Back to Journals » Clinical Interventions in Aging » Volume 17
The Frail Depressed Patient: A Narrative Review on Treatment Challenges
Authors Aprahamian I , Borges MK , Hanssen DJC, Jeuring HW, Oude Voshaar RC
Received 11 April 2022
Accepted for publication 16 June 2022
Published 22 June 2022 Volume 2022:17 Pages 979—990
DOI https://doi.org/10.2147/CIA.S328432
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Ivan Aprahamian,1,2 Marcus K Borges,3 Denise J C Hanssen,2 Hans W Jeuring,2 Richard C Oude Voshaar2
1Group of Investigation on Multimorbidity and Mental Health in Aging (GIMMA), Geriatrics Division, Department of Internal Medicine, Jundiaí Medical School, Jundiaí, Brazil; 2University of Groningen, University Medical Center Groningen, Department of Psychiatry, Groningen, the Netherlands; 3Federal University of Paraná, Department of Psychiatry, Curitiba, Brazil
Correspondence: Ivan Aprahamian, Group of Investigation on Multimorbidity and Mental Health in Aging (GIMMA), Division of Geriatrics, Department of Internal Medicine, Jundiaí Medical School, Jundiaí, Brazil, Email [email protected]
Abstract: Although the public importance of frailty is widely acknowledged by the World Health Organization, physical frailty is still largely neglected in geriatric mental health care. Firstly in this narrative review, we summarize the knowledge on the epidemiology of the association between depression and frailty, whereafter implications for treatment will be discussed. Even though frailty and depression have overlapping diagnostic criteria, epidemiological studies provide evidence for distinct constructs which are bidirectionally associated. Among depressed patients, frailty has predictive validity being associated with increased mortality rates and an exponentially higher fall risk due to antidepressants. Nonetheless, guidelines on the treatment of depression neither consider frailty for risk stratification nor for treatment selection. We argue that frailty assessment enables clinicians to better target the pharmacological and psychological treatment of depression as well as the need for interventions targeting primarily frailty, for instance, lifestyle interventions and reduction of polypharmacy. Applying a frailty informed framework of depression treatment studies included in a meta-analysis reveals that the benefit–harm ratio of antidepressants given to frail depressed patients can be questioned. Nonetheless, frail-depressed patients should not withhold antidepressants as formal studies are not available yet, but potential adverse effects should be closely monitored. Dopaminergic antidepressants might be preferable when slowness is a prominent clinical feature. Psychotherapy is an important alternative for pharmacological treatment, especially psychotherapeutic approaches within the movement of positive psychology, but this approach needs further study. Finally, geriatric rehabilitation, including physical exercise and nutritional advice, should also be considered. In this regard, targeting ageing-related abnormalities underlying frailty that may also be involved in late-life depression such as low-grade inflammation might be a promising target for future studies. The lack of treatment studies precludes firm recommendations, but more awareness for frailty in mental health care will open a plethora of alternative treatment options to be considered.
Keywords: frailty, depression, depressive disorder
Introduction
Depression is a debilitating condition in older adults. In the geriatric population, depression is associated with a lower level of wellbeing and quality of life, functional decline, cognitive impairment, premature nursing home admission, and even premature death by suicide or due to chronic somatic diseases.1 These negative health consequences are associated with a depressive disorder according to criteria of diagnostic classification systems like the Diagnostic and Statistical Manual of Mental Disorder (DSM-5) and the International Classification of Diseases (ICD-11) as well as to clinically relevant depressive symptoms (CRDS) measured by self-reported rating scales. Diagnosing depression may become increasingly difficult in later life as psychological symptoms such as low mood or feeling guilty may be expressed less explicitly, while the somatic-affective symptoms of depression symptoms may be difficult to differentiate from somatic multimorbidity.2 Indeed, a recent review of depression rating scales found that overreliance on somatic items overestimates the prevalence of depression due to a higher prevalence of somatic diseases in later life.3 As outlined in this review, the prevalence of depression may also be overestimated among frail elderly.
Frailty is a condition of loss of homeostasis due to multiple system dysregulation yielding to a lower biological reserve against different forms of stressors.4 Frailty predicts future adverse health outcomes, like falls and fractures, physical disability, restricted activities of daily living, hospitalization, and in particular mortality.5 Although operational definitions of frailty are still a matter of debate, geriatric societies generally agree that biomedical frailty is clinically characterized by diminished strength, endurance, and a reduced physiologic function of several organ systems.6
Since symptoms and signs of frailty partly overlap with those of depression, the assessment of frailty in depression, or inversely, depression in frailty, is challenging. Nonetheless, unraveling this Gordian Knot systematically is critical to guide clinical practice and relevant regarding the progressively aging population worldwide. The objectives of this narrative review are twofold. First, we will summarize the knowledge on the epidemiology of the association between depression and frailty. We will build a case on why assessment of frailty within the context of depression is valid and clinically relevant, despite being mutual confounding. Second, we will formulate treatment rationales for depression in the presence of frailty based on the limited and often indirect evidence available.
Epidemiology
Depression
The pooled prevalence rate of major depressive disorder was estimated at 1.8% for community-dwelling adults aged ≥55 years in one meta-analysis,7 and at 7.2% when combining community-dwelling and institutionalized older adults aged ≥75 years in another.8 These two meta-analyses found a pooled prevalence rates of clinically relevant depressive symptoms at 13.5% and 17.1%, respectively.7,8 When interpreting these numbers, we should keep in mind that clinically relevant depressive symptoms simply rely on a cut-off score of a (generally self-report) depression rating scale that weighs all symptoms and signs similarly. Thus, one can imagine that the presence of frailty may result in an overestimation of clinically relevant depressive symptoms in the oldest outpatients, although this has not been evaluated empirically yet. In contrast, depressive disorder can only be diagnosed when the core symptoms of depression, being a low mood or lack of interest (anhedonia), are present in addition to a minimum number of secondary depressive symptoms and when the patient is functionally impaired or significantly distressed. Classification systems (DSM-5, ICD-11) state that a symptom or sign only qualifies as a criterion for depression when this symptom or sign cannot be explained by an underlying somatic condition. This rule prevents diagnostic overlap between depressive disorder and frailty, but in clinical practice it can be very challenging, for instance, to assign a symptom like fatigue to either depression or frailty.
Frailty
Over the past two decades, a plethora of frailty assessment instruments has been developed based on different conceptual models of frailty. Within the geriatric literature there is an increasing preference to assess frailty either through the frailty phenotype criteria (Fried’s criteria) or the frailty index.9 The frailty phenotype criteria is a syndromic approach in which a person is considered frail when at least three out of five criteria are met, ie, unwanted weight loss, muscle weakness, slowness, exhaustion, and a low activity level.10 According to the frailty index, which is based on the deficit accumulation model, a person is frail when at least 25% out of a list of ≥30 potential health deficits are present.11 Since the frailty index is the proportion of potential health deficits that are present (theoretically ranging from 0 to 1), the frailty index is a dimensional scale assessing frailty severity which enables to evaluate frailty trajectories over time. Among community-dwelling older people aged ≥50 years, the prevalence of frailty has been estimated at 12% when operationalized according to the Fried criteria and at 24% when operationalized according to the frailty index (10).12 Despite the different frailty operationalizations, all frailty definitions predict mortality over and above chronological age, the most widely used validity measure.
Frailty-Depression Association
About 10 years ago, epidemiologists had already pointed to similarities between many of the symptoms, consequences and risk factors for frailty and depression in older persons.13 Nonetheless, both constructs can be disentangled in large population-based cohort studies. Within the Baltimore Epidemiologic Catchment Area Study, confirmatory latent class analysis including the frailty phenotype criteria and the DSM-criteria for a depressive disorder supported the separation of frailty and depression as distinct latent constructs, as opposed to a single construct.14 Notwithstanding, classifying people according to depression criteria (2.9% severe depressed, 19.4% mildly depressed, 77.7% not depressed) and to frailty criteria (21.1% frail, 78.9% not frail) resulted in highly overlapping classes, with a kappa value of 0.66 (95% CI: 0.57–0.74).14 Moreover, all persons classified as severely depressed were also classified as being frail.14 In the Health and Retirement Study, confirmatory factor analysis of either depression criteria or frailty criteria confirmed the presence of both dimensions.15 Nonetheless, depression severity highly correlated with frailty severity, regardless of whether based on frailty phenotype parameters (ρ=0.68) or the frailty index (ρ=0.70).15 When the factor analyses were repeated but accounted for shared symptoms, ie, depression criteria were allowed to load on the frailty dimension, and vice versa, the correlations weakened somewhat (frailty phenotype parameters: ρ=0.45; frailty index: ρ=0.56).15 Altogether, these initial findings show that frailty and depression are distinct but overlapping constructs that can be difficult to distinguish in clinical practice.
After these initial studies, meta-analyses of cross-sectional comorbidity rates have shown that among frail older adults, the pooled prevalence of depression was 38.6%, while inversely among depressed older persons the prevalence of frailty was 40.4%.16 The high comorbidity rates between frailty and depression may be partly explained by confounding, as only 3 of the 24 studies included had diagnosed depression according to formal diagnostic criteria.16 Within samples of depressed patients seeking mental health treatment, about one in four patients meet the frailty phenotype criteria for being frail.17,18 Nevertheless, another systematic review showed that the risk of being frail secondary to depression was consistent across study methodology and not conditional on specific depression criteria, frailty criteria, or covariate adjustment.19
Causality
Longitudinal studies investigating the causal relationship between depression and frailty in the literature are scarce (n = 12), as shown in Table 1.20–32
 |
Table 1 Longitudinal Studies on Relationship Between Depression and Frailty |
The seven longitudinal studies with the highest methodological quality found that depressed older adults were approximately 2 to 3 times more likely to develop frailty than their non-depressed counterparts over a 3 to 5-year follow-up.20–26 These findings were independent of the exact operationalization of frailty, being mainly the frailty phenotype criteria, but also the frailty index24 and other frailty instruments.22,24,25 Nonetheless, only one of these studies assessed depressive disorder based on a well-validated semi-structured diagnostic interview,24 whereas the other studies only used screening scales. On the other hand, a large study with a 3-year follow-up found a significant dose-response relationship between severity of depressive symptoms and incidence of frailty (OR = 2.2 for severe symptoms, and OR = 1.3 for moderate symptoms), giving further credential for a causal association.20
Acknowledging potential (long-term) side-effect of antidepressants, two of these studies also evaluated the association between antidepressant treatment and the onset of frailty.20,22 In the Women’s Health Initiative Observational Study, the incidence of frailty was significantly higher among non-depressed antidepressant drug users (OR = 1.7 [95% CI: 1.4–2.1]), depressed patients not using antidepressants (OR = 2.1 [95% CI: 1.8–2.5]), and highest among depressed ones, antidepressant users (OR = 3.6 [95% CI: 2.4–5.5]) when compared to a reference group with no depression and no antidepressant drug use.20 Furthermore, this risk differed between the type of antidepressants with an OR of 1.5 (95% CI: 1.2–2.0) for tricyclic agents, of 1.9 (95% CI: 1.4–1.5) for selective serotonin reuptake inhibitors (SSRIs), and 2.9 (95% CI: 1.9–4.7) for the combination of two or more agents, whereas duration of antidepressant drug use had no impact on the risk for frailty.20 Among psychiatric outpatients, depression and monotherapy with SSRIs were significantly associated with frailty at baseline (OR = 2.8 [95% CI: 1.7–4.7]) as well as at a 12-month follow-up (OR = 2.8 [95% CI: 1.8–4.1]).22
On the other hand, six studies have examined whether frail community-dwelling older adults are exposed to a higher risk of becoming depressed.27–32 Frail older adults were 2 to 4 times more likely to develop clinically significant depressive symptoms over a 2 to 4-year follow-up, although one study did not find any association.31 Studies were rather homogeneous regarding frailty and depression assessment as all five studies had assessed frailty according to the frailty phenotype criteria and all used a self-report depression screening scale (eg, Geriatric Depression Scale).27–29,31,32 Only one of these studies had a priori excluded antidepressant users to prevent reverse causation.27
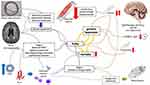
In summary, it can be concluded that frailty and depression are bidirectionally associated over time. The pathophysiologic mechanisms underlying this reciprocal association, however, remain complex and difficult to trace back to linear causality patterns (Figure 1). Most, if not all, physiological or molecular biomarkers associated with frailty have also been linked to depressive disorder in middle-aged and older persons, in particular immune-metabolic dysregulation, autonomic nervous system dysregulation, dopamine depletion, and mitochondrial dysfunction.33,34 Also, these phenomena seem to be related to complex bidirectional interactions, which are beyond the scope of the present review.
Predictive Validity
Overlapping diagnostic criteria for frailty and depression as well as shared underlying pathophysiological mechanism (Figure 1), may question the predictive validity of frailty in late-life depression. This might be especially true acknowledging the huge amount of cohort studies showing that depression itself is a risk factor for premature mortality35 since frailty has been introduced in geriatric medicine to identify patients at increased risk of adverse health effects, with premature death as the most important validator. Moreover, the prospective association of frailty with mortality differs between disease clusters, with the highest predictive value among patients with pulmonary and heart diseases36,37 and lowest predictive value for patients with neuropsychiatric disorders.37,38 Yet, using data of the Netherlands Study of Depression in Older persons (NESDO), we showed that frailty at baseline was highly predictive of premature mortality among depressed older patients, irrespective whether frailty was operationalized according to the frailty phenotype criteria39 or the deficits accumulation model underlying the frailty index.40 In sum, these studies support the (predictive) validity of frailty assessment in depression and thus for risk stratification in geriatric mental health care. In the second part of this review, implications for treatment will be discussed.
Pharmacological Treatment
Pharmacological treatment of depressive disorder is less favorable among older patients compared to their younger counterparts. First, a meta-analysis of randomized controlled trials on the efficacy of antidepressants has shown that these agents are less effective in older compared to younger patients.41 Moreover, an observational study including 1042 depressed patients aged 18–88 years showed that a higher age was associated with a more chronic course of depression, prolonged time to remission, and higher symptom severity over a two-year follow-up.42 Finally, pooled results of 34 randomized controlled trials including only depressed older patients aged ≥60 years showed that the efficacy of antidepressants decreases with increasing age.43
We postulate that these findings may be explained by an increasing prevalence of frailty with age. A systematic review of 27 randomized clinical trials (RCTs) evaluating the effectiveness of antidepressants for older adults has evaluated to what extent geriatric characteristics, such as frailty, multimorbidity, disability, malnutrition, and cognitive impairment were considered in the study design.44 Remarkably, frailty and malnutrition have never been considered. Furthermore, when considered, most geriatric characteristics were used as an exclusion criterion. To get hard evidence on the impact of frailty on treatment outcome, RCTs should perform randomization stratified in the presence of frailty. Due to the paucity of knowledge on the impact of frailty in the treatment of depression, the results of another meta-analysis on antidepressants for the treatment of late-life depression have been subjected to a frailty-informed framework to consider how the evidence could be applied to frailty.45 This frailty-informed framework estimates to what extent frailty might have affected the results of the meta-analysis by interdisciplinary discussions regarding 1) characteristics of the trial population; 2) outcomes; 3) timeline for benefit; 4) harms; and 5) other relevant evidence.46,47 The authors concluded that frail older adults might be overtreated with antidepressants with little to no benefit at the cost of highly relevant side-effects for frail older persons and thus advocated “less is more” in management of depressed frail patients. According to that conclusion, we observed that SSRI monotherapy and depression were independently associated with a higher risk of falling during a one-year follow-up among 811 geriatric outpatients.48 Interestingly, the risk of falling related to SSRI usage and frailty interacted significantly giving rise to an exponential increase in fall risk.48
To our knowledge, only two observational studies that have examined prospectively the impact of frailty on the course of depression among depressed older patients referred to mental health treatment. In both studies, frailty was assessed according to the frailty phenotype criteria10 and both confirmed the presence of a depressive disorder at baseline with a diagnostic interview and examined the course of depressive symptoms over time.30,49 Among 285 depressed patients, those meeting the frailty phenotype criteria for frailty, 46.6% remitted according to DSM-criteria, which was comparable to a remission rate of 48.5% for pre-frail patients, but significantly less than 69.4% among robust patients.30 Interestingly, frailty was associated with significantly greater improvement of depressive symptom severity over time (due to higher baseline levels of these symptoms). In other words, frail depressed patients greatly improved with respect to depressive symptoms, but after two years they still suffered from a higher level of (residual?) depressive symptoms compared to their non-frail depressed counterparts.30 These findings were replicated in a smaller American study including 100 depressed patients.49 An intriguing question that remains unanswered by these empirical data is how we should classify these patients at follow-up. Should we consider them as suffering from residual depressive symptoms? In that case, switching or augmenting antidepressants should be considered for further improvement and/or relapse prevention. Or should we consider them as frail primarily? In that case, frailty management or geriatric rehabilitation may be more appropriate and the use of polypharmacy and/or psychotropic drug should be prevented (as discussed below). In other words, both diagnostic perspectives refer to different treatment guidelines applied in different health-care settings4 and misclassifying frailty for depression or the other way around may place patients at risk of inappropriate treatment.
Due to the lack of antidepressant treatment studies among frail depressed patients, we can only recommend being critical whether initiating an antidepressant is really needed as well as paying extra attention to the monitoring of side-effects. Regarding choices for specific antidepressants, antidepressants targeting slowness and/or exhaustion more specifically might be preferred based on the clinical profile of the frail depressed patient. In this regard, dopaminergic antidepressants may be preferred. Indeed, pharmacologic augmentation of dopaminergic neurotransmission, such as dopamine agonists (ie, L-dopa or pramipexole) as well as stimulants (ie, methylphenidate) have been shown to improve slowing and depressive symptoms in late life depression.50–52
Psychological Treatment
In contrast to a decreasing efficacy of antidepressants with increasing age, age has not been identified consistently as an effect modifier for the outcome of psychological treatment of depression. A large meta-analysis of the effectiveness of psychotherapy in different age groups was not able to demonstrate clinically relevant differences between middle-aged (≥24–55 years), older (≥55–75 years), and very old (75 years and older) patients but firm conclusions were limited due to large heterogeneity between trials and methodological shortcomings.53 Although circumstantial, these findings fit with two meta-analyses that demonstrated good effectiveness of psychotherapy for depression in patients with somatic comorbidity.54,55 Nonetheless, observational data of 28,498 patients referred for psychotherapy showed that patients with long-term somatic illnesses had similar outcomes compared to those without somatic comorbidity, excepted those with musculoskeletal condition, chronic obstructive pulmonary disease, and diabetes, considering that cases with somatic comorbidity needed more therapy sessions to achieve the same results.56,57
One explanation for the lack of an age-effect might be that psychotherapy studies often include persons with clinically relevant depressive symptoms irrespective of whether these persons meet the diagnostic criteria for a depressive disorder. These milder subtypes of depression can often be considered as a psychological response to a stressor – an age-related somatic disease, for example. Psychotherapy may thus effectively target these maladaptive psychological responses irrespective of age. Pharmacological studies more often apply stringent diagnostic inclusion criteria that pertain to dysfunctional brain circuitries less amenable to change in later life. Regardless of whether these explanations hold true, the empirical results suggest that older persons that do not meet the criteria of a depressive disorder but pass the cut-off for clinically relevant depressive symptoms due to an emotional response to their frailty status may still benefit from a psychotherapeutic approach. Moreover, most older patients prefer psychotherapy over antidepressant drug use.57,58
Psychological aspects of frailty have received little attention in the literature, while available studies show that frailty is associated with low levels of well-being.59 Due to the gradual and slow progression of frailty, frail older adults often face a lower quality of life for longer periods of time compared to patients with terminal illnesses. This decline in subjective well-being, life satisfaction, and overall health is more closely related to the distance from death than to chronological age and generally starts around 3–5 years before death.60,61 When facing the latest stages of life, well-being becomes much more important from a patient’s perspective than the actual level of functioning. The very gradual transition from fitness to frailty is often accompanied by maladaptive psychological responses.59,62 To this end, it has been argued that prevention programs aimed at reducing frailty should also evaluate meaning in life and include meaningful activities to ameliorate social connectedness.63 Psychological therapies, however, have never been studied in frail people to improve their quality of life and well-being. In geriatric medicine, psychosocial interventions are often advised, but generally directed to primary health care for implementation. General practitioners often prioritize physical health over mental health in frail patients due to limited time in consultations and the complexity of their needs.64 As a result, frail patients will not receive psychotherapy or will be coached by nurses or mental health workers who often lack formal psychotherapy skills.
In our opinion, especially the movement of positive psychology, such as acceptance and commitment therapy (ACT), mindfulness-based stress reduction (MBSR), and life review, might be relevant to target maladaptive (psychological) responses to frailty as outlined below.
Acceptance and Commitment Therapy (ACT) is a promising psychotherapy to improve quality of life and well-being of frail older adults since ACT focuses on possibilities acknowledging the presence of losses and handicaps. The objective of ACT is not to eliminate difficult or unpleasant feelings, which are inevitable for frail persons, but rather to stimulate them to embrace what life brings them now and to move toward valued behavior.65 ACT helps people to clarify their personal values, stimulates them to take the appropriate actions, and also to increase meaning in their life within their possibilities.66 The evidence of ACT is well established for the treatment of several psychiatric disorders.67,68 Moreover, ACT is increasingly being applied as a psychological intervention to improve functioning, well-being, and quality of life for people living with chronic somatic conditions, like diabetes, cancer, and chronic pain.69–71 Among people with chronic pain, older patients were more likely to respond to ACT whereas younger patients better responded to cognitive therapy,72 an indirect argument to favor ACT above cognitive therapy for frail older adults.
Mindfulness is the process of paying attention to the present moment in a receptive and non-judgmental attitude.73 Several studies have shown that mindfulness protects people from the well-known harmful effects of stress on mental health74 and might even dampen cellular aging.75 Mindfulness-Based Stress Reduction (MBSR) and mindfulness practice can cultivate an awareness of life, moment by moment, and supports an older person to face frailty and physical losses with presence and equanimity. While MBSR has been developed as a specific therapy protocol, training in mindfulness is also part of most ACT protocols. Two systematic reviews showed that MBSR is acceptable and feasible for older adults for the treatment of stress-related conditions, even though its effectiveness in later life is still debated due to methodological flaws of the studies conducted thus far.76,77 Moreover, the impact of MBSR on mental well-being in frail older persons specifically has not been examined yet.
Life Review Therapy has been conceptualized as a natural process of recalling, evaluating, and analyzing past experiences to achieve a more profound self-concept in people who approach the end of their life.78 In Life Review Therapy, an individual’s forgotten, but influential, experiences are revealed; their negative experiences are analyzed logically; and their positive experiences are discussed to make them feel useful and important again.79 Life Review Therapy addresses subjects that, due to unresolved conflicts, are hard to analyze by oneself without feeling upset, disgusted, or guilty. An RCT comparing Life Review Therapy to usual care among 74 frail nursing home residents found a significantly larger improvement of life satisfaction compared to usual care, but no impact on personal meaning.80
Frailty Management in Depression
In the previous sections on treatment of frail depressed patients, we focus on the potential adaptation of traditional mental health treatment guidelines. But what can be learned from geriatric frailty prevention and treatment programs?
Multidomain intervention programs, including physical exercise, nutritional intervention, and psychosocial support, are generally advocated to prevent, stabilize, or even reverse physical frailty.81 The evidence, however, is only sufficient for physical exercise to implement in clinical practice, with some evidence for combined exercise-nutrition interventions.82 This knowledge suggests that psychiatric treatment should be enriched with physical exercise and a nutritional advice to target physical frailty among older patients in specialized mental health care. This strategy might be relevant and effective for several reasons. First, psychiatric patients generally have an unfavorable lifestyle compared to the general population, which is relatively sedentary, with less physical activity, a less healthy diet, and less social contact. Therefore, frailty management may achieve even greater benefit in psychiatric patients compared to the general population. Second, patients with psychiatric disorders are often excluded from community or primary care-based life-style interventions and if not, often refuse participation. Implementing these programs within mental health treatment may overcome some barriers these patients face when offering life-style interventions in primary care or their neighborhood. Third, meta-analyses have shown that physical exercise also reduces depressive symptoms in later life with a small to moderate effect-size.83,84 Actually, a win-win situation for frail depressed patients. The implementation of physical exercise can be initiated in home care, together with training of a family member or caregiver, increasing the cost-effectiveness of the intervention.85 Generally, this type of intervention is quickly implemented and seen as favorable by patients and families.85 In addition, interventions of this type and with low resistive load (initially) are shown to be favorable and effective in patients with cognitive impairment associated with frailty, which constitutes an additional barrier to the improvement of frail patients with depression.86,87 Cognitive impairment should also be addressed in the treatment plan of depressed frail patients, as impaired cognition could mediate adverse outcomes.88
In addition to the clinical approach of frailty, targeting shared pathophysiological mechanisms (Figure 1) underlying frailty and depression have also been proposed as promising avenues for future research, especially mitochondrial dysfunction, dopamine depletion, and inflammation.34 A recent systematic review found that several anti-inflammatory agents can improve the effect of antidepressants.91 In this line, a potential agent would be metformin due to its broad action in several pathways involved in the pathophysiology of frailty and depression. Some studies are under development.89,90 Unfortunately, data were too limited to specify which patients profit most from this strategy.
Conclusion
Although the public importance of frailty is widely acknowledged by the World Health Organization,92 physical frailty is still largely neglected in geriatric mental health care.44 This should be changed since depressed older patients are two to four times more likely to be physically frail compared to the general population,16,29 and functional recovering is achieved only in the minority (~20%) of cases.93 As pointed out in this review, treatment studies on frail depressed patients are lacking. In clinical practice, comorbidity between frailty and depression can be quite complex, and physical frailty can be detected using self-reported questionnaires or simple physical maneuvers.94,95 Overlap between diagnostic criteria may easily result in overdiagnosing depression among frail elderly, but also depressed older patients might be considered “pseudo-frail” when exhausted and slowed down by their depression. Nonetheless, we hope that this review may stimulate the awareness of frailty and its detection in mental health care and may stimulate collaboration between health-care settings to allow clinicians to consider the plethora of potential treatment options available, including their potential benefits and pitfalls for the individual patient.
Acknowledgments
Prof I. Aprahamian receives a national public grant level two for frailty research from the National Council for Scientific and Technological Development (Ministry of Science, Technology, Innovation and Communications, Brazil). Dr. D.J.C. Hanssen and prof. R.C. Oude Voshaar have received funding by ZonMW to develop an ACT protocol for treatment of frailty (grant number 10390052010008).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Taylor WD, Solomon CG; Clinical practice. Depression in the elderly. N Engl J Med. 2014;371(13):1228–1236. doi:10.1056/NEJMcp1402180
2. Préville M, Mechakra Tahiri SD, Vasiliadis HM, et al. Association between perceived social stigma against mental disorders and use of health services for psychological distress symptoms in the older adult population: validity of the STIG scale. Aging Ment Health. 2015;19(5):464–474. doi:10.1080/13607863.2014.944092
3. Balsamo M, Cataldi F, Carlucci L, Padulo C, Fairfield B. Assessment of late-life depression via self-report measures: a review. Clin Interv Aging. 2018;13:2021–2044. doi:10.2147/CIA.S178943
4. Dent E, Morley JE, Cruz-Jentoft AJ, et al. Physical frailty: ICFSR International clinical practice guidelines for identification and management. J Nutr Health Aging. 2019;23(9):771–787. doi:10.1007/s12603-019-1273-z
5. Vermeiren S, Vella-Azzopardi R, Beckwée D, et al.; Gerontopole Brussels Study group. Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc. 2016;17(12):
6. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. doi:10.1016/j.jamda.2013.03.022
7. Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174(4):307–311. doi:10.1192/bjp.174.4.307
8. Luppa M, Sikorski C, Luck T, et al. Age- and gender-specific prevalence of depression in latest-life–systematic review and meta-analysis. J Affect Disord. 2012;136(3):212–221. doi:10.1016/j.jad.2010.11.033
9. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–1375. doi:10.1016/S0140-6736(19)31786-6
10. Fried LP, Tangen CM, Walston J, et al.; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol a Biol Sci Med Sci. 2001;56(3):M146–56. doi:10.1093/gerona/56.3.m146
11. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001;1:323–336. doi:10.1100/tsw.2001.58
12. O’Caoimh R, Sezgin D, O’Donovan MR, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2021;50(1):96–104. doi:10.1093/ageing/afaa219
13. Mezuk B, Edwards L, Lohman M, Choi M, Lapane K. Depression and frailty in later life: a synthetic review. Int J Geriatr Psychiatry. 2012;27(9):879–892. doi:10.1002/gps.2807
14. Mezuk B, Lohman M, Dumenci L, Lapane KL. Are depression and frailty overlapping syndromes in mid- and late-life? A latent variable analysis. Am J Geriatr Psychiatry. 2013;21(6):560–569. doi:10.1016/j.jagp.2012.12.019
15. Lohman M, Dumenci L, Mezuk B. Depression and frailty in late life: evidence for a common vulnerability. J Gerontol B Psychol Sci Soc Sci. 2016;71(4):630–640. doi:10.1093/geronb/gbu180
16. Soysal P, Veronese N, Thompson T, et al. Relationship between depression and frailty in older adults: a systematic review and meta-analysis. Ageing Res Rev. 2017;36:78–87. doi:10.1016/j.arr.2017.03.005
17. Brown PJ, Roose SP, O’Boyle KR, et al. Frailty and its correlates in adults with late life depression. Am J Geriatr Psychiatry. 2020;28(2):145–154. doi:10.1016/j.jagp.2019.10.005
18. Collard RM, Comijs HC, Naarding P, Oude Voshaar RC. Physical frailty: vulnerability of patients suffering from late-life depression. Aging Ment Health. 2014;18(5):570–578. doi:10.1080/13607863.2013.827628
19. Chu W, Chang SF, Ho HY, Lin HC. The relationship between depression and frailty in community-dwelling older people: a systematic review and meta-analysis of 84,351 older adults. J Nurs Scholarsh. 2019;51(5):547–559. doi:10.1111/jnu.12501
20. Lakey SL, LaCroix AZ, Gray SL, et al. Antidepressant use, depressive symptoms, and incident frailty in women aged 65 and older from the women’s health initiative observational study. J Am Geriatr Soc. 2012;60:854–861. doi:10.1111/j.1532-5415.2012.03940.x
21. Paulson D, Lichtenberg PA. Vascular depression: an early warning sign of frailty. Aging Ment Health. 2013;17(1):85–93. doi:10.1080/13607863.2012.692767
22. Aprahamian I, Suemoto CK, Lin SM, et al. Depression is associated with self-rated frailty in older adults from an outpatient clinic: a prospective study. Int Psychogeriatr. 2018;13:1–10. doi:10.1017/S104161021800100X
23. Zhang N, Shi GP, Wang Y, et al. Depressive symptoms are associated with incident frailty in a Chinese population: the Rugao Longevity and Aging Study. Aging Clin Exp Res. 2020;32(11):2297–2302. doi:10.1007/s40520-019-01409-x
24. Borges MK, Romanini CV, Lima NA, et al. Longitudinal association between Late-Life Depression (LLD) and Frailty: findings from a Prospective Cohort Study (MiMiCS-FRAIL). J Nutr Health Aging. 2021;25(7):895–902. doi:10.1007/s12603-021-1639-x
25. Da Mata FAF, Miranda Forte Gomes M, Lício Ferreira Santos J, Apareci da de Oliveira Duarte Y, Gomes PM. Depression and frailty in older adults: a population-based cohort study. PLoS One. 2021;16(3):e0247766. doi:10.1371/journal.pone.0247766
26. Prina AM, Stubbs B, Veronese N, et al. Depression and incidence of frailty in older people from six Latin American Countries. Am J Geriatr Psychiatry. 2019;27(10):1072–1079. doi:10.1016/j.jagp.2019.04.008
27. Feng L, Nyunt MSZ, Feng L, Yap KB, Ng TP. Frailty predicts new and persistent depressive symptoms among community-dwelling older adults: findings from Singapore longitudinal aging study. J Am Med Dir Assoc. 2014;15(1):
28. Makizako H, Shimada H, Doi T, et al. Physical frailty predicts incident depressive symptoms in elderly people: prospective findings from the Obu Study of Health Promotion for the Elderly. J Am Med Dir Assoc. 2015;16(3):194–199. doi:10.1016/j.jamda.2014.08.017
29. Collard RM, Comijs HC, Naarding P, et al. Frailty as a predictor of the incidence and course of depressed mood. J Am Med Dir Assoc. 2015;16(6):509–514. doi:10.1016/j.jamda.2015.01.088
30. Collard RM, Arts MHL, Schene AH, Naarding P, Oude Voshaar RC, Comijs HC. The impact of frailty on depressive disorder in later life: findings from the Netherlands Study of depression in older persons. Eur Psychiatry. 2017;43:66–72. doi:10.1016/j.eurpsy.2017.01.003
31. Veronese N, Solmi M, Maggi S, et al. Frailty and incident depression in community-dwelling older people: results from the ELSA study. Int J Geriatr Psychiatry. 2017;32(12):e141–e149. doi:10.1002/gps.4673
32. Chu XF, Zhang N, Shi GP, et al. Frailty and incident depressive symptoms in a Chinese sample: the Rugao Longevity and Ageing Study. Psychogeriatrics. 2020;20(5):691–698. doi:10.1111/psyg.12565
33. Diniz BS. The molecular intersection between senescence and major depression in the elderly. Am J Geriatr Psychiatry. 2018;26(11):1097–1105. doi:10.1016/j.jagp.2018.07.005
34. Brown PJ, Rutherford BR, Yaffe K, et al. The depressed frail phenotype: the clinical manifestation of increased biological aging. Am J Geriatr Psychiatry. 2016;24(11):1084–1094. doi:10.1016/j.jagp.2016.06.005
35. Miloyan B, Fried E. A reassessment of the relationship between depression and all-cause mortality in 3,604,005 participants from 293 studies. World Psychiatry. 2017;16(2):219–220. doi:10.1002/wps.20439
36. Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry. 2014;171(4):453–462. doi:10.1176/appi.ajp.2013.13030325
37. Oude Voshaar RC, Jeuring HW, Borges MK, et al. Course of frailty stratified by physical and mental multimorbidity patterns: a 5-year follow-up of 92,640 participants of the LifeLines cohort study. BMC Med. 2021;19(1):29. doi:10.1186/s12916-021-01904-x
38. Nguyen QD, Wu C, Odden MC, et al. Multimorbidity patterns, frailty, and survival in community-dwelling older adults. J Gerontol a Biol Sci Med Sci. 2019;75(8):1265–1270. doi:10.1093/gerona/gly205
39. Arts MHL, van den Berg KS, Marijnissen RM, et al. Frailty as a predictor of mortality in late-life depression: a prospective clinical cohort study. J Clin Psychiatry. 2021;82(3):20m13277. doi:10.4088/JCP.20m13277
40. Oude Voshaar RC, Dimitriadis M, vandenBrink RHS, et al. A 6-year prospective clinical cohort study on the bidirectional association between frailty and depressive disorder. Int J Geriatr Psychiatry. 2021;36(11):1699–1707. doi:10.1002/gps.5588
41. Tedeschini E, Levkovitz Y, Iovieno N, Ameral VE, Nelson JC, Papakostas GI. Efficacy of antidepressants for late-life depression: a meta-analysis and meta-regression of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72(12):1660–1668. doi:10.4088/JCP.10r06531
42. Schaakxs R, Comijs HC, Lamers F, Kok RM, Beekman ATF, Penninx BWJH. Associations between age and the course of major depressive disorder: a 2-year longitudinal cohort study. Lancet Psychiatry. 2018;5(7):581–590. doi:10.1016/S2215-0366(18)30166-4
43. Calati R, Salvina Signorelli M, Balestri M, et al. Antidepressants in elderly: metaregression of double-blind, randomized clinical trials. J Affect Disord. 2013;147(1–3):1–8. doi:10.1016/j.jad.2012.11.053
44. Benraad CE, Kamerman-Celie F, van Munster BC, Oude Voshaar RC, Spijker J, Olde Rikkert MG. Geriatric characteristics in randomised controlled trials on antidepressant drugs for older adults: a systematic review. Int J Geriatr Psychiatry. 2016;31(9):990–1003. doi:10.1002/gps.4443
45. Mallery L, MacLeod T, Allen M, et al. Systematic review and meta-analysis of second-generation antidepressants for the treatment of older adults with depression: questionable benefit and considerations for frailty. BMC Geriatr. 2019;19(1):306. doi:10.1186/s12877-019-1327-4
46. Mallery LH, Ransom T, Steeves B, Cook B, Dunbar P, Moorhouse P. Evidence-informed guidelines for treating frail older adults with type 2 diabetes: from the Diabetes Care Program of Nova Scotia (DCPNS) and the Palliative and Therapeutic Harmonization (PATH) program. J Am Med Dir Assoc. 2013;14(11):801–808. doi:10.1016/j.jamda.2013.08.002
47. Mallery LH, Moorhouse P, McLean Veysey P, Allen M, Fleming I. Severely frail elderly patients do not need lipid-lowering drugs. Cleve Clin J Med. 2017;84(2):131–142. doi:10.3949/ccjm.84a.15114
48. Lin SM, Borges MK, de Siqueira ASS, et al. Serotonin receptor inhibitor is associated with falls independent of frailty in older adults. Aging Ment Health. 2021;25(2):219–224. doi:10.1080/13607863.2019.1675143
49. Brown PJ, Ciarleglio A, Roose SP, et al. Frailty worsens antidepressant treatment outcomes in late life depression. Am J Geriatr Psychiatry. 2021;29(9):944–955. doi:10.1016/j.jagp.2020.12.024
50. Rutherford BR, Slifstein M, Chen C, et al. Effects of L-DOPA monotherapy on psychomotor speed and [11C]raclopride binding in high-risk older adults with depression. Biol Psychiatry. 2019;86(3):221–229. doi:10.1016/j.biopsych.2019.04.007
51. Lavretsky H, Reinlieb M, St Cyr N, Siddarth P, Ercoli LM, Senturk D. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015;172(6):561–569. doi:10.1176/appi.ajp.2014.14070889
52. Tundo A, de Filippis R, De Crescenzo F. Pramipexole in the treatment of unipolar and bipolar depression. A systematic review and meta-analysis. Acta Psychiatr Scand. 2019;140(2):116–125. doi:10.1111/acps.13055
53. Cuijpers P, Karyotaki E, Eckshtain D, et al. Psychotherapy for depression across different age groups: a systematic review and meta-analysis. JAMA Psychiatry. 2020;77(7):694–702. doi:10.1001/jamapsychiatry.2020.0164
54. Beltman MW, Voshaar RC, Speckens AE. Cognitive-behavioural therapy for depression in people with a somatic disease: meta-analysis of randomised controlled trials. Br J Psychiatry. 2010;197(1):11–19. doi:10.1192/bjp.bp.109.064675
55. Miguel C, Karyotaki E, Ciharova M, Cristea IA, Penninx BWJH, Cuijpers P. Psychotherapy for comorbid depression and somatic disorders: a systematic review and meta-analysis. Psychol Med. 2021;18:1–11. doi:10.1017/S0033291721004414
56. Delgadillo J, Dawson A, Gilbody S, Böhnke JR. Impact of long-term medical conditions on the outcomes of psychological therapy for depression and anxiety. Br J Psychiatry. 2017;210(1):47–53. doi:10.1192/bjp.bp.116.189027
57. Hetlevik O, Garre-Fivelsdal G, Bjorvatn B, Hjørleifsson S, Ruths S. Patient-reported depression treatment and future treatment preferences: an observational study in general practice. Fam Pract. 2019;36:771–777. doi:10.1093/fampra/cmz026
58. Wuthrich VM, Frei J. Barriers to treatment for older adults seeking psychological therapy. Int Psychogeriatr. 2015;27(7):1227–1236. doi:10.1017/S1041610215000241
59. Andrew MK, Fisk JD, Rockwood K. Psychological well-being in relation to frailty: a frailty identity crisis? Int Psychogeriatr. 2012;24(8):1347–1353. doi:10.1017/S1041610212000269
60. Cohen-Mansfield J, Skornick-Bouchbinder M, Brill S. Trajectories of end of life: a systematic review. J Gerontol B Psychol Sci Soc Sci. 2018;73(4):564–572. doi:10.1093/geronb/gbx093
61. Vogel N, Schilling OK, Wahl H-W, Beekman ATF, Penninx BWJH. Time-to-death-related change in positive and negative affect among older adults approaching the end of life. Psychol Aging. 2013;28(1):128–141. doi:10.1037/a0030471
62. Fillit H, Butler RN. The frailty identity crisis. J Am Geriatr Soc. 2009;57(2):348–352. doi:10.1111/j.1532-5415.2008.02104.x
63. Duppen RD, Machielse A, Verté RD, Dury S, De Donder L, Consortium DS. Meaning in life for socially frail older adults. J Community Health Nurs. 2019;36(2):65–77. doi:10.1080/07370016.2019.1582160
64. Frost R, Beattie A, Bhanu C, Walters K, Ben-Shlomo Y. Management of depression and referral of older people to psychological therapies: a systematic review of qualitative studies. Br J Gen Pract. 2019;69(680):e171–e181. doi:10.3399/bjgp19X701297
65. Hayes SC, Strosahl KD, Wilson KG, eds. Acceptance and Commitment Therapy: The Process and Practice of Mindful Change.
66. Zettle RD. The evolution of a contextual approach to therapy: from comprehensive distancing to ACT. Int J Behav Consult Ther. 2005;1:77–89. doi:10.1037/h0100736
67. Twohig MP, Levin ME. Acceptance and commitment therapy as a treatment for anxiety and depression: a review. Psychiatr Clin North Am. 2017;40(4):751–770. doi:10.1016/j.psc.2017.08.009
68. Bai Z, Luo S, Zhang L, Wu S, Chi I. Acceptance and Commitment Therapy (ACT) to reduce depression: a systematic review and meta-analysis. J Affect Disord. 2020;260:728–737. doi:10.1016/j.jad.2019.09.040
69. Hughes LS, Clark J, Colclough JA, Dale E, McMillan D. Acceptance and Commitment Therapy (ACT) for chronic pain: a systematic review and meta-analyses. Clin J Pain. 2017;33(6):552–568. doi:10.1097/AJP.0000000000000425
70. Maghsoudi Z, Razavi Z, Razavi M, Javadi M. Efficacy of acceptance and commitment therapy for emotional distress in the elderly with type 2 diabetes: a randomized controlled trial. Diabetes Metab Syndr Obes. 2019;12:2137–2143. doi:10.2147/DMSO.S221245
71. Zhao C, Lai L, Zhang L, et al. The effects of acceptance and commitment therapy on the psychological and physical outcomes among cancer patients: a meta-analysis with trial sequential analysis. J Psychosom Res. 2021;140:110304. doi:10.1016/j.jpsychores.2020.110304
72. Wetherell JL, Petkus AJ, Alonso-Fernandez M, Bower ES, Steiner AR, Afari N. Age moderates response to acceptance and commitment therapy vs. cognitive behavioral therapy for chronic pain. Int J Geriatr Psychiatry. 2016;31(3):302–308. doi:10.1002/gps.4330
73. Kabat-Zinn J. Mindfulness-based interventions in context: past, present, and future. Clin Psychol. 2003;144–156. doi:10.1093/clipsy.bpg016
74. de Frias CM, Whyne E. Stress on health-related quality of life in older adults: the protective nature of mindfulness. Aging Ment Health. 2015;19(3):201–206. doi:10.1080/13607863.2014.924090
75. Epel E, Daubenmier J, Moskowitz JT, Folkman S, Blackburn E. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172(1):34–53. doi:10.1111/j.1749-6632.2009.04414.x
76. Li SYH, Bressington D. The effects of mindfulness-based stress reduction on depression, anxiety, and stress in older adults: a systematic review and meta-analysis. Int J Ment Health Nurs. 2019;28(3):635–656. doi:10.1111/inm.12568
77. Geiger PJ, Boggero IA, Brake CA, et al. Mindfulness-based interventions for older adults: a review of the effects on physical and emotional well-being. Mindfulness. 2016;7(2):296–307. doi:10.1007/s12671-015-0444-1
78. Butler RN. The life review: an interpretation of reminiscence in the aged. Psychiatry. 1963;26(1):65–76. doi:10.1080/00332747.1963.11023339
79. Butler RN. Successful aging and the role of the life review. J Am Geriatr Soc. 1974;22(12):529–535. doi:10.1111/j.1532-5415.1974.tb04823.x
80. Lan X, Xiao H, Chen Y, Zhang X. Effects of life review intervention on life satisfaction and personal meaning among older adults with frailty. J Psychosoc Nurs Ment Health Serv. 2018;56(7):30–36. doi:10.3928/02793695-20180305-01
81. Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394(10206):1376–1386. doi:10.1016/S0140-6736(19)31785-4
82. Negm AM, Kennedy CC, Thabane L, et al. Management of frailty: a systematic review and network meta-analysis of randomized controlled trials. J Am Med Dir Assoc. 2019;20(10):1190–1198. doi:10.1016/j.jamda.2019.08.009
83. Klil-Drori S, Klil-Drori AJ, Pira S, Rej S. Exercise intervention for late-life depression: a meta-analysis. J Clin Psychiatry. 2020;81(1):19r12877. doi:10.4088/JCP.19r12877
84. Pérez-López FR, Martínez-Domínguez SJ, Lajusticia H, Chedraui P; Health Outcomes Systematic Analyses Project. Effects of programmed exercise on depressive symptoms in midlife and older women: a meta-analysis of randomized controlled trials. Maturitas. 2017;106:38–47. doi:10.1016/j.maturitas.2017.09.001
85. Swan L, Horgan NF, Cummins V, et al. Embedding physical activity within community home support services for older adults in Ireland a qualitative study of barriers and enablers. Clin Interv Aging. 2022;17:223–234. doi:10.2147/CIA.S351431
86. Murukesu RR, Singh DKA, Shahar S, Subramaniam P. Physical activity patterns, psychosocial well-being and coping strategies among older persons with cognitive frailty of the “WE-RISE” trial throughout the COVID-19 movement control order. Clin Interv Aging. 2021;16:415–429. doi:10.2147/CIA.S290851
87. Cezar NOC, Aprahamian I, Ansai JH, et al. Feasibility of reducing frailty components in older adults with Alzheimer’s dementia: a randomized controlled home-based exercise trial (AD-HOMEX). Exp Gerontol. 2021;150:111390. doi:10.1016/j.exger.2021.111390
88. Ge ML, Simonsick EM, Dong BR, Kasper JD, Xue QL. Frailty, with or without cognitive impairment, is a strong predictor of recurrent falls in a US Population-representative sample of older adults. J Gerontol a Biol Sci Med Sci. 2021;76(11):e354–e360. doi:10.1093/gerona/glab083
89. Espinoza SE, Musi N, Wang CP, et al. Rationale and study design of a randomized clinical trial of metformin to prevent frailty in older adults with prediabetes. J Gerontol a Biol Sci Med Sci. 2020;75(1):102–109. doi:10.1093/gerona/glz078
90. Hazuda HP, Pan Q, Florez H, et al. Association of intensive lifestyle and metformin interventions with frailty in the diabetes prevention program outcomes study. J Gerontol a Biol Sci Med Sci. 2021;76(5):929–936. doi:10.1093/gerona/glaa295
91. Köhler-Forsberg O, Lydholm CN, Hjorthøj C, Nordentoft M, Mors O, Benros ME. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139(5):404–419. doi:10.1111/acps.13016
92. Decade of healthy ageing: baseline report. Geneva: World Health Organization; 2020. Available from: https://www.who.int/publications/i/item/9789240023307.
93. Wassink-Vossen S, Collard RM, Wardenaar KJ, et al. Trajectories and determinants of functional limitations in late-life depression: a 2-year prospective cohort study. Eur Psychiatry. 2019;62:90–96. doi:10.1016/j.eurpsy.2019.09.003
94. Aprahamian I, Suemoto CK, Lin SM, et al. Depression is associated with self-rated frailty in older adults from an outpatient clinic: a prospective study. Int Psychogeriatr. 2019;31(3):425–434. doi:10.1017/S104161021800100X
95. Jung HW, Jin T, Baek JY, et al. Functional age predicted by electronic short physical performance battery can detect frailty status in older adults. Clin Interv Aging. 2020;15:2175–2182. doi:10.2147/CIA.S280542
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.