alexandra Wissle for her support in facilitating the survey. Editorial support (in the form of writing assistance, including development of the initial draft based on author direction, assembling tables and figures, collating authors’ comments, grammatical editing, and referencing) was provided by Ewa Kilinska, of Fishawack Communications Limited, part of Fishawack Health, UK, and was funded by Bayer. The authors would also like to acknowledge Sharon Eastwood, of Bayer, for editorial support during the development of the manuscript.
Disclosure
MW received honoraria for advisory boards from Bayer, BioMarin, Bioverativ, Catalyst Biosciences, CSL Behring, Genentech, Novo Nordisk, and Takeda; CN has received grants/research support/honoraria or consultation fees from Bayer, BioMarin, Catalyst Biosciences, CSL Behring, Freeline, Novo Nordisk, Pfizer, Roche, Sanofi, Sobi, Spark Therapeutics, Takeda, Uniqure; FD is an employee of Bayer; CG is a full-time employee of The Lewin Group, and has been assigned to projects with various life sciences companies, including AbbVie, Alkermes, Amgen, Bayer, BioMarin, bluebird bio, Genentech, GlaxoSmithKline, Janssen, Medtronic, Merck, Mitsubishi Tanabe, and Roche, as well as not-for-profit organizations including Hemophilia Alliance and National Hemophilia Foundation; MS reports grants and personal fees from Bayer, BioMarin, Roche/Genentech, Takeda, Novo Nordisk, and Sanofi; grants from Freeline, UniQure, and Sobi; other from Spark and Pfizer, outside the submitted work; and is a Member of the Institute for Clinical and Economic Review (ICER) Governing Board, Blue Cross Blue Shield Medical Advisory Panel, Consultant for National Hemophilia Foundation and the National Hemophilia Foundation’s Medical and Scientific Advisory Council (MASAC) Member. The authors report no other conflicts of interest in this work.
References
1. Iorio A, Stonebraker JS, Chambost H, et al. Establishing the prevalence and prevalence at birth of hemophilia in males: a meta-analytic approach using national registries. Ann Intern Med. 2019;171(8):540–546. doi:10.7326/M19-1208
2. Srivastava A, Santagostino E, Dougall A, et al. WFH Guidelines for the Management of Hemophilia. Haemophilia. 2020;26(S6):1–158. doi:10.1111/hae.14046
3. Hermans C, Noone D, Benson G, et al. Hemophilia treatment in 2021: choosing the “optimal” treatment using an integrative, patient-oriented approach to shared decision-making between patients and clinicians. Blood Rev. 2021;1:100890. doi:10.1016/j.blre.2021.100890
4. Knobe K, Berntorp E. Haemophilia and joint disease: pathophysiology, evaluation, and management. J Comorbidity. 2011;1(1):51–59. doi:10.15256/joc.2011.1.2
5. Aledort L, Mannucci PM, Schramm W, Tarantino M. Factor VIII replacement is still the standard of care in haemophilia A. Blood Transfus. 2019;17(6):479–486. doi:10.2450/2019.0211-19
6. Mannucci PM. Hemophilia therapy: the future has begun. Haematologica. 2020;105(3):545–553. doi:10.3324/haematol.2019.232132
7. Pipe SW. Delivering on the promise of gene therapy for haemophilia. Haemophilia. 2021;27(S3):114–121. doi:10.1111/hae.14027
8. Batty P, Lillicrap D. Hemophilia gene therapy: approaching the first licensed product. HemaSphere. 2021;5(3). doi:10.1097/HS9.0000000000000540
9. Perrin GQ, Herzog RW, Markusic DM. Update on clinical gene therapy for hemophilia. Blood. 2019;133(5):407–414. doi:10.1182/blood-2018-07-820720
10. Aiyegbusi OL, Macpherson K, Elston L, et al. Patient and public perspectives on cell and gene therapies: a systematic review. Nat Commun. 2020;11(1):6265. doi:10.1038/s41467-020-20096-1
11. Casey GA, Papp KM, MacDonald IM. Ocular gene therapy with adeno-associated virus vectors: current outlook for patients and researchers. J Ophthalmic Vis Res. 2020;15(3):396–399. doi:10.18502/jovr.v15i3.7457
12. Horgan D, Bolanos N, Mastris K, Mendao L, Malats N. Health literacy: read all about it. Biomed Hub. 2017;2(Suppl 1):44–47. doi:10.1159/000481129
13. Yeoman G, Furlong P, Seres M, et al. Defining patient centricity with patients for patients and caregivers: a collaborative endeavour. BMJ Innovations. 2017;3(2):76. doi:10.1136/bmjinnov-2016-000157
14. Safeer RS, Keenan J. Health literacy: the gap between physicians and patients. Am Fam Physician. 2005;72(3):463–468.
15. Carpenter DM, Geryk LL, Chen AT, Nagler RH, Dieckmann NF, Han PKJ. Conflicting health information: a critical research need. Health Expect. 2016;19(6):1173–1182. doi:10.1111/hex.12438
16. Suarez-Lledo V, Alvarez-Galvez J. Prevalence of health misinformation on social media: systematic review. J Med Internet Res. 2021;23(1):e17187. doi:10.2196/17187
17. DeFrank JT, Berkman ND, Kahwati L, Cullen K, Aikin KJ, Sullivan HW. Direct-to-consumer advertising of prescription drugs and the patient-prescriber encounter: a systematic review. Health Commun. 2020;35(6):739–746. doi:10.1080/10410236.2019.1584781
18. Hersh L, Salzman B, Snyderman D. Health literacy in primary care practice. Am Fam Physician. 2015;92(2):118–124.
19. Lea DH, Kaphingst KA, Bowen D, Lipkus I, Hadley DW. Communicating genetic and genomic information: health literacy and numeracy considerations. Public Health Genomics. 2011;14(4–5):279–289. doi:10.1159/000294191
20. Gringeri A, Doralt J, Valentino LA, Crea R, Reininger AJ. An innovative outcome-based care and procurement model of hemophilia management. Expert Rev Pharmacoecon Outcomes Res. 2016;16(3):337–345. doi:10.1080/14737167.2016.1178066
21. Miesbach W, O’Mahony B, Key NS, Makris M. How to discuss gene therapy for haemophilia? A patient and physician perspective. Haemophilia. 2019;25(4):545–557. doi:10.1111/hae.13769
22. Valentino LA, Blanchette V, Negrier C, et al. Personalising haemophilia management with shared decision making. J Haemophilia Practice. 2021;8(1):69–79. doi:10.17225/jhp00178
23. Kunneman M, Montori VM. When patient-centred care is worth doing well: informed consent or shared decision-making. BMJ Qual Saf. 2017;26(7):522–524. doi:10.1136/bmjqs-2016-005969
24. Hart DP, Branchford BR, Hendry S, et al. Optimizing language for effective communication of gene therapy concepts with hemophilia patients: a qualitative study. Orphanet J Rare Dis. 2021;16(1):189. doi:10.1186/s13023-020-01555-w
25. National Academies of Sciences E, and Medicine; Health and Medicine; Division; Board on Population Health and Public Health Practice; Roundtable on Health Literacy; Alexis Wojtowicz and Melissa G. French, Rapporteurs. The National Academies Collection: reports funded by National Institutes of Health. In: French MG, Wojtowicz A, editors. Health Literacy in Clinical Research: Practice and Impact: Proceedings of a Workshop. Washington (DC): National Academies Press (US); 2020.
26. NHF. Frequently Asked Questions. Available from: https://www.hemophilia.org/bleeding-disorders-A-z/treatment/future-therapies/frequently-asked-questions.
27. Laferton JAC, Kube T, Salzmann S, Auer CJ, Shedden-Mora MC. Patients’ expectations regarding medical treatment: a critical review of concepts and their assessment. Front Psychol. 2017;8:233. doi:10.3389/fpsyg.2017.00233
28. Pierce GF, Coffin D. Members of the WFHGTRTPC, Organizing C. The 1st WFH Gene Therapy Round Table: understanding the landscape and challenges of gene therapy for haemophilia around the world. Haemophilia. 2019;25(2):189–194. doi:10.1111/hae.13673
29. Peyvandi F, Lillicrap D, Mahlangu J, et al. Hemophilia gene therapy knowledge and perceptions: results of an international survey. Res Pract Thromb Haemost. 2020;4(4):644–651. doi:10.1002/rth2.12326
30. Iorio A, Skinner MW, Clearfield E, et al. Core outcome set for gene therapy in haemophilia: results of the coreHEM multistakeholder project. Haemophilia. 2018;24(4):e167–e172. doi:10.1111/hae.13504
31. van Balen EC, Wesselo ML, Baker BL, et al. Patient perspectives on novel treatments in haemophilia: a qualitative study. Patient. 2020;13(2):201–210. doi:10.1007/s40271-019-00395-6
32. van Overbeeke E, Michelsen S, Hauber B, et al. Patient perspectives regarding gene therapy in haemophilia: interviews from the PAVING study. Haemophilia. 2021;27(1):129–136. doi:10.1111/hae.14190
33. Sturgis P, Brunton-Smith I, Jackson J. Trust in science, social consensus and vaccine confidence. Nat Human Behav. 2021;5(11):1528–1534. doi:10.1038/s41562-021-01115-7
34. Offit PA, Coffin SE. Communicating science to the public: MMR vaccine and autism. Vaccine. 2003;22(1):1–6. doi:10.1016/s0264-410x(03)00532-2
35. Rush L, Patterson C, McDaid L, Hilton S. Communicating antimicrobial resistance and stewardship in the national press: lessons from sepsis awareness campaigns. J Infect. 2019;78(2):88–94. doi:10.1016/j.jinf.2018.09.001
36. Wise J. Covid-19: how AstraZeneca lost the vaccine PR war. Br Med J. 2021;1:373. doi:10.1136/bmj.n921
37. Oliffe M, Thompson E, Johnston J, Freeman D, Bagga H, Wong PKK. Assessing the readability and patient comprehension of rheumatology medicine information sheets: a cross-sectional Health Literacy Study. BMJ Open. 2019;9(2):e024582. doi:10.1136/bmjopen-2018-024582
38. Stossel LM, Segar N, Gliatto P, Fallar R, Karani R. Readability of patient education materials available at the point of care. J Gen Intern Med. 2012;27(9):1165–1170. doi:10.1007/s11606-012-2046-0
39. Khair K, Steadman L, Chaplin S, Holland M, Jenner K, Fletcher S. Parental perspectives on gene therapy for children with haemophilia: the Exigency study. Haemophilia. 2021;27(1):120–128. doi:10.1111/hae.14188
40. Reiss UM, Zhang L, Ohmori T. Hemophilia gene therapy-New country initiatives. Haemophilia. 2021;27 Suppl 3:132–141. doi:10.1111/hae.14080
41. Kaczmarek R, Pierce GF, Noone D, O’Mahony B, Page D, Skinner MW. Eliminating Panglossian thinking in development of AAV therapeutics. Mol Ther. 2021;29(12):3325–3327. doi:10.1016/j.ymthe.2021.10.025
42. Pierce GF. Uncertainty in an era of transformative therapy for haemophilia: addressing the unknowns. Haemophilia. 2021;27(S3):103–113. doi:10.1111/hae.14023
43. Pierce GF, Kaczmarek R, Noone D, O’Mahony B, Page D, Skinner MW. Gene therapy to cure haemophilia: is robust scientific inquiry the missing factor? Haemophilia. 2020;26(6):931–933. doi:10.1111/hae.14131
44. Rosen S, Tiefenbacher S, Robinson M, et al. Activity of transgene-produced B-domain–deleted factor VIII in human plasma following AAV5 gene therapy. Blood. 2020;136(22):2524–2534. doi:10.1182/blood.2020005683
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
