Back to Journals » Vascular Health and Risk Management » Volume 19
The Evolving Management and Treatment Options for Patients with Pulmonary Hypertension: Current Evidence and Challenges
Authors Swisher JW , Weaver E
Received 29 October 2022
Accepted for publication 1 February 2023
Published 3 March 2023 Volume 2023:19 Pages 103—126
DOI https://doi.org/10.2147/VHRM.S321025
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
John W Swisher, Eric Weaver
East Tennessee Pulmonary Hypertension Center, StatCare Pulmonary Consultants, Knoxville, TN, USA
Correspondence: John W Swisher, Email [email protected]
Abstract: Pulmonary hypertension may develop as a disease process specific to pulmonary arteries with no identifiable cause or may occur in relation to other cardiopulmonary and systemic illnesses. The World Health Organization (WHO) classifies pulmonary hypertensive diseases on the basis of primary mechanisms causing increased pulmonary vascular resistance. Effective management of pulmonary hypertension begins with accurately diagnosing and classifying the disease in order to determine appropriate treatment. Pulmonary arterial hypertension (PAH) is a particularly challenging form of pulmonary hypertension as it involves a progressive, hyperproliferative arterial process that leads to right heart failure and death if untreated. Over the last two decades, our understanding of the pathobiology and genetics behind PAH has evolved and led to the development of several targeted disease modifiers that ameliorate hemodynamics and quality of life. Effective risk management strategies and more aggressive treatment protocols have also allowed better outcomes for patients with PAH. For those patients who experience progressive PAH with medical therapy, lung transplantation remains a life-saving option. More recent work has been directed at developing effective treatment strategies for other forms of pulmonary hypertension, such as chronic thromboembolic pulmonary hypertension (CTEPH) and pulmonary hypertension due to other lung or heart diseases. The discovery of new disease pathways and modifiers affecting the pulmonary circulation is an ongoing area of intense investigation.
Keywords: pulmonary arterial hypertension, chronic thromboembolic pulmonary hypertension, nitric oxide pathway, endothelin antagonism, prostacyclin analogue
Introduction
Pulmonary hypertension (PH) is defined as a mean pulmonary artery pressure (PAP) greater than or equal to 20 mmHg as measured by right heart catheterization.1,3 Several mechanisms behind the development of elevated pulmonary artery pressures have led to the World Health Organization (WHO) classification of pulmonary hypertensive diseases into five distinct groups.1 One of the foremost challenges in approaching a patient with pulmonary hypertension is the task of correctly diagnosing and classifying the disease in order to determine appropriate treatment. In some cases, optimal management of an associated disease is the primary treatment of choice, but in other situations targeted medications or surgical measures may be indicated. Over the last two decades, a substantial scientific effort has been invested in understanding the pathobiology behind WHO Group 1 pulmonary arterial hypertension (PAH). PAH is a disease affecting the pulmonary arteries and characterized by specific changes in arterial morphology and rising pulmonary vascular resistance that can lead to right heart failure and death. Pathophysiologic pathways involved in PAH have been defined.2,3 A number of novel mechanisms and mediators are under investigation. The discovery of specific disease-causing mechanisms has fostered development of targeted therapies that are effective in controlling the progressive arteriopathy and symptoms of PAH. Several investigations are underway to elucidate if these same targeted modifiers might be beneficial in the treatment of pulmonary hypertension in other WHO Groups. This review will describe the basic mechanisms involved in the classification of pulmonary hypertensive diseases, elaborate in more detail on the state-of-the-art understanding of PAH pathobiology, outline current management strategies to achieve optimal outcomes for patients with PAH, and summarize more recent efforts to identify solutions for the treatment of PH in other WHO Groups.
Diagnosis and Classification of Pulmonary Hypertensive Diseases
The pulmonary hypertensive diseases were first classified by the World Health Organization according to pathological and clinical features in 1973 during the 1st World Symposium on Pulmonary Hypertension in Geneva, Switzerland.4 The WHO classification system has been further refined over time with the most recent updates coming from the 6th World Symposium in 2018 and the 2022 ESC/ERS (European Society of Cardiology/European Respiratory Society) Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension.1,3 Classification is based on the primary mechanisms causing elevated pulmonary arterial pressure (Figure 1). Patients with WHO Group 1 PAH have a distinct arteriopathy characterized by excessive proliferation of the cellular components of the vascular wall, smooth muscle hypertrophy, in situ thrombosis and formation of plexiform lesions that occlude the vessel lumen.5,6 Hyperproliferative changes that occur in this group may also develop in pulmonary venous structures. WHO Group 2 PH results primarily from rising postcapillary pressures as seen in diseases affecting the left side of the heart, such as cardiomyopathies or valvular heart disease. A third mechanism causing pulmonary hypertension is mediated by hypoxia and associated vasoconstriction. Hypoxic vasoconstriction in WHO Group 3 PH may be associated with high altitude, sleep apnea and other lung diseases, such as pulmonary fibrosis or emphysema. Circulatory flow obstruction, resulting from thromboembolic or other embolic events, can result in WHO Group 4 pulmonary hypertension. The final classification, WHO Group 5, consists of disorders that are associated with pulmonary hypertension, without any unifying mechanistic features.
The presence of pulmonary hypertension may be suspected from medical history, symptoms, and findings on electrocardiogram or chest radiographs.7 A careful history of other disease states is essential in determining causal relationships and classification according to WHO criteria. Connective tissue disease, portal hypertension, human immunodeficiency virus infection, congenital heart disease, and certain drug or toxin exposures have been associated with development of the same arteriopathy seen in PAH.8 Symptoms are nonspecific and overlap with those seen in many other cardiopulmonary diseases. Data from the REVEAL (Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Management) registry indicate that shortness of breath and fatigue are the most common presenting symptoms. Chest pain is often noted but less prevalent. Edema and syncope are ominous symptoms that usually indicate a more advanced stage of pulmonary hypertension.9,10 Physical manifestations of pulmonary hypertension on exam may include an accentuated second pulmonic heart sound, a systolic murmur due to tricuspid regurgitation, and a right ventricular lift. Jugular venous distension, ascites and edema represent manifestations of advanced pulmonary vascular disease.11 Signs of right ventricular hypertrophy or right axis deviation on ECG are indicators of underlying pulmonary hypertension, as are enlarged central pulmonary arteries, peripheral vascular pruning and obliteration of the retrosternal clear space on chest radiographs.7,12
When history, exam findings and basic diagnostic studies suggest pulmonary hypertension, the suspicion can be confirmed by further screening with an echocardiogram.13–15 The standard transthoracic echocardiogram can provide an estimate of pulmonary artery pressure from velocity of tricuspid regurgitation. The echocardiogram is also helpful in distinguishing left heart causes for pulmonary hypertension and provides useful prognostic information from assessment of right ventricular characteristics and presence or absence of pericardial effusion. After echocardiography, the diagnostic algorithm includes a process of eliminating causes of secondary pulmonary hypertension (Figure 2). Current guidelines recommend an algorithmic approach which includes bloodwork to screen for autoimmune connective tissue disease, HIV infection, liver and thyroid dysfunction, screening for sleep apnea, CT scanning to exclude fibrotic or emphysematous parenchymal lung disease, a VQ scan to eliminate thromboembolic disease and pulmonary function testing to identify the presence of obstructive or restrictive lung disease.3,16
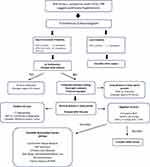 |
Figure 2 Algorithm for the diagnosis of pulmonary arterial hypertension. Abbreviations: ECG, electrocardiogram; CXR, chest x-ray; VTR, velocity tricuspid regurgitation; LV, left ventricle; PH, pulmonary hypertension; PAH, pulmonary arterial hypertension; VQ, ventilation perfusion; CT, CT scan; RHC, right heart catheterization; mPAP, mean pulmonary artery pressure; CWP, capillary wedge pressure; PVR, pulmonary vascular resistance; WU, Wood Units; HIV, human immunodeficiency virus. Notes: Swisher JW andKailash S. Advances in management of pulmonary hypertension associated with systemic sclerosis. In: New Insights Into Systemic Sclerosis. (Michal Tomcik, ed.) InTech Open, London, UK.© 2019 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License, which permits unrestricted use, distribution, and reproduction in any medium.17 |
Right heart catheterization provides the most accurate measurement of pulmonary arterial pressure and is the final confirmatory diagnostic study. A complete right heart study also determines vasoreactivity, evaluates post-capillary pulmonary circulatory pressure, identifies intracardiac left to right shunts and provides valuable prognostic data.18 The traditional hemodynamic definition of precapillary PAH was updated at the 6th World Symposium on Pulmonary Hypertension in 2018 to include: a) mean PA pressure >20 mmHg, b) pulmonary capillary wedge pressure ≤15 mmHg, and c) pulmonary vascular resistance (PVR) ≥3 WU (Wood Units).1 The recently released 2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension further modify this hemodynamic definition by lowering the PVR criterion to any value >2 WU.3 Figure 3 includes a summary of the most recent hemodynamic definitions for PH from the ESC/ERS Guidelines. In patients with idiopathic, heritable or drug-related PAH vasoreactivity testing identifies a small subset of PAH patients who may respond well to treatment with calcium blockers. Vasoreactivity testing can be performed using inhaled nitric oxide, adenosine or intravenous epoprostenol. A positive vasoreactivity study is determined by a decrease in mean PAP by at least 10 mmHg and an absolute value of mean PAP less than 40 mmHg.19 Adjunctive testing with exercise, straight leg raise or fluid challenge during right heart catheterization can unmask postcapillary pulmonary hypertension seen with heart failure and preserved ejection fraction.20–22 Certain data collected during right heart catheterization, in particular the right atrial pressure and cardiac index, have been shown to be important predictors of long-term survival.23,24
Epidemiology
Most demographic data reported for pulmonary hypertension pertain to those patients with WHO Group 1 PAH. There has been little detailed information available about epidemiology in other WHO Groups. Perhaps, the most comprehensive report of epidemiology in WHO Groups 1 through 4 comes from a large population-based cohort study in Ontario, Canada.25 Data pertaining to the incidence, prevalence, comorbidities and mortality in this report were extracted from hospitalization, emergency room visit and universal coverage health service records for 50,529 residents of Ontario from 1993 to 2012. It is important to note that reliability of data in this report was dependent on the accuracy of diagnostic coding. Further, only 40.9% of incident patients in this cohort underwent a detailed diagnostic workup that included right heart catheterization. With these limitations in mind, the investigation revealed the mean age of patients with pulmonary hypertension of any etiology was 68.5 years and 54.5% were female. The overall group included 13.8% with WHO Group 1, 68.5% with Group 2, 47% with Group 3 and 9% with Group 4 PH. Mixed WHO Group characteristics were suggested in 35.4% of patients who had diagnosis codes signifying more than one WHO Group. This study demonstrated a rise in both incidence and prevalence of PH during the decade from 2002 to 2012. Mortality rates for the overall PH population were 12.8%, 35.9% and 61.5% at 30 days, 1 year and 5 years, respectively. Patients with WHO Group 1 PH had the lowest mortality rate, while those with WHO Group 2 and 3 had the highest risk of death. Other reports also indicate that left heart disease is the most common cause of PH with COPD being the second most common worldwide.26
Population registries from France and the United States have illustrated the epidemiologic makeup of WHO Group 1 PAH.27,28 As noted previously, pulmonary arterial hypertension can be subclassified depending on whether it is heritable, idiopathic or associated with other conditions which are considered risk factors for PAH. Entities considered risk factors for development of PAH include drug and toxin exposure, connective tissue disease, HIV infection, portal hypertension, congenital heart disease and schistosomiasis. There is wide variability in reports of incidence, prevalence, mortality and other characteristics of PAH depending on geographic differences in target populations, economic determinants, differences in health-care systems and clinical practice.29–33 The source for data acquisition can be an important factor contributing to variability as well. A recent comprehensive review of the literature pertaining to WHO Group 1 PAH and WHO Group 4 chronic thromboembolic pulmonary hypertension (CTEPH) underscores this point.29 Based on publications reflecting data from national registries, clinical databases and claims/administrative databases patients with PAH had a mean age ranging from 43 to 76 years of which 55% to 81% were female. The incidence of PAH was 1.5 to 32 cases per million of the general population and prevalence ranged from 12.4 to 268 cases per million. Similarly, with CTEPH wide variability was reported with mean age at diagnosis 58 to 73 years and 37% to 70% female. The incidence of CTEPH from this review ranged from 0.9 to 39 cases per population million and prevalence ranged 14.5 to 144 per million.
Pathophysiology Behind Pulmonary Arterial Hypertension (WHO Group 1)
Patients classified with WHO Group 1 PAH share a vasculopathy of the small precapillary arteries characterized by excessive endothelial proliferation, smooth muscle proliferation and hypertrophy, in situ thrombosis, formation of vascular plexiform lesions.5,34 These vascular changes can also be found in the postcapillary vessels in certain disorders, such as systemic sclerosis and pulmonary veno-occlusive disease. In addition to characteristic remodeling of the pulmonary vasculature, there is a loss of precapillary arteries and exaggerated infiltration of the perivascular space with inflammatory cells.2 The pathogenesis of these changes is a complex process with evidence for genetic factors, cytokines and growth factors, ion channel dysfunction, and vascular injury with endothelial dysfunction resulting in imbalance of endogenous vasomotor regulation and cell proliferation in the pulmonary vascular bed.2,35,36
A genetic basis for PAH was first uncovered in 2000 with discovery of a bone morphogenetic protein receptor II (BMPR2) gene mutation in patients with heritable PAH.37,38 This mutation is identified in 70–80% of patients with heritable PAH and 15–25% with idiopathic PAH.39,40 BMPR2 is a member of the transforming growth factor-beta (TGF-B) gene superfamily and serves to limit smooth muscle cell proliferation and endothelial cell apoptosis.41 Sequence variations in genes encoding BMPR2-related downstream SMAD (Suppressor of Mothers Against Decapentaplegic) signaling intermediaries (SMAD1, SMAD4, SMAD8, SMAD9) have also been linked to the pathogenesis of PAH.42–44 Mutations involving activin receptor-like kinase 1 (ACVRL1 or ALK1) and endoglin (ENG) account for 80% of cases of hereditary hemorrhagic telangiectasia (HHT) with 15–20% of these patients also developing pulmonary hypertension.45,46 Other PAH-linked mutations unrelated to BMPR2 signaling involve KCNK3 and CAV1. KCNK3 (potassium channel subfamily K member 3) encodes a pH sensitive potassium channel that influences vascular tone.47 CAV1 (caveolin-1) encodes a membrane protein essential for creation of lipid rafts or caveoli that function to position BMP receptors.48 Up to 25% of sporadic cases of pulmonary capillary hemangiomatosis and pulmonary veno-occlusive disease have been attributed to mutations in EIF2AK4 (eukaryotic translation initiation factor 2 alpha kinase 4).49,50 In addition to mutations of the genome, epigenetic mechanisms affecting cellular function have also been a focus of recent research. These mechanisms may involve DNA methylation, micro RNAs or modification of histone proteins which alter expression of growth factor levels or gene expression and thereby influence cell growth and proliferation.51–54
Inflammatory cell infiltration is observed in proximity to areas of vascular remodeling in PAH raising interest in the potential role of cytokines and growth factors in the vascular disease process. It is unclear if the presence of inflammatory cell infiltrates represents a consequence of hypoxia, or alternatively if inflammatory mediators promote vascular cell injury and dysfunction. Examination of vascular lesions has demonstrated lymphocyte, macrophage, mast and dendritic cell invasion.55–57 While a deficiency of regulatory T cells has been noted in lungs of idiopathic PAH patients,58 an expansion of ectopic pulmonary lymphoid tissue and presence of autoantibodies suggest excessive B cell activation.59 Ectopic tissue adjacent to pulmonary arteries in PAH may be linked to the vascular remodeling process. Dendritic cells release cytokines that attract T and B lymphocytes, enhance their survival and contribute to an inflammatory environment.60 Macrophages located in the vicinity of vascular lesions are susceptible to the effects of IL-6 released by activated adventitial fibroblasts and assume a phenotype with proinflammatory characteristics.61 Macrophages are a source of platelet-derived growth factor (PDGF) which is a potent mitogen and chemoattractant for vascular cells.62 Although the exact role of immune cell biology and inflammation in the pathogenesis of PAH is yet to be defined, the presence of similar pulmonary vascular changes in both PAH and a significant number of patients with primary autoimmune disorders supports a role for immune cell mediated events in pulmonary vascular remodeling.
Aside from the impact of genetic and immune or inflammatory factors on structural remodeling, there is evidence that endothelial injury and dysfunction cause imbalances in the production of endogenous mediators of vascular tone, platelet aggregation and cellular proliferation. Immunohistochemical studies have demonstrated significant reductions in nitric oxide synthase and prostacyclin synthase levels in pulmonary vascular endothelium where these enzymes serve critical roles in the production of nitric oxide and prostacyclin, both of which have vasodilatory and antiproliferative effects on pulmonary vascular cells.63,64 While production of nitric oxide and prostacyclin by pulmonary vascular endothelium is reduced, the production of endothelin-1 by endothelial cells is increased in PAH.65 Endothelin-1 promotes opposing properties including vasoconstriction and cell proliferation. Endothelin-1, survivin and vascular endothelial growth factor (VEGF) have been isolated in vascular plexiform lesions and are believed to enhance endothelial and smooth muscle cell proliferation while inhibiting apoptosis.65–67 Thromboxane production by the pulmonary endothelium is also increased leading to vasoconstriction and in situ thrombus formation within the pulmonary arteries.68
Other factors with potential roles in the pathogenesis of PAH include serotonin, autoantibodies, dysfunctional voltage-gated potassium channels, and cancer-like patterns of cellular proliferation and apoptosis resistance. Serotonin may promote vasoconstriction and vascular remodeling by stimulating smooth muscle cell (SMC) and fibroblast proliferation.69–71 Anti-endothelial cell and anti-fibroblast antibody expression against specific target antigens have been observed in idiopathic and scleroderma-related PAH, although the role of these autoimmune effectors in pathogenesis of PAH is unclear.72,73 Smooth muscle cell proliferation may be stimulated by serotonin transporter activation of the platelet-derived growth factor-beta (PDGF-B) receptor.74 Smooth muscle intracellular calcium regulates not only contraction, but also proliferation and resistance to apoptosis. Downregulation and dysfunction of voltage-gated potassium channels affect membrane polarization and increase calcium influx, which in turn promotes smooth muscle cell contraction and enhances proliferation by driving cells into the cell cycle.39,75,76 A number of cancer-like cellular behaviours have been observed in pulmonary vascular endothelial cells, SMCs and fibroblasts including monoclonal expansion of endothelial cells in idiopathic PAH, presence of unstable short DNA microsatellite sequences in plexiform lesions, and a persistent hyperproliferative and apoptosis-resistant state when endothelial cells are removed from the in vivo environment.77–79 Pulmonary vascular endothelial cells, SMCs and fibroblasts are more dependent on glycolysis for energy production.80–82 Mitochondria shift from glucose oxidation to uncoupled aerobic glycolysis in a manner similar to cancer cells, thus enhancing creation of precursors for DNA synthesis and rapid cell proliferation.83 Vascular remodeling in PAH is distinguished from cancer by the fact that pulmonary vascular cells have not been shown to reproduce in clonal fashion without control.
Pathophysiology Behind Pulmonary Hypertension in Other WHO Groups
Although pulmonary hypertension in non-PAH WHO Groups is typically thought to arise via mechanisms specific to other disease processes and their effects on the pulmonary circulation, there is evidence for similarities in pathophysiology across WHO Groups that stem from endothelial damage or dysfunction like that seen in PAH.35 Pulmonary hypertension in left heart disease (PH-LHD) is considered a consequence of the retrograde transmission of elevated left atrial pressure to the pulmonary circulation. Elevated left atrial pressure may be a consequence of systolic or diastolic left ventricular dysfunction or valvular heart disease. In some WHO Group 2 patients, vascular remodeling with characteristics similar to PAH may develop in distal pulmonary arteries and venules and persist even after correction of the underlying cause of left atrial hypertension.84 As an example, patients with valvular heart disease have been shown to exhibit indicators of persistent pulmonary hypertension after valvular repair and removal of the source of elevated post-capillary pressures.85 Endothelial dysfunction resulting from prolonged retrograde increases in pulmonary vascular pressure is thought to mediate many of the observed alterations in vascular structure and function.89 Elevated plasma endothelin levels are reported in patients having PH-LHD and nitric oxide production is reduced in heart failure.87,90 The pulmonary vasculopathy in PH-LHD is known to involve activation of fibroblasts, proliferation of the vascular intima and media, perivascular infiltration with inflammatory cells, and increased cytokine and growth factor expression all similar to features seen in PAH.86–88 These findings correlate with observations of vascular remodeling resembling that seen in PAH.
WHO Group 3 pulmonary hypertension due to lung disease and hypoxia is often considered a result of hypoxic vasoconstriction and loss of small vessels and capillaries. While this is true, chronic hypoxia has also been linked to the activity of several mediators affecting vascular structure and function similar to the vascular remodeling noted in PAH.91,92 Supplemental oxygen may be helpful in reversing hypoxic vasoconstriction in early stages, but as disease progresses and hypoxia becomes a more chronic fixture, vascular remodeling becomes a more significant cause of rising pulmonary vascular resistance. As with PAH, endothelial biology plays a key role in the pulmonary vascular response to hypoxia and is influenced by TGF-B1 and hypoxia-inducible factor 1-alpha (HIF-1a) signaling pathways.81,91,93,94 In experimental models, chronic hypoxia induces expression of vascular endothelial growth factor A (VEGFA) and its receptor VEGFR2 via HIF-1a.91,95,96 Hypoxia also induces expression of TGF-B1 and thereby enhances production of PDGF-B. PDGF-B has been shown to promote VEGFA expression and hence hypoxia-induced endothelial proliferation.91,97 Chronic hypoxia is a stimulus for serotonin release, which in turn stimulates vasoconstriction and promotes endothelial proliferation and smooth muscle hypertrophy.92,98 Hypoxia is reported to decrease endothelial cell nitric oxide production and increase endothelin release.92,99 As a clinical correlate, impaired endothelial-dependent SMC relaxation has been observed in patients with COPD of varying severity.100 There is considerable interest in the relationship of tobacco smoking to vascular remodeling and pulmonary hypertension in chronic lung disease. Smoking by its own virtue appears to have multiple effects on lung vascular biology including enhanced VEGF expression, reduced nitric oxide synthase levels, increased infiltration of inflammatory cells, endothelial hyperplasia and disrupted mitochondrial function.101–103 Although poorly understood overall, it is clear that the root causes of pulmonary hypertension in the patient with chronic lung disease and hypoxia go much deeper than hypoxic vasoconstriction and loss of small vessels.
Pulmonary hypertension is a well-known complication of acute pulmonary thromboembolism. In the acute phase of thromboembolism pulmonary vascular resistance may be increased by significant clot burden obstructing the pulmonary vascular bed. Typically, with thrombolysis and/or anticoagulation, the obstructing clot will be eliminated and normal vascular resistance restored. However, even with continuous anticoagulation, lung perfusion defects can persist beyond 3 months from an acute event in over 50% of cases.104 A small percentage of these patients will go on to develop WHO Group 4 chronic thromboembolic pulmonary hypertension (CTEPH). Studies estimate the annual incidence of CTEPH after an acute pulmonary embolism to range from 0.4% to 6.2%.105 The true incidence is uncertain though, because current estimates of CTEPH incidence after acute PE may include patients that already had chronic thromboembolism prior to the acute event. CTEPH may be diagnosed several months or years after continuous anticoagulation and without symptomatic recurrent acute events. Pulmonary hypertension in this population is, in part, a consequence of nonresolving thrombus. In contrast to the characteristic fresh clot seen in acute pulmonary embolism, the chronic flow limiting material in CTEPH consists of a yellow fibrotic material that adheres tightly to the vessel wall and is composed of collagen, elastin, inflammatory cells and recanalization vessels.105
There are several proposed theories pertaining to the non-resolution of thrombotic material in patients who develop CTEPH. When the thromboembolic burden is large, the intrinsic lytic system may be unable to achieve complete resolution due to insufficient lytic capacity or ability to reach the entire mass of clot. Another possibility is that patients who are treated may not be anticoagulated adequately or long enough. Underlying autoimmune disorders, non-O blood group, history of splenectomy, ventriculo-atrial shunts, thyroid replacement and a history of malignancy were all associated with higher CTEPH risk in a large study comparing patients with CTEPH to those with other forms of pulmonary hypertension.106 Higher levels of several inflammatory markers and mediators of inflammation have been reported in CTEPH suggesting that underlying inflammation is somehow involved.107,108 Mutations causing abnormal fibrinogen and hence abnormal fibrin structure and resistance to plasmin-mediated lysis have been identified.109–111 Abnormalities of platelet function and dysfunctional angiogenesis and recanalization of thrombus have also been suggested to explain nonresolution of thrombus in CTEPH.112,113
Nonresolution of clot alone does not explain the development of pulmonary hypertension in CTEPH. Small vessel remodeling similar to that described in idiopathic PAH is also observed in CTEPH and involves vessels with unobstructed flow, as well as those distal to flow-limiting thrombi. Further, the characteristics of arterial vasculopathy extend to involve venules and small veins. The development of vasculopathy in unobstructed arteries has been considered a consequence of redirected flow with increased pressure and shear stress leading to endothelial injury.114 However, this mechanism would not explain development of an arteriopathy distal to obstructive thrombi. Anastamoses between the bronchial and pulmonary arteries have been identified that may allow flow distal to pulmonary arterial obstructions.115 It is theorized that exposing small arteries distal to thrombus to systemic level pressures may promote vascular remodeling in these arteries. Aside from these mechanical considerations, there is evidence that molecular factors favoring vascular remodeling also exist in CTEPH. Levels of the endogenous vasodilator, nitric oxide, are known to be reduced in both patients with PAH and CTEPH.116 Further, levels of the nitric oxide synthase inhibitor, asymmetric dimethylarginine, are increased and may limit the favorable vasorelaxant and antiproliferative actions of nitric oxide at the vascular level.117 Increased levels of endothelin, as noted PAH, have also been observed in CTEPH patients.118 Even though thrombosis is the primary event in CTEPH, there is growing evidence that development of pulmonary hypertension involves aberrations in a complex molecular signalling system similar in many ways to that seen in PAH.
Risk Assessment and Treatment of WHO Group 1 PAH
Treatment options for pulmonary arterial hypertension have rapidly evolved in recent years. Prior to 1995 treatment options for patients with PAH included diuretics, anticoagulation, digoxin, calcium channel blockers and oxygen. Those patients who had unfettered disease progression might ultimately require lung transplantation. In 1995 epoprostenol was the first targeted therapy approved by the United States Federal Drug Administration for treatment of PAH. Several additional targeted therapies have been approved since that time, expanding the number of non-surgical options available to patients. Currently available treatment agents for PAH target pathophysiologic mechanisms in nitric oxide, endothelin, or prostacyclin pathways. As more medical therapies have become available, so has evidence that combining agents and targeting multiple pathways simultaneously is often more beneficial than using them individually. Extending the use of WHO Group 1 PAH targeted therapies for treatment in other WHO Groups has met with limited success, leaving a paucity of good options for treating these patients. Therefore, most of the focus on available treatments in this review will pertain to options for WHO Group 1 PAH and Group 4 CTEPH with mention of recent investigations into extension of the agents for use in other WHO Groups.
Choosing from among the available medical therapies to devise an effective PAH treatment plan involves an ongoing process of risk assessment and outcome monitoring. PAH is a progressive disease, even in the current era of targeted therapy, and careful risk and outcome monitoring is an essential part of the treatment process. The observation that there is a strong correlation between survival and certain clinical characteristics in PAH, such as 6 minute walk distance, functional class, hemodynamic measures and other parameters, has led to the development of several risk assessment tools.119 Right ventricular function is widely recognized as a determinant of outcome in PAH. Noninvasive measures of right ventricular structure and function by echocardiography or cardiac MRI are useful in risk stratification and have been incorporated into management guidelines.120,121 The recent European Society of Cardiology/European Respiratory Society guidelines include right atrial area, tricuspid annular plane systolic excursion/systolic pulmonary artery pressure (TAPSE/sPAP) and presence of pericardial effusion among risk stratification variables.3 Risk assessment tools integrate multiple clinical parameters to predict whether a patient is at low, moderate or high risk of near-term death from PAH, and as such, guide the approach to medical treatment design. Two of the most commonly used risk assessment tools are the ESC/ERS risk assessment algorithm3 and the REVEAL risk calculator.122 The REVEAL risk calculator was created from data acquired in the American REVEAL PAH Registry which was a 3-year longitudinal registry of 2967 WHO Group 1 PAH patients with data collected pertaining to clinical characteristics, evaluation, treatment and outcomes.123 A validated revision of the REVEAL calculator (REVEAL 2.0) includes an abridged version (REVEAL Lite 2) that improves usefulness in outpatient settings were regular follow up care is provided.124,125 Risk assessment is typically performed at each regular follow up visit, and adjustments to the treatment plan are made as needed to achieve and maintain low risk status. Based on REVEAL 2.0 risk stratification, patients in the low-risk group have a > 94% predicted 1-year survival, moderate-risk 70 to <94% 1-year survival and high-risk <70% 1-year survival. Current treatment guidelines incorporate risk assessment in the algorithm to modify treatment plans.
Nitric Oxide Pathway
Nitric oxide (NO) is an endogenous vasodilator produced by the pulmonary endothelium. Nitric oxide synthase (NOS) converts L-arginine to NO which acts on adjacent smooth muscle cells where it catalyzes conversion of guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP) by guanylate cyclase. Cyclic GMP in turn promotes smooth muscle relaxation and vasodilation, but also regulates cell proliferation, apoptosis and inflammation.126,127 Cyclic GMP is neutralized by endogenous phosphodiesterase-5 (PDE-5), thus limiting its effects. Patients with PAH have deficient NOS activity in the pulmonary vasculature leading to low levels of NO production.63 Favorable outcomes in the treatment of PAH have been achieved by targeting the NO-cGMP pathway with agents that inhibit PDE-5 (PDE-5 inhibitors) or directly stimulate guanylate cyclase (soluble guanylate cyclase stimulators).
Phosphodiesterase-5 inhibitors limit the inactivation of cGMP, thereby augmenting its impact in vascular smooth muscle. Three PDE-5 inhibitors, sildenafil, tadalafil and vardenafil, have been shown to improve pulmonary hemodynamics, exercise capacity and symptoms in patients with PAH, including those with connective tissue disease.128–130 Additionally, tadalafil has been shown to increase the time to clinical worsening.129 These agents may precipitate severe hypotension if used in conjunction with nitroglycerin, and therefore the use of nitroglycerin with PDE-5 inhibitors is contraindicated. Safety in pregnant humans has not been established; however, fetal harm was not noted in animal studies.
Guanylate cyclase can be directly stimulated to produce cGMP by soluble guanylate cyclase (sGC) stimulators. Riociguat is the only sGC stimulator approved for the treatment of pulmonary hypertension to date, and has been approved by the US Federal Drug Administration for both PAH and CTEPH. The benefits of riociguat for pulmonary hypertension have been elucidated in two randomized clinical trials. In PATENT-1, riociguat improved hemodynamics, exercise capacity and time to clinical worsening in patients with PAH.131 In CHEST-1 riociguat was associated with significant improvement in exercise capacity and pulmonary vascular resistance in patients with CTEPH.132 Riociguat is currently the only therapeutic agent approved for treatment of CTEPH (WHO Group 4 PH). Like the PDE-5 inhibitors, riociguat can cause hypotension if used with nitroglycerin. Thus, using riociguat and nitroglycerin together is contraindicated. Further, riociguat is teratogenic and should not be used in pregnant humans. Females of childbearing age are required to have monthly pregnancy testing and follow careful contraceptive measures while taking riociguat.
Endothelin Pathway
Endothelin-1 is a vasoconstrictor and mitogenic mediator produced by endothelial cells and present at increased levels in plasma and lung tissue of patients with PAH.65,133 Endothelin exerts its effects by binding to two G protein-coupled receptors, types A and B, which are located on the smooth muscle cell surface. The type A receptor mediates vasoconstriction, cell growth and inflammation, while the B type receptor promotes vasodilation and natriuresis while limiting cell proliferation and inflammation. There are three compounds in this group used for the treatment of PAH, including bosentan, ambrisentan, and macitentan.
Bosentan blocks both type A and B receptors to improve exercise capacity, time to clinical worsening, hemodynamics and echocardiographic abnormalities in PAH.134 Bosentan use was associated with liver transaminase elevations in about 10% of patients in clinical trials leading to a requirement for liver function monitoring on a monthly basis. In addition, bosentan exposure has been associated with edema and anemia. The drug is teratogenic and should not be used in pregnant humans. Females of reproductive age are required to undergo monthly pregnancy testing and practice careful contraception.
In contrast to bosentan, ambrisentan is a selective type A receptor antagonist which was associated with significant improvements in symptoms, exercise capacity, clinical worsening and hemodynamics in clinical trials including patients with idiopathic PAH or PAH related to connective tissue disease or HIV infection.135,136 The risk of liver injury is minimal with ambrisentan, and monthly liver function testing is not required. However, using ambrisentan in patients with chronic liver disease should be avoided. Again, ambrisentan is teratogenic leading to the same pregnancy and contraceptive precautions as with bosentan.
The third, and newest, endothelin receptor antagonist is macitentan. The benefits of macitentan in patients with PAH was demonstrated in a large event-driven trial during which macitentan reduced the risk of disease progression by 45% and the risk of death or hospitalization due to PAH by 50%.137 A significant number of patients were on background therapy with other agents in this study and, even so, demonstrated significant benefit from macitentan. The major adverse effects of this drug include anemia and fluid retention. Monitoring for liver injury is not required, however like other endothelin receptor antagonists, macitentan is teratogenic and should not be used in pregnant females. Pregnancy monitoring and careful contraceptive measures are required in females with reproductive capacity.
Prostacyclin Pathway
Prostaglandin I2 produced from arachidonic acid in the pulmonary endothelial cell mediates smooth muscle cell relaxation by catalyzing the production of cyclic adenosine monophosphate (cAMP) from adenosine triphosphate (ATP) in smooth muscle cells. Cyclic AMP mediates smooth muscle relaxation, vasodilation and antiproliferative activities in the pulmonary vascular bed. The prostacyclin analogues are potent vasodilators of the pulmonary vasculature, inhibitors of platelet aggregation and inhibitors of cell proliferation that exert their effect by interaction with prostaglandin receptors.138 Pulmonary endothelial prostacyclin synthase expression is reduced in PAH resulting in reduced production of prostaglandin I2 from arachidonic acid and thereby reduced smooth muscle cyclic AMP production.64 Synthetic prostacyclin analogues can offset the deficiency of endogenous prostaglandin I2 production in PAH and are available as oral, inhaled, and subcutaneously or intravenously delivered disease-modifying therapies.
Epoprostenol was the first prostacyclin analogue shown to be effective in reducing symptoms, improving exercise capacity, and improving hemodynamics in idiopathic PAH and PAH associated with systemic sclerosis.139,140 Further, treatment with epoprostenol was noted to reduce mortality in one randomized trial involving idiopathic PAH patients.140 Sustained long-term benefits of epoprostenol have been demonstrated in idiopathic PAH, PAH associated with other disorders and inoperable CTEPH.141–145 Epoprostenol is only available as a continuous IV therapy and has a short half-life of 3–5 minutes. Tachyphylaxis occurs with the continuous infusion requiring dose escalation over time. The infusion is typically well tolerated, although adverse effects including jaw pain, headache, flushing, nausea and diarrhea may be experienced for a short period after dose escalation. There is a serious risk of rebound vasoconstriction and even death if the infusion is disrupted.
Treprostinil is a newer prostacyclin analogue that is available in oral, inhaled, and subcutaneously or intravenously infused forms. Treprostinil was first investigated using a subcutaneous delivery method in a large group of patients with idiopathic PAH and PAH associated with connective tissue disease. Although dose titration was limited by adverse effects, such as infusion site pain, the study demonstrated improvement in exercise tolerance and hemodynamics.146,147 The use of treprostinil for PAH was later extended to include an intravenous application. The infused forms of treprostinil are associated with tachyphylaxis, similar to epoprostenol, requiring ongoing monitoring and dose escalation. The half-life of treprostinil is 3–4 hours. Adverse effects are similar to those noted with epoprostenol and include the added risk of infusion site pain for those using the subcutaneous route of treatment. The inhaled formulation of treprostinil is an effective treatment option for those patients who may not be suited for a continuous infusion system. Significant improvements in 6 minute walk distance, NT-proBNP levels and quality of life indicators were observed in a randomized clinical trial of inhaled treprostinil that included patients already on background therapy with bosentan or sildenafil.148 Inhaled treprostinil is administered four times daily and, as such, is not associated with development of tachyphylaxis. Inhaled treprostinil is well tolerated with the most common adverse effects including sore throat, cough and headache. An oral formulation of treprostinil has also been shown in a randomized clinical trial of treatment naïve PAH patients to improve 6 minute walk distance.149 Oral treprostinil is delivered on a b.i.d. or t.i.d. schedule with gradual dose escalation to achieve and maintain optimal effect. Use of oral treprostinil may be complicated by anorexia, nausea and diarrhea. The oral formulation should not be used in patients with Child Pugh Class 3 hepatic impairment.
Iloprost is a prostacyclin analogue available in Europe in intravenous and inhaled formulations but only available in the inhaled form in the United States. The inhaled formulation was compared with placebo in patients with PAH and CTEPH and led to improved symptoms, exercise capacity and hemodynamics.150 Benefits of intravenous iloprost were found to be similar to those of epoprostenol in a small group of patients with either PAH or CTEPH.151 Inhaled iloprost is delivered with a specifically designed nebulizer and requires treatment 6 to 9 times a day. Each dose is effective for 30–90 minutes and may be associated with cough, flushing or jaw pain. Hypotension may occur, so caution should be exercised in patients with lower baseline blood pressure.
In addition to exogenous delivery of prostacyclin analogues, the unfavorable effect of prostacyclin synthase deficiency in PAH can also be overcome with a newer targeted therapy class, the prostacyclin receptor agonist. Prostanoid receptors within the pulmonary artery include IP, EP3 and TP receptors. The IP receptor mediates vasodilation and limits proliferation, while the others promote vasoconstriction and cell proliferation.152–154 To date, selexipag, is the only commercially available prostacyclin receptor agonist, although a second agent, ralinepag, is currently under investigation in patients with PAH.
Selexipag is metabolized to an active form that is 37 times more potent than the parent compound. In a long-term event-driven investigation, selexipag reduced the risk of a composite morbidity and mortality endpoint by 40%.155,156 A similar composite endpoint risk reduction of 41% was noted in a subgroup of patients with PAH related to connective tissue disease.157 Composite endpoint events included need for atrial septostomy or lung transplantation, initiation of chronic oxygen or parenteral prostanoid therapy, hospitalization for a PAH related reason, other indicator of disease progression, or death. A large majority (80%) of patients were on background therapy with an endothelin receptor blocker, PDE- inhibitor or both. A consistent treatment effect was noted irrespective of background therapy. Common adverse effects of this particular agent include headache, flushing, nausea, diarrhea, myalgia, and arthralgia.
Combination Therapy
While the various therapeutic agents used to treat PAH are effective in their own right, data has accumulated indicating that combinations of agents from different classes may be even more effective in limiting disease progression. There have been numerous clinical trials in recent years that have examined the benefits of combination therapy and demonstrated variable degrees of success.148,158,159 One of the more pivotal trials in support of combination therapy was the Ambrisentan and Tadalafil in Patients with Pulmonary Hypertension (AMBITION) Trial comparing up front combination therapy with ambrisentan and tadalafil to each agent used as monotherapy.160 The combination of ambrisentan and tadalafil resulted in a 50% reduction in composite morbidity/mortality events when compared with either agent used alone. In a retrospective analysis of 106 patients, upfront combination of ambrisentan and tadalafil improved REVEAL 2.0 risk score, lowered PVR and preserved stroke volume after two years of treatment, although 57% of patients remained in intermediate or high REVEAL 2.0 risk categories.161 In the TRITON study (The Efficacy and Safety of Initial Triple Versus Initial Dual Oral Combination Therapy in Patients With Newly Diagnosed Pulmonary Arterial Hypertension) patients were randomized to receive initial combination therapy with macitentan, tadalafil and placebo or macitentan, tadalafil and selexipag.162 At week 26, patients receiving either dual or triple combination therapy had significant improvement in the primary endpoint, PVR. However, there was no significant difference in PVR between the two groups. Likewise, there was improvement in the secondary endpoints, 6 minute walk distance and NT-proBNP level, but again no significant difference between the groups. Exploratory analysis suggested a lower risk of disease progression in the triple combination therapy group. Investigations of newer treatments, such as riociguat, macitentan and selexipag, have included significant numbers of patients on background therapies. Even so, these studies have shown additive improvements in symptoms, exercise capacity and clinical worsening.131,137,156 Significant improvements in PVR, mPAP, cardiac index, right atrial pressure and mixed venous oxygen saturation have been reported with combination regimens.163 Benefits such as these have led to the inclusion of combination therapy recommendations in current treatment guidelines.3,164
Chronic Thromboembolic Pulmonary Hypertension
While much of the scientific effort in recent years has focused on understanding the pathobiology behind PAH and developing effective disease-modifying treatments, advances have also been made in the understanding and treatment of CTEPH. The pathogenesis of CTEPH is not well-defined; however, it is characterized by accumulation of chronically organized thrombus in the pulmonary arteries associated with vascular remodeling that may involve ineffective fibrinolysis, endothelial dysfunction and abnormal angiogenesis as discussed earlier in this review.165 CTEPH is often considered a consequence of persistent thromboembolism following an acute pulmonary embolism, however the rates of acute pulmonary embolism prior to diagnosis of CTEPH range from 15% to 75% depending on geographic location.166,167 A recent multicenter observational cohort study from Germany (FOCUS Study) monitored 1017 patients after acute pulmonary embolism for 24 months and reported a cumulative 2-year CTEPH incidence of 2.3%.168 It is clear that pulmonary hypertension associated with chronic thromboembolism can be a disease not only involving thrombotic flow obstruction, but also progressive vascular remodelling, rising pulmonary vascular resistance, and right heart failure.
CTEPH is confirmed by right heart catheterization and demonstration of arterial occlusions on chest imaging studies after at least 3 months of effective anticoagulation. Diagnosis may be challenging as symptoms are not always preceded by an acute pulmonary embolism. Presenting symptoms are nonspecific and may mimic PAH or other cardiopulmonary diseases, thus often delaying detection of CTEPH.169 Diagnosis depends on right heart catheterization that confirms pre-capillary pulmonary hypertension and imaging confirmation that may include mismatched perfusion defects on ventilation-perfusion (VQ) imaging or characteristic findings on CT angiography, MRI or traditional pulmonary angiography. VQ scanning is the preferred imaging modality for diagnosis of CTEPH with a reported 96–97% sensitivity and 90–95% specificity.170 Advances in CT angiography have improved diagnostic accuracy making it comparable to VQ scanning in one study with 100% sensitivity, 93.7% specificity, and 96.5% accuracy for VQ scanning compared to 96.1% sensitivity, 95.2% specificity and 95.6% accuracy with CT angiography.171
Surgical pulmonary endarterectomy (PEA) is the recommended treatment of choice and may be curable for patients with operable occlusive disease. Advances in surgical technique allow endarterectomy to the level of subsegmental branches in experienced centers. Peri-operative mortality rates are reported below 2.5%.172–174 Long term survival after endarterectomy exceeds that for non-surgical treatment with 3-year survival at 90% after endarterectomy and 70% with non-surgical care in one report.175 Similarly, 5-year survival has been reported at 83% in patients with proximal disease undergoing endarterectomy compared to 53% in those who declined surgery.176 Despite endarterectomy being an effective definitive treatment for CTEPH in most, about 25% of patients undergoing surgery will have persistent pulmonary hypertension.177 Patients diagnosed with CTEPH should be evaluated in a center with experience in pulmonary endarterectomy to determine if they are a candidate for surgical treatment.
For those patients who may not be candidates for PEA, balloon pulmonary angioplasty (BPA) may be an alternative treatment approach. BPA is considered an option in those patients with distal thromboembolic disease or persistent pulmonary hypertension following PEA. This procedure has been shown to improve exercise capacity, hemodynamics and right ventricular function.178–181 BPA is performed in multiple sessions with dilation of a limited number of lesions per session.182 BPA may be used as an adjunct in patients with persistent or recurrent pulmonary hypertension after PEA or in patients with unilateral interoperability.183 While effective in experienced hands, the procedure is not without significant potential complications including vascular injury from wire perforation or balloon overinflation, lung injury, hemoptysis, and hypoxia. The decision to pursue BPA should be part of a multidisciplinary evaluation and be performed only in CTEPH centers with a high level of BPA experience.
A significant proportion of CTEPH patients may be considered inoperable or opt not to undergo PEA. Targeted pulmonary vasodilators have proven beneficial in this population. Significant improvements in 6 minute walk distance, PVR, NT-proBNP level and WHO functional class were observed in the CHEST-1 trial comparing riociguat to placebo in patients with inoperable CTEPH or persistent pulmonary hypertension following PEA.132 The CHEST-2 extension study demonstrated sustained 6 minute walk and functional class benefits for up to 48 months.184 In the Merit-1 trial, macitentan improved 6 minute walk distance and PVR in a Phase 2 investigation comparing placebo to 10 mg macitentan in patients with inoperable CTEPH.185 The safety and efficacy of macitentan 75 mg in inoperable CTEPH or persistent pulmonary hypertension after PEA is currently under Phase 3 investigation. Subcutaneous treprostinil has also been shown to improve 6 minute walk distance in CTEPH patients with inoperable disease or persistent pulmonary hypertension after endarterectomy.186 The role of medical therapy in conjunction with PEA and BPA is a topic of ongoing discussion and investigation. There is some evidence to suggest that medical treatment as a bridge to PEA may delay definitive surgical treatment without providing clinical benefit.187 In contrast, selected patients may benefit from pretreatment with targeted PAH therapies to optimize hemodynamics prior to surgical endarterectomy.188
Treatment Evolution in Other WHO Groups
With the success of targeted therapies in WHO Group 1 and 4 patients, interest has evolved in finding similar solutions for patients with pulmonary hypertension secondary to other heart and lung disease processes (WHO Groups 2 and 3). As previously noted, there are features of vascular remodeling that appear common across WHO Groups that likely result from pulmonary vascular injury. Multiple studies employing targeted PAH therapies in WHO Group 2 and 3 patients have yielded often inconsistent and overall disappointing results.
Pulmonary hypertension due to left heart disease (WHO Group 2) is the most common form of pulmonary hypertension and may result from left ventricular systolic or diastolic dysfunction, valvular disease or congenital disorders causing left ventricular inflow or outflow limitation. Optimizing treatment of the primary cardiac disease process remains the cornerstone of treatment in WHO Group 2 patients. Even though multiple studies have employed targeted PAH therapies, success in finding a suitable target to safely and effectively reverse vascular remodeling in this group of patients has been elusive. One small placebo-controlled trial of epoprostenol in patients with heart failure and reduced ejection fraction (HFrEF) demonstrated improvement in 6 minute walk distance without significant adverse events.189 Improvements in cardiac index and capillary wedge pressure noted in a much larger international study randomizing HFrEF patients to epoprostenol or placebo were negated by increased mortality.190 Studies with endothelin receptor antagonists have also been disappointing. One of the most notable recent studies in this regard was the MELODY-1 study comparing macitentan and placebo in patients with combined pre- and post-capillary pulmonary hypertension (CpcPH). There were no significant improvements in PVR, PAWP or NT-proBNP levels with macitentan, and macitentan was associated with significant fluid retention.191 The nitric oxide pathway has also been targeted in patients with PH-LHD. The effect of the PDE-5 inhibitor sildenafil has shown more positive benefit in HFrEF than heart failure with preserved ejection fraction (HFpEF) patients with studies demonstrating improvements in hemodynamic measures, oxygen consumption and exercise capacity.192 One small study comparing sildenafil and placebo in HFpEF was able to confirm improvements in mPA pressure and RV function but not in breathlessness or fatigue.193 Yet another study comparing 60 mg sildenafil t.i.d. to placebo for 12 weeks did not show improvement in the primary endpoint, mPA pressure.194 Similarly, in the RELAX trial sildenafil had no impact on activity tolerance or quality of life in patients with HFpEF.195 A comparative investigation of riociguat and placebo in PH due to HFrEF demonstrated improvement in PVR and cardiac index without change in mPA pressure.196 Additional investigation of nitric oxide pathway modifiers for PH-LHD is warranted to clarify the role of these agents in WHO Group 2 PH.
As is the case with WHO Group 2 PH, there are limited data pertaining to the use of PAH therapies for treatment of WHO Group 3 PH, and findings in studies addressing this population are mixed. For instance, there is evidence in patients with COPD that PAH therapies may be beneficial in some cases, while harmful in others. Improvements in hemodynamic parameters and activity tolerance compared to baseline were reported from the ASPIRE and COMPERA registries when patients having severe PH and COPD were treated with PDE-5 inhibitors.197,198 Significant improvements in hemodynamics, exercise capacity and dyspnea were observed without deterioration in oxygenation in a large randomized, controlled, multicenter trial of sildenafil compared to placebo in patients with severe PH and COPD.199 In contrast, in a study of tadalafil in patients with advanced COPD and mild PH, there were no observed improvements in activity tolerance or quality of life.200 Bosentan use in a patient group with mild PH and severe COPD was associated with declining functional status and gas exchange.201 The heterogeneity of patients with COPD and varying degrees of airflow obstruction and airspace damage may explain the discrepant results of studies to date. Future work in this group may need to focus on specific subpopulations with heterogeneous phenotypic manifestations of COPD.
Efforts to identify an effective treatment for patients in WHO Group 3 with interstitial lung disease (ILD) have also met with mixed results. A clinical trial investigating the use of riociguat in PH associated with idiopathic pulmonary fibrosis was discontinued due to increased clinical worsening and risk of mortality.202 In contrast to these disappointing results, notable benefits were observed in the INCREASE trial comparing inhaled treprostinil with placebo in patients with PH-ILD.203 In this study, patients treated with inhaled treprostinil experienced a 31 meter improvement in 6 minute walk at 16 weeks, significant improvement in NT-proBNP levels and fewer clinical worsening events compared to placebo. The use of inhaled treprostinil was also associated with an unexpected improvement in forced vital capacity.
Lung Transplantation and Atrial Septostomy
Although advances in medical therapy for PAH have reduced the need for lung transplantation in the PH patient population, those who fail medical therapy and those with PH due to other lung diseases and hypoxia may be candidates for lung transplantation. Bilateral lung transplantation is the standard approach for PAH patients. Indications for transplant referral include REVEAL risk score >7 or ESC/ERS intermediate or high risk status on appropriate PAH medications, use of parenteral prostanoids, rapidly progressive disease, and confirmed or suspected pulmonary veno-occlusive disease or pulmonary capillary hemangiomatosis, and signs of liver or kidney dysfunction due to PAH.204 In general, PAH patients who survive 1 year post transplant have a median survival of 10 years.204 One recent study reported survival rates of 93% at 3 months, 90% at 1 year, 87% at 3 years and 87% at 5 years.205 Survival has improved with advances in perioperative management, such as use of ECMO rather than cardiopulmonary bypass.206 The option of lung transplantation should be considered early, as these disease entities can progress rapidly, and response to current medical therapies is unpredictable Early referral allows for a more complete and careful pre-transplant evaluation and time for patients to consider the requirements and impact lung transplantation will have on themselves and their families. Atrial septostomy is a consideration as a bridge to lung transplantation when medical therapy fails. Creation of a right to left inter atrial shunt may serve to decompress right heart chambers and improve oxygen transport despite lowering oxygen saturation.207
The Challenge of Covid Infection in Patients with Pulmonary Hypertension
During the early months of the COVID-19 pandemic observational reports surfaced suggesting that patients with PAH may fare better with lower hospitalization and mortality rates than the general population.208–210 Data from a cohort of 13 patients from 32 US PH centers revealed a hospitalization rate of 53.8% and mortality at 7.7%.208 While hospitalization rate was higher in small cohorts from Italy (100% of 4 patients) and Spain (70% of 10 patients), mortality was 0% in both.209,210 Several theories were advanced to explain these early observations, including endothelial-protective effects of targeted PAH therapies, limited viral entry consequent to lower angiotensin-converting enzyme-2 (ACE-2) expression in PAH, and reduced responsiveness to changes in lung perfusion in PAH.211 However, as experience with COVID-19 in patients with PAH accumulated, it was apparent that outcomes were less favorable than initially thought. In late 2020 a report emerged from a survey of 47 PH centers around the world collectively reporting 70 cases of COVID-19 infection in PAH and CTEPH patients from April 17, 2020 through May 10, 2020. In this cohort, 63% were hospitalized (46% conventional ward, 17% ICU) and the mortality rate was 20% in those with PAH.212 Another survey was conducted in the United States at that time in which 50 patients with PAH or CTEPH and COVID-19 infection between April 17, 2020 and May 1, 2020 were identified by 58 PH center directors.213 In this group, the incidence of COVID-19 infection in the patient population was similar to that in the general population, 30% of patients were hospitalized, and there were 12% non-survivors. Data from a much larger French Pulmonary Hypertension Network cohort of 211 patients with PH (123 PAH, 47 CTEPH, 41 other PH) and COVID-19 infection from February 1, 2020 through April 30, 2021 provided additional insight into outcomes associated with the infection in this population.214 Overall mortality in this cohort was 24.6% with in-hospital mortality at 41.3%. A majority of patients were hospitalized (59.7%) with 32.2% managed on a conventional hospital ward and 27.5% requiring ICU admission. Patient characteristics associated with a higher mortality included older age, male gender, diabetes, hypertension, other chronic respiratory diseases, chronic cardiac disease, and chronic renal failure. PAH therapy use was similar in survivors and non-survivors.
Endothelial dysfunction is central to the development of PAH, and may be further complicated by damage invoked with COVID-19 viral invasion. Infection with SARS-CoV-2 causes diffuse alveolar damage and perivascular lymphocytic infiltration, but also specific patterns of endothelial injury.215 Notable findings include cellular swelling, loss of contact with basal membrane, and disruption of intercellular junctions. Endothelial membrane ACE-2 protein is thought to be a receptor for the COVID-19 virus which has been demonstrated within pulmonary vascular endothelial cells.216 In addition to the direct effects of the virus on endothelial cells, post-mortem histologic examination reveals vascular thrombosis, microangiopathy and areas of occlusion in the alveolar capillary bed.217,218 There is evidence under other circumstances that disruption of normal vascular endothelial function by inflammatory and infectious events may result in activation of thrombogenic pathways, failure of normal vasomotor regulation in favor of vasoconstriction, impairment of normal endothelial oxidative defenses, and loss of barrier integrity leading to capillary leak in the microvascular compartment.219 Pathologic effects of COVID-19 infection on pulmonary vascular endothelial biology could therefore amplify endothelial dysfunction in patients with PAH or CTEPH.
Patients with pulmonary hypertension should be encouraged to receive COVID-19 vaccination. They should also be advised to practice measures that can limit exposure to the virus during everyday life. Successful management of pulmonary hypertension depends on regular monitoring of the patient’s clinical progress with face-to-face visits, exercise assessment and diagnostic testing. During the height of the COVID-19 pandemic telehealth platforms limited interruptions in regular patient assessment to some degree and allowed patients to continue receiving vital support from PH experts. Treatment with PAH targeted therapies should be continued in the event of COVID-19 infection. While COVID-19 infection should be managed according to contemporary treatment guidelines, potential interactions between COVID-19 and PAH drug treatments should be taken into consideration.211 High risk patients who can be managed on an outpatient basis, yet are susceptible to unfavorable outcomes with infection, may benefit from treatment with newer antivirals, such as molnupiravir and nirmatrelvir-ritonavir. There are no significant interactions reported between molnupiravir and currently available PAH therapies. Nirmatrelvir-ritonavir, however, may alter the effects of phosphodiesterase-5 inhibitors, bosentan, riociguat and calcium channel blockers. The coadministration of these PAH therapies and nirmatrelvir-ritonavir is not advisable. Hospitalization may be required for those patients with severe infection, especially when associated with significant hypoxemia. While adequate oxygenation may be achievable with nasal or heated high flow cannulas, noninvasive ventilation may be required to offset hypercapnic respiratory failure. Intubation and positive pressure ventilation may further compromise right ventricular function and should be considered as a last resort to support gas exchange in those with PAH and impaired right ventricular function. Continuation of targeted PAH therapies may be a challenge in hospitalized patients who are unable to take oral medications and may require transition to intravenous or inhaled options. A PAH expert can provide valuable management guidance and should be consulted when PAH patients are hospitalized with serious COVID-19 infections. Expert centers are often able to provide remote teleconsultation or accept transfer of patients when a local PAH expert is not available.
Therapeutic Horizon
Several therapeutic agents are under investigation that may provide additional options for medical treatment of PAH in the near future. Ralinepag is a non-prostanoid, selective prostacyclin (IP) receptor agonist currently under phase 3 investigation. In a 22-week randomized, placebo-controlled phase 2 study of patients with PAH on single or dual oral background therapy, patients receiving ralinepag experienced significant reductions in PVR compared to placebo (−163.9 dyn-s-cm−5 with ralinepag vs +0.7 dyn-s-cm−5 with placebo; p = 0.02).220 Improvements in secondary endpoints with ralinepag, including 6 minute walk distance, clinical worsening, and NT-proBNP levels, were not statistically significant.
Sotatercept is a first-in-class fusion protein under investigation for treatment of PAH. This novel protein includes the extracellular domain of human activin receptor type IIA fused with the Fc domain of human IgG1. Sotatercept acts as a ligand trap for transforming growth factor-beta (TGF-B) superfamily members and rebalances growth differentiation and inhibitory pathways. In the 24 week, multicenter, randomized, double-blind, placebo-controlled phase 2 PULSAR study, statistically significant improvements in the primary endpoint, PVR, were noted at 0.3 and 0.7 mg doses compared to placebo.221 Reductions in PVR were noted in patients on single, double or triple background therapies including infused prostacyclins. In addition, improvements in 6 minute walk distance and NT-proBNP levels were observed. Thrombocytopenia and increased hemoglobin levels were notable adverse effects of special interest in the PULSAR study. A phase 3 investigation is currently ongoing.
Tyrosine kinase inhibitors have been shown to improve hemodynamic parameters in preliminary studies as an add on treatment for patients with disease refractory to combination regimens with nitric oxide, endothelin and/or prostacyclin pathway drugs. There was considerable interest in imatinib after significant improvements in PVR and cardiac output were noted in a phase 2, placebo-controlled study in patients with inadequate response on one or more targeted PAH therapies.222 Imatinib has an inhibitory effect on platelet-derived growth factor (PDGF) signaling thought to play an important role in the pathobiology of PAH.223 Further study of imatinib in the 24-week placebo-controlled Imatinib in Pulmonary Arterial Hypertension, a Randomized, Efficacy Study (IMPRES) confirmed significant improvement in 6 minute walk distance and PVR in patients on two or more PAH therapies receiving imatinib.224 However, serious adverse effects and discontinuations were considerably more frequent in the treatment arm including two subdural hematomas in patients receiving imatinib and anticoagulation. The extension study was terminated early due to frequent serious and unexpected adverse events, including six additional subdural hematomas.225 Additional investigations of imatinib are in progress currently to evaluate benefits of a newly formulated oral and a dry-powder formulation of the drug. Sorafenib, an inhibitor of tyrosine and serine/threonine kinases, has also been associated with improvements in functional class and mean PAP in preliminary work and is undergoing further investigation as an option for refractory PAH.226
Other agents under investigation include inhaled vardenafil and rodatristat ethyl. Inhaled vardenafil is under development to be used on an as-needed basis for sporadic symptom exacerbations. In an open label, phase 2a escalating dose trial, inhaled vardenafil lowered PVR rapidly with the effect persisting at least 1 hour after inhalation.227 Rodatristat ethyl is a prodrug for rodatristat which potently inhibits tryptophan hydroxylase, the rate limiting enzyme responsible for synthesis of serotonin from tryptophan.228 As noted previously in this review, serotonin has been implicated in the vasoconstriction and vascular remodeling characteristic of PAH.69
Conclusion
The management and treatment of patients with pulmonary hypertension continues to evolve as we gain an increased understanding of the complex mechanisms behind the pathobiology and resulting changes in pulmonary vascular function. Patients with pulmonary arterial hypertension have a number of effective medical therapies that can significantly impact their quality of life and survival when careful risk and outcome monitoring are employed. Outcomes for patients with pulmonary hypertension associated with other cardiopulmonary comorbidities are not as favorable as those for PAH, although there is ongoing investment in a search for solutions for these patients. The outlook for patients with CTEPH has improved with advances in surgical and medical approaches to this unique WHO Group. Ongoing research is uncovering new mechanisms and mediators of PH pathobiology and is exploring the benefits of several new agents under investigation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Simmoneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):18001913.
2. Humbert M, Guignabert C, Bonnet S, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53(1):1801887. doi:10.1183/13993003.01887-2018
3. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2022:2200879. doi:10.1183/13993003.00879-2022
4. Lüthy E. Proceedings: the epidemic of primary pulmonary hypertension in Europe. Pathol Microbiol. 1975;43(2–O):246–247.
5. Ishibashi-Ueda H, Ohta-Ogo K. Human pathology. In: Fukumoto Y, editor. Diagnosis and Treatment of Pulmonary Hypertension. Singapore: Springer; 2017. doi:10.1007/978-981-287-840-3_8
6. Gaine S. Pulmonary hypertension. J Am Med Assoc. 2000;284(24):3160–3168. doi:10.1001/jama.284.24.3160
7. Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53(1):1801904. doi:10.1183/13993003.01904-2018
8. Humbert M, Nunes H, Sitbon O, et al. Risk factors for pulmonary arterial hypertension. Clin Chest Med. 2001;22(3):459–475. doi:10.1016/S0272-5231(05)70284-7
9. Elliott CG, Farber H, Frost A, et al. Reveal registry: medical history and time to diagnosis of enrolled patients. Chest. 2007;132(4):631A. doi:10.1378/chest.132.4_MeetingAbstracts.631a
10. Brown LM, Chen H, Halpern S, et al. Delay in recognition of pulmonary arterial hypertension. Chest. 2011;140(1):19–26. doi:10.1378/chest.10-1166
11. Rich JD, Rich S. Clinical diagnosis of pulmonary hypertension. Circulation. 2014;130(20):1825–1830. doi:10.1161/CIRCULATIONAHA.114.006971
12. Rich S, Dantzker DR, Ayers SM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107:216–223. doi:10.7326/0003-4819-107-2-216
13. Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiog. 2010;23:685–713. doi:10.1016/j.echo.2010.05.010
14. Hellenkamp K, Unsold B, Mushemi-Blake S, et al. Echocardiographic estimation of mean pulmonary artery pressure: a comparison of different approaches to assign the likelihood of pulmonary hypertension. J Am Soc Echocardiog. 2018;31(1):89–98. doi:10.1016/j.echo.2017.09.009
15. Topyła-Putowska W, Tomaszewski M, Wysokiński A, et al. Echocardiography in pulmonary arterial hypertension: comprehensive evaluation and technical considerations. J Clin Med. 2021;10(15):3229. doi:10.3390/jcm10153229
16. Hassoun PM. Pulmonary arterial hypertension. N Engl J Med. 2021;385:2361–2376. doi:10.1056/NEJMra2000348
17. Swisher JW and Kailash S Advances in management of pulmonary hypertension associated with systemic sclerosis. In: New Insights Into Systemic Sclerosis. (Tomcik M, editor)
18. Rosenfranz S, Preston IR. Right heart catheterization: best practices and pitfalls in pulmonary hypertension. Eur Respir Rev. 2015;24:642–652.
19. Sitbon O, Humbert M, Jais X, et al. Long-term response to calcium blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–3111.
20. Kovacs G, Herve P, Barbera JA, et al. An official European respiratory society statement: pulmonary haemodynamics during exercise. Eur Respir J. 2017;50(5):1700578. doi:10.1183/13993003.00578-2017
21. Van de Bovenkamp AA, Wijkstra N, Oosterveer FPT, et al. The value of passive leg raise during right heart catheterization in diagnosing heart failure with preserved ejection fraction. Circ Heart Fail. 2022;15(4):e008935. doi:10.1161/CIRCHEARTFAILURE.121.008935
22. Vachiery JL, Teford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi:10.1183/13993003.01897-2018
23. Benza RL, Miller DP, Gomber-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and long-term pulmonary Arterial hypertension disease management (REVEAL). Circulation. 2010;122:164–172. doi:10.1161/CIRCULATIONAHA.109.898122
24. Bouchy A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50:1700889. doi:10.1183/13993003.00889-2017
25. Wijeratne DT, Lajkosz K, Brogly SB, et al. Increasing incidence and prevalence of World Health Organization Groups 1 to 4 pulmonary hypertension: a population-based cohort study in Ontario, Canada. Circ Cardiovasc Qual Outcomes. 2018;11(2):e003973. doi:10.1161/CIRCOUTCOMES.117.003973
26. Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med. 2016;4(4):306–322. doi:10.1016/S2213-2600(15)00543-3
27. Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173(9):1023–1030. doi:10.1164/rccm.200510-1668OC
28. Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137(2):376–387. doi:10.1378/chest.09-1140
29. Leber L, Beaudet A, Muller A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: identification of the most accurate estimates from a systematic literature review. Pulm Circ. 2021;11(1):1–12. doi:10.1177/2045894020977300
30. Strange G, Playford D, Stewart S, et al. Pulmonary hypertension: prevalence and mortality in the Armadale echocardiography cohort. Heart. 2012;98(24):1805–1811. doi:10.1136/heartjnl-2012-301992
31. Escribano-Subias P, Blanco I, Lopez-Meseguer M, et al. Survival in pulmonary hypertension in Spain: insights from the Spanish registry. Eur Respir J. 2012;40(3):596–603. doi:10.1183/09031936.00101211
32. Yang X, Mardekian J, Sanders KN, et al. Prevalence of pulmonary arterial hypertension in patients with connective tissue diseases: a systematic review of the literature. Clin Rheumatol. 2013;32(10):1519–1531. doi:10.1007/s10067-013-2307-2
33. Gillmeyer KR, Lee -M-M, Link A, et al. Accuracy of algorithms to identify pulmonary arterial hypertension in administrative data: a systematic review. Chest. 2019;155(4):680–688. doi:10.1016/j.chest.2018.11.004
34. Graham BB, Zhang L, Tuder RM, et al. Histological and pathological diagnosis of pulmonary hypertension: pathological classification of pulmonary vascular lesions. In: editor, Yuan -JX-J. Textbook of Pulmonary Vascular Disease. Springer Science +Business Media, LLC; 2011. doi:10.1007/978-0-387-87429-6_101
35. Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109(2):159–165. doi:10.1161/01.CIR.0000102381.57477.50
36. Hassoun PM, Mouthon L, Barbera JA, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54(Suppl 1):S10–S19. doi:10.1016/j.jacc.2009.04.006
37. Deng Z, Morse JH, Slager SL, et al. Familial primary pulmonary hypertension (Gene PPH1) is caused by mutations in the bone morphogenetic protein receptor–II gene. Am J Hum Genet. 2000;67(3):737–744. doi:10.1086/303059
38. Lane KB, Machado RD, Pauciulo MW, et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. doi:10.1038/79226
39. Thenappan T, Ormiston ML, Ryan JJ, et al. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. 2018;360:j5492. doi:10.1136/bmj.j5492
40. Thomson JR, Machado RD, Pauciulo MW, et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II. J Med Genet. 2000;37:741–745. doi:10.1136/jmg.37.10.741
41. Austin ED, Loyd JE. The genetics of pulmonary arterial hypertension. Circ Res. 2014;115(1):189–202. doi:10.1161/CIRCRESAHA.115.303404
42. Nasim MT, Ogo T, Ahmed M, et al. Molecular genetic characterization of SMAD signaling molecules in pulmonary arterial hypertension. Hum Mutat. 2011;32(12):1385–1389. doi:10.1002/humu.21605
43. Shintani M, Yagi H, Nakayama T, et al. A new nonsense mutation of SMAD 8 associated with pulmonary arterial hypertension. J Med Genet. 2009;46:331–337. doi:10.1136/jmg.2008.062703
44. Graf S, Haimel M, Bleda M, et al. Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat Commun. 2018;9(1):1416. doi:10.1038/s41467-018-03672-4
45. Trembath RC, Thomson JR, Machado RD, et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Eng J Med. 2001;345(5):325–334. doi:10.1056/NEJM200108023450503
46. Chaouat A, Coulet F, Favre C, et al. Endoglin germline mutation in a patient with hereditary hemorrhagic telangiectasia and dexfenfluramine associated pulmonary arterial hypertension. Thorax. 2004;59:446–448. doi:10.1136/thx.2003.11890
47. Ma L, Roman-Campos D, Austin ED, et al. A novel channelopathy in pulmonary arterial hypertension. N Eng J Med. 2013;369(4):351–361. doi:10.1056/NEJMoa1211097
48. Austin ED, Ma L, LeDuc C, et al. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ Cardiovasc Genet. 2012;5(3):336–343. doi:10.1161/CIRCGENETICS.111.961888
49. Best DH, Sumner KL, Austin ED, et al. EIF2AK4 mutations in pulmonary capillary hemangiomatosis. Chest. 2014;145(2):231–236. doi:10.1378/chest.13-2366
50. Eyries M, Montani D, Girerd B, et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet. 2014;46(1):mm65–mm69. doi:10.1038/ng.2844
51. Kim GH, Ryan JJ, Marsboom G, et al. Epigenetic mechanisms of pulmonary hypertension. Pulm Circ. 2011;1(3):347–356. doi:10.4103/2045-8932.87300
52. Wang Y, Kahaleh B. Epigenetic repression of bone morphogenetic protein receptor II expression in scleroderma. J Cell Mol Med. 2013;17(10):1291–1299. doi:10.1111/jcmm.12105
53. Zhao L, Chen C-N, Hajji N, et al. Histone deacetylation inhibition in pulmonary hypertension: therapeutic potential of valproic acid and suberoylanilide hydroxamic acid. Circulation. 2012;126(4):455–467. doi:10.1161/CIRCULATIONAHA.112.103176
54. Kim J, Kang Y, Kojima Y, et al. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med. 2013;19:74–82.
55. Tuder RM, Marcki JC, Richter A, et al. Pathology of pulmonary hypertension. Clin Chest Med. 2007;28(1):23–42. doi:10.1016/j.ccm.2006.11.010
56. Heath D, Yacoub M. Lung mast cells in plexogenic pulmonary arteriopathy. J Clin Pathol. 1991;44:1003–1006. doi:10.1136/jcp.44.12.1003
57. Perros F, Dorfmuller P, Souza R, et al. Dendritic cell recruitment in lesions of human and experimental pulmonary hypertension. Eur Respir J. 2007;29(3):462–468. doi:10.1183/09031936.00094706
58. Savai R, Pullamsetti SS, Kolbe J, et al. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186(9):897–908. doi:10.1164/rccm.201202-0335OC
59. Perros F, Dorfmuller P, Montani D, et al. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185(3):311–321. doi:10.1164/rccm.201105-0927OC
60. Van Uden D, Boomars K, Kool M. Dendritic cell subsets and effector function in idiopathic and connective tissue disease-associated pulmonary arterial hypertension. Front Immunol. 2019;10:11. doi:10.3389/fimmu.2019.00011
61. El Kasmi KC, Pugliese SC, Riddle SR, et al. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J Immunol. 2014;193(2):597–609. doi:10.4049/jimmunol.1303048
62. Antoniu SA. Targeting PDGF pathway in pulmonary arterial hypertension. Expert Opin Ther Targets. 2012;16(11):1055–1063. doi:10.1517/14728222.2012.719500
63. Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333(4):214–221. doi:10.1056/NEJM199507273330403
64. Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159(6):1925–1932. doi:10.1164/ajrccm.159.6.9804054
65. Giaid A, Yanagisawa M, Langleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328(24):1732–1739. doi:10.1056/NEJM199306173282402
66. McMurtry MS, Archer SL, Altieri DC, et al. Gene therapy targeting surviving selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest. 2005;115:1479–1491. doi:10.1172/JCI23203
67. Tuder RM, Chacon M, Alger LA, et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol. 2001;195(3):367–374. doi:10.1002/path.953
68. Christman B, McPherson C, Newman J, et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med. 1992;327(2):70–75. doi:10.1056/NEJM199207093270202
69. MacLean MR, Herve P, Eddahibi S, et al. 5-hydroxytryptamine and the pulmonary circulation: receptors, transporters and relevance to pulmonary arterial hypertension. Br J Pharmacol. 2000;131(2):161–168. doi:10.1038/sj.bjp.0703570
70. Welsh DJ, Harnett M, MacLean M, et al. Proliferation and signaling in fibroblasts: role of 5-hydroxytryptamine2A receptor and transporter. Am J Respir Crit Care Med. 2004;170(3):252–259. doi:10.1164/rccm.200302-264OC
71. Lee SL, Wang WW, Lanzillo JJ, et al. Serotonin produces both hyperplasia and hypertrophy of bovine pulmonary artery smooth muscle cells in culture. Am J Physiol. 1994;266(1 Pt 1):L46–L52. doi:10.1152/ajplung.1994.266.1.L46
72. Tamby MD, Chanseaud Y, Humbert M, et al. Anti-endothelial cell antibodies in idiopathic and systemic sclerosis associated pulmonary arterial hypertension. Thorax. 2005;60:765–772. doi:10.1136/thx.2004.029082
73. Dib H, Tamby MC, Bussone G, et al. Targets of anti-endothelial cell antibodies in pulmonary hypertension and scleroderma. Eur Respir J. 2012;39(6):1405–1414. doi:10.1183/09031936.00181410
74. Ren W, Watts SW, Fanburg BL. Serotonin transporter interacts with the PDGF beta receptor in PDGF-BB-induced signaling and mitogenesis in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2011;300(3):L486–L497. doi:10.1152/ajplung.00237.2010
75. Yuan X-J, Wang J, Juhaszova M, et al. Attenuated K+ channel gene transcription in primary pulmonary hypertension. Lancet. 1998;351(9104):726–727. doi:10.1016/S0140-6736(05)78495-6
76. Yuan X-J, Aldinger AM, Juhaszova M, et al. Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation. 1998;98:1400–1406. doi:10.1161/01.CIR.98.14.1400
77. Archer SL, Gomberg-Maitland M, Maitland ML, et al. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-KV1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294(2):H570–H578. doi:10.1152/ajpheart.01324.2007
78. Caruso P, Dunmore BJ, Schlosser K, et al. Identification of miR-124 as a major regulator of enhanced endothelial cell glycolysis in pulmonary arterial hypertension via PTBP1 and PKM2. Circulation. 2017;136(25):2451–2467. doi:10.1161/CIRCULATIONAHA.117.028034
79. Zhang H, Wang D, Li M, et al. The metabolic and proliferative state of vascular adventitial fibroblasts in pulmonary hypertension is regulated through a miR-124/PTBP1/PKM axis. Circulation. 2017;136(25):2468–2485. doi:10.1161/CIRCULATIONAHA.117.028069
80. Xu W, Koeck T, Lara AR, et al. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci USA. 2007;104(4):1342–1347. doi:10.1073/pnas.0605080104
81. Tuder RM, Davis LA, Graham BB. Targeting energetic metabolism: a new frontier in the pathogenesis and treatment of pulmonary hypertension. Am J Respir Crit Care Med. 2012;185(3):260–266. doi:10.1164/rccm.201108-1536PP
82. Sutendra G, Michelakis ED. The metabolic basis of pulmonary arterial hypertension. Cell Metab. 2014;19(4):558–573. doi:10.1016/j.cmet.2014.01.004
83. Ryan JJ, Archer SL. Emerging concepts in the molecular basis of pulmonary arterial hypertension: part 1: metabolic plasticity and mitochondrial dynamics in the pulmonary circulation and right ventricle in pulmonary arterial hypertension. Circulation. 2015;131(19):1691–1702. doi:10.1161/CIRCULATIONAHA.114.006979
84. Fernandez AI, Yotti R, Gonzalez-Mansilla A, et al. The biological bases of group 2 pulmonary hypertension. Int J Mol Sci. 2019;20(23):5884. doi:10.3390/ijms20235884
85. Aris A, Camara ML. Long-term results of mitral valve surgery in patient with severe pulmonary hypertension. Ann Thorac Surg. 1996;61:1583–1584. doi:10.1016/0003-4975(96)00114-2
86. Gerges C, Gerges M, Lang MB, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest. 2013;143(3):758–766. doi:10.1378/chest.12-1653
87. Cody RJ, Haas GJ, Binkley PF, et al. Plasma endothelin correlates with the extent of pulmonary hypertension in patients with chronic congestive heart failure. Circulation. 1992;85(2):504–509. doi:10.1161/01.CIR.85.2.504
88. Pietra GG, Capron F, Stewart S, et al. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol. 2004;43(12 Suppl S):25S–32S. doi:10.1016/j.jacc.2004.02.033
89. Dayeh NR, Ledoux J, Dupuis J. Lung capillary stress failure and arteriolar remodelling in pulmonary hypertension associated with left heart disease (Group 2 PH). Prog Cardiovasc Dis. 2016;59:11–21. doi:10.1016/j.pcad.2016.05.002
90. Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation. 2016;133(13):1240–1248. doi:10.1161/CIRCULATIONAHA.115.020207
91. Singh N, Dormuller P, Shlobin O, et al. Group 3 pulmonary hypertension: from bench to bedside. Circ Res. 2022;130:1404–1422. doi:10.1161/CIRCRESAHA.121.319970
92. McGettrick M, Peacock A. Group 3 pulmonary hypertension: challenges and opportunities. Glob Cardiol Sci Pract. 2020;6. doi:10.21542/gcsp.2020.6
93. Lu A, Zuo C, He Y, et al. EP3 receptor deficiency attenuates pulmonary hypertension through suppression of Rho/TGF-B1 signaling. J Clin Invest. 2015;125:1228–1242. doi:10.1172/JCI77656
94. Gilbane AJ, Derrett-Smith E, Trindar SL, et al. Impaired bone morphogenetic protein receptor II signaling in a transforming growth factor-B-dependent mouse model of pulmonary hypertension and in systemic sclerosis. Am J Respir Crit Care Med. 2015;191:665–677. doi:10.1164/rccm.201408-1464OC
95. Voelkel NF, Gomez-Arroyo J. The role of vascular endothelial growth factor in pulmonary arterial hypertension. The angiogenesis paradox. Am J Respir Cell Mol Biol. 2014;51(4):474–484. doi:10.1165/rcmb.2014-0045TR
96. Christou H, Yoshida A, Arthur V, et al. Increased vascular endothelial growth factor production in the lungs of rats with hypoxia-induced pulmonary hypertension. Am J Respir Cell Mol Biol. 1998;18(6):768–776. doi:10.1165/ajrcmb.18.6.2980
97. Liang S, Yu H, Chen X, et al. PDGF-BB/KLF4/VEGF signaling axis in pulmonary artery endothelial cell angiogenesis. Cell Physiol Biochem. 2017;41:2333–2349.
98. Eddahibi S, Hanoun N, Lanfumey L, et al. Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene. J Clin Invest. 2000;105(11):1555–1562. doi:10.1172/JCI8678
99. Bourque SL, Davidge ST, Adams MA. The interaction between endothelin-1 and nitric oxide in the vasculature: new perspectives. Am J Physiol Regul Integr Comp Physiol. 2011;300(6):R1288–R1295. doi:10.1152/ajpregu.00397.2010
100. Dinh-Xuan AT, Higenbottam TW, Clelland CA, et al. Impairment of endothelium-dependent pulmonary artery relaxation in chronic obstructive lung disease. N Engl J Med. 1991;324:1539–1547. doi:10.1056/NEJM199105303242203
101. Santos S, Peinado VI, Ramirez J, et al. Characterization of pulmonary vascular remodeling in smokers and patients with mild COPD. Eur Respir J. 2002;19:632–638. doi:10.1183/09031936.02.00245902
102. Barbera JA, Peinado VI, Santos S, et al. Reduced expression of endothelial nitric oxide synthase in pulmonary arteries of smokers. Am J Respir Crit Care Med. 2001;164:709–713. doi:10.1164/ajrccm.164.4.2101023
103. Wang Z, White A, Wang X, et al. Mitochondrial fission mediated cigarette smoke-induced pulmonary endothelial injury. Am J Respir Cell Mol Biol. 2020;63:637–651. doi:10.1165/rcmb.2020-0008OC
104. Wartski M, Collignon MA. Incomplete recovery of lung perfusion after 3 months in patients with acute pulmonary embolism treated with antithrombotic agents. THESEE Study Group. Tinzaparin ou heparin standard: evaluation dans l’Embolie pulmonaire study. J Nucl Med. 2000;41:1043–1048.
105. Simonneau G, Torbicki A, Dorfmuller P, et al. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143):160112. doi:10.1183/16000617.0112-2016
106. Bonderman D, Wilkens H, Wahounig S, et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2009;33:325–331. doi:10.1183/09031936.00087608
107. Zabini D, Heinemann A, Foris V, et al. Comprehensive analysis of inflammatory markers in chronic thromboembolic pulmonary hypertension patients. Eur Respir J. 2014;44(4):951–962. doi:10.1183/09031936.00145013
108. Quarck R, Wynants M, Verbeken E, et al. Contribution of inflammation and impaired angiogenesis to the pathobiology of chronic thromboembolic pulmonary hypertension. Eur Respir J. 2015;46(2):431–443. doi:10.1183/09031936.00009914
109. Le Gal G, Delahousse B, Lacut K, et al. Fibrinogen Aa-Thr312Ala and factor XIII-A Val34Leu polymorphisms in idiopathic venous thromboembolism. Thromb Res. 2007;121:333–338. doi:10.1016/j.thromres.2007.05.003
110. Morris TA, Marsh JJ, Chiles PG, et al. High prevalence of dysfibrinogenemia among patients with chronic thromboembolic pulmonary hypertension. Blood. 2009;114:1929–1936. doi:10.1182/blood-2009-03-208264
111. Marsh JJ, Chiles PG, Liang NC, et al. Chronic thromboembolic pulmonary hypertension- associated dysfibrinogenemias exhibit disorganized fibrin structure. Thromb Res. 2013;132:729–734. doi:10.1016/j.thromres.2013.09.024
112. Remkova A, Simkova I, Valkovicova T. Platelet abnormalities in chronic thromboembolic pulmonary hypertension. Int J Clin Exp Med. 2015;8:9700–9707.
113. Alias S, Redwan B, Panzenbock A, et al. Defective angiogenesis delays thrombus resolution: a potential pathogenetic mechanism underlying chronic thromboembolic pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2014;34:810–819. doi:10.1161/ATVBAHA.113.302991
114. Moser KM, Bloor CM. Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest. 1993;103:685–692. doi:10.1378/chest.103.3.685
115. Dorfmuller P, Gunther S, Ghigna MR, et al. Microvascular disease in chronic thromboembolic pulmonary hypertension: a role for pulmonary veins and systemic vasculature. Eur Respir J. 2014;44:1275–1288. doi:10.1183/09031936.00169113
116. Stasch JP, Evgenov OV. Soluble guanylate cyclase stimulators in pulmonary hypertension. Handb Exp Pharmacol. 2013;218:279–313.
117. Skoro-Sajer N, Mittermayer F, Panzenboeck A, et al. Asymmetric dimethylarginine is increased in chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2007;176:1154–1160. doi:10.1164/rccm.200702-278OC
118. Reesink HJ, Meijer RC, Lutter R, et al. Hemodynamic and clinical correlates of endothelin-1 in chronic thromboembolic pulmonary hypertension. Circ J. 2006;70:1058–1063. doi:10.1253/circj.70.1058
119. Galie N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53:1801889. doi:10.1183/13993003.01889-2018
120. Fauvel C, Raitiere O, Boucly A, et al. Interest of TAPSE/sPAP ratio for noninvasive pulmonary arterial hypertension risk assessment. J Heart Lung Transplant. 2022;41(12):1761–1772. doi:10.1016/j.healun.2022.09.005
121. Farmakis IT, Demerouti E, Karyofyllis P, et al. Echocardiography in pulmonary arterial hypertension: is it time to reconsider its prognostic utility? J Clin Med. 2021;10(13):2868. doi:10.3390/jcm10132826
122. Benza RL, Gomberg-Maitland M, Miller DP, et al. The REVEAL registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest. 2012;141:354–362. doi:10.1378/chest.11-0676
123. McGoon MD, Miller DP. REVEAL: a contemporary US pulmonary arterial hypertension registry. Eur Respir Rev. 2012;123(123):8–18. doi:10.1183/09059180.00008211
124. Benza RL, Gomberg-Maitland M, Elliott CG, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest. 2019;156(2):323–337. doi:10.1016/j.chest.2019.02.004
125. Benza RL, Kanwar MK, Raina A, et al. Development and validation of an abridged version of the REVEAL 2.0 risk score calculator, REVEAL Lite 2, for use in patients with pulmonary arterial hypertension. Chest. 2021;159(1):337–346. doi:10.1016/j.chest.2020.08.2069
126. Chen C, Watson G, Zhao L. Cyclic guanosine monophosphate signalling pathway in pulmonary arterial hypertension. Vascul Pharmacol. 2013;58(3):211–218. doi:10.1016/j.vph.2012.09.001
127. Donaldson S, Ogunti R, Kibreab A, et al. Riociguat in the treatment of chronic thromboembolic pulmonary hypertension: an evidence-based review of its place in therapy. Core Evid. 2020;15:31–40. doi:10.2147/CE.S172791
128. Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353(20):2148–2157. doi:10.1056/NEJMoa050010
129. Galie N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119(22):2894–2903. doi:10.1161/CIRCULATIONAHA.108.839274
130. Jing ZC, Yu ZX, Shen JY, et al. Vardenafil in pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med. 2011;183(12):1723–1729. doi:10.1164/rccm.201101-0093OC
131. Ghofrani HA, Galie’ N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330–340. doi:10.1056/NEJMoa1209655
132. Ghofrani HA, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319–329. doi:10.1056/NEJMoa1209657
133. Galie’ N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovasc Res. 2004;61:227–237. doi:10.1016/j.cardiores.2003.11.026
134. Channick RN, Simmoneau G, Sitbon O, et al. Effects of the dual endothelin receptor antagonist bosentan in patients with pulmonary hypertension: a randomized placebo-controlled study. Lancet. 2001;358(9288):1119–1123. doi:10.1016/S0140-6736(01)06250-X
135. Galie’ N, Badesch BD, Oudiz R, et al. Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2005;46(3):529–535. doi:10.1016/j.jacc.2005.04.050
136. Galie’ N, Olschewski H, Oudiz R, et al. Ambrisentan for the treatment of pulmonary arterial hypertension. Results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117(23):3010–3019. doi:10.1161/CIRCULATIONAHA.107.742510
137. Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369(9):809–818. doi:10.1056/NEJMoa1213917
138. Galie’ N, Manes A, Branzi A. Prostanoids for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2003;2:123–137.
139. Rubin LJ, Mendoza J, Hood M, et al. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trial. Ann Intern Med. 1990;112:485–491. doi:10.7326/0003-4819-112-7-485
140. Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334(5):296–302. doi:10.1056/NEJM199602013340504
141. Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40(4):780–788. doi:10.1016/S0735-1097(02)02012-0
142. McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106(12):1477–1482. doi:10.1161/01.CIR.0000029100.82385.58
143. Rosenzweig EB, Kerstein D, Barst RJ. Long-term prostacyclin for pulmonary hypertension with associated congenital heart defects. Circulation. 1999;99(14):1858–1865. doi:10.1161/01.CIR.99.14.1858
144. Krowka MJ, Frantz RP, McGoon MD, et al. Improvement in pulmonary hemodynamics during intravenous epoprostenol (prostacyclin): a study of 15 patients with moderate to severe portopulmonary hypertension. Hepatology. 1999;30(3):641–648. doi:10.1002/hep.510300307
145. Cabrol S, Souza R, Jais X, et al. Intravenous epoprostenol in inoperable chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant. 2007;26(4):357–362. doi:10.1016/j.healun.2006.12.014
146. Simmoneau G, Barst RJ, Galie’ N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:800–804. doi:10.1164/ajrccm.165.6.2106079
147. Barst RJ, Galie’ N, Naeije R, et al. Long-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinil. Eur Respir J. 2006;28:1195–1203. doi:10.1183/09031936.06.00044406
148. McLaughlin V, Benza RL, Rubin LJ, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized, controlled trial. J Am Coll Cardiol. 2010;55(18):1915–1922. doi:10.1016/j.jacc.2010.01.027
149. Jing ZC, Parikh K, Pulido T, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation. 2013;127:624–633. doi:10.1161/CIRCULATIONAHA.112.124388
150. Olschewski H, Simmoneau G, Galie’ N, et al. Inhaled iloprost in severe pulmonary hypertension. N Engl J Med. 2002;347:322–329. doi:10.1056/NEJMoa020204
151. Higenbottam T, Butt AY, McMahon A, et al. Long-term intravenous prostaglandin (epoprostenol or iloprost) for treatment of severe pulmonary hypertension. Heart. 1998;80(2):151–155. doi:10.1136/hrt.80.2.151
152. Norel X. Prostanoid receptors in the human vascular wall. Sci World J. 2007;7:1359–1374. doi:10.1100/tsw.2007.184
153. Humbert M, Lau EM, Montani D, et al. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation. 2014;130(24):2189–2208. doi:10.1161/CIRCULATIONAHA.114.006974
154. Mubarek KK. A review of prostaglandin analogues in the management of patients with pulmonary arterial hypertension. Respir Med. 2010;104:9–21. doi:10.1161/01.CIR.103.1.10
155. McLaughlin VV, Channick R, Chin KM, et al. Effect of selexipag on morbidity/mortality in pulmonary arterial hypertension: results of the GRIPHON study. J Am Coll Cardiol. 2015;65(Suppl A):A380. doi:10.1016/S0735-1097(15)61538-8
156. Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373(26):2522–2533. doi:10.1056/NEJMoa1503184
157. Gaine S, Chin K, Coghlan G, et al. Selexipag for the treatment of connective tissue disease-associated pulmonary arterial hypertension. Eur Respir J. 2017;50:1602493. doi:10.1183/13993003.02493-2016
158. Simmoneau G, Rubin LJ, Nazzareno G, et al. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med. 2008;149(8):521–530. doi:10.7326/0003-4819-149-8-200810210-00004
159. Barst RJ, Oudiz RJ, Beardsworth A, et al. Tadalafil monotherapy as an add-on to background bosentan in patients with pulmonary arterial hypertension. J Heart Lung Transplant. 2011;30(6):632–643. doi:10.1016/j.healun.2010.11.009
160. Galie’ N, Barbara JA, Frost A, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;379:834–844. doi:10.1056/NEJMoa1413687
161. D’Alto M, Badagliacca R, Giudice FL, et al. Hemodynamics and risk assessment 2 years after the initiation of upfront ambrisentan-tadalafil in pulmonary arterial hypertension. J Heart Lung Transplant. 2020;39(12):1389–1397. doi:10.1016/j.healun.2020.08.016
162. Chin KM, Sitbon O, Doelberg M, et al. Three- versus two-drug therapy for patients with newly diagnosed pulmonary arterial hypertension. J Am Coll Cardiol. 2021;78:1393–1403. doi:10.1016/j.jacc.2021.07.057
163. Farmakis IT, Vrana E, Mouratoglou S-A, et al. Haemodynamic effects of initial combination therapy in pulmonary arterial hypertension: a systematic review and meta-analysis. ERJ Open Res. 2022;8(4):00313–2022. doi:10.1183/23120541.00313-2022
164. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults. CHEST guideline and expert panel report. Chest. 2014;146(2):449. doi:10.1378/chest.14-0793
165. Lang IM, Pesavento R, Bonderman D, et al. Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: a current understanding. Eur Respir J. 2013;41(2):462–468. doi:10.1183/09031936.00049312
166. Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124:1973–1981.
167. Tanabe N. Analysis of Chronic Thromboembolic Pulmonary Hypertension (Intractable Disease Database). Tokyo: Ministry of Health, Wealth and Labor; 2008.
168. Valerio L, Mavromanoli AC, Barco S, et al. Chronic thromboembolic pulmonary hypertension and impairment after pulmonary embolism: the FOCUS study. Eur Heart J. 2022;43:3387–3398.
169. Pepke-Zaba J, Hoeper MM, Humbert M. Chronic thromboembolic pulmonary hypertension: advances from bench to patient management. Eur Respir J. 2013;41(1):8–9. doi:10.1183/09031936.00181212
170. Tunariu N, Gibbs SJR, Win Z, et al. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med. 2007;48:680–684. doi:10.2967/jnumed.106.039438
171. He J, Fang W, Lv B, et al. Diagnosis of chronic thromboembolic pulmonary hypertension: comparison of ventilation/perfusion scanning and multidetector computed tomography pulmonary angiography with pulmonary angiography. Nucl Med Commun. 2012;33(5):459–463. doi:10.1097/MNM.0b013e32835085d9
172. Madani MM, Auger WR, Preorius V, et al. Pulmonary endarterectomy: recent changes in a single institution’s experience of more than 2700 patients. Ann Thorac Surg. 2012;94:97–103. doi:10.1016/j.athoracsur.2012.04.004
173. D’Armini AM, Morsolini M, Mttiucci G, et al. Pulmonary endarterectomy for distal chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg. 2014;148:1005–1011. doi:10.1016/j.jtcvs.2014.06.052
174. Lankeit M, Krieg V, Hobohm L, et al. Pulmonary endarterectomy in chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant. 2018;37(2):250–258. doi:10.1016/j.healun.2017.06.011
175. Delcroix M, Lang I, Pepke-Zaba J, et al. Long-term outcome of patient with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. Circulation. 2016;133:859–871. doi:10.1161/CIRCULATIONAHA.115.016522
176. Quadery SR, Swift AJ, Billings CG, et al. The impact of patient choice on survival in chronic thromboembolic pulmonary hypertension. Eur Respir J. 2018;52:1800589. doi:10.1183/13993003.00589-2018
177. Hsieh WC, Jansa P, Huang WC, et al. Residual pulmonary hypertension after pulmonary endarterectomy: a meta-analysis. J Thorac Cardiovasc Surg. 2018;156(3):1275–1287. doi:10.1016/j.jtcvs.2018.04.110
178. Ogo T. Balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension. Curr Opin Pulm Med. 2015;21(5):425–431. doi:10.1097/MCP.0000000000000188
179. Fukui S, Ogo T, Goto Y, et al. Exercise intolerance and ventilatory inefficiency improve early after balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Int J Cardiol. 2015;180:66–68. doi:10.1016/j.ijcard.2014.11.187
180. Feinstien JA, Goldhaber SZ, Lock JE, et al. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation. 2001;103:10–13.
181. Darocha S, Pietura R, Pietrasik A, et al. Improvement in quality of life and hemodynamics in chronic thromboembolic pulmonary hypertension treated with balloon pulmonary angioplasty. Circ J. 2017;81(4):552–557. doi:10.1253/circj.CJ-16-1075
182. Mahmud E, Behnamfar O, Ang L, et al. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Interv Cardiol Clin. 2018;7:103–117. doi:10.1016/j.iccl.2017.09.003
183. Wiedenroth CB, Liebetrau C, Breithecker A, et al. Combined pulmonary endarterectomy and balloon angioplasty in patients with chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant. 2016;35:591–596. doi:10.1016/j.healun.2015.10.030
184. Simmonneau G, D’Armini AM, Ghofrani H-A, et al. Riociguat for the treatment of thromboembolic pulmonary hypertension: a long-term extension study (CHEST-2). Eur Respir J. 2015;45(5):1293–1302. doi:10.1183/09031936.00087114
185. Ghofrani HA, Simmoneau G, D’Armini AM, et al. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): results from the multicentre, phase 2, randomized, double-blind, placebo-controlled study. Lancet Respir Med. 2017;5:785–794. doi:10.1016/S2213-2600(17)30305-3
186. Sadushi-Kolici R, Jansa P, Kopec G, et al. Subcutaneous treprostinil for the treatment of severe non-operable chronic thromboembolic pulmonary hypertension (CTEPH): a double-blind, phase 3, randomized controlled trial. Lancet Respir Med. 2019;7:239–248. doi:10.1016/S2213-2600(18)30367-9
187. Jensen KW, Kerr KM, Fedullo PF, et al. Pulmonary hypertensive medical therapy in chronic thromboembolic pulmonary hypertension before pulmonary thromboendarterectomy. Circulation. 2009;120(13):1248–1254. doi:10.1161/CIRCULATIONAHA.109.865881
188. Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141(3):702–710. doi:10.1016/j.jtcvs.2010.11.024
189. Sueta CA, Gheorghiade M, Adams KF, et al; Epoprostenol Multicenter Research Group. Safety and efficacy of epoprostenol in patients with severe congestive heart failure. Am J Cardiol. 1995;75:34A–43A.
190. Califf RM, Adams KF, McKenna WJ, et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: the Flolan International Randomized Survival Trial (FIRST). Am Heart J. 1997;134(1):44–54. doi:10.1016/S0002-8703(97)70105-4
191. Vachiery JL, Delcroix M, Al-Hiti H, et al. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J. 2018;51:1701886. doi:10.1183/13993003.01886-2017
192. Zhuang XD, Long M, Li F, et al. PDE5 inhibitor sildenafil in the treatment of heart failure: a meta-analysis of randomized controlled trials. Int J Cardiol. 2014;172(3):581–587. doi:10.1016/j.ijcard.2014.01.102
193. Guazzi M, Vicenzi M, Arena R, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124(2):164–174. doi:10.1161/CIRCULATIONAHA.110.983866
194. Hoendermis ES, Liu LCY, Hummel YM, et al. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur Heart J. 2015;36(38):2565–2573. doi:10.1093/eurheartj/ehv336
195. Redfield MM, Chen HH, Borlaug BA, et al. RELAX trial. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309(12):1268–1277. doi:10.1001/jama.2013.2024
196. Bonderman D, Ghio S, Felix SB, et al. Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation. 2013;128(5):502–511. doi:10.1161/CIRCULATIONAHA.113.001458
197. Hurdman J, Condliffe R, Elliot CA, et al. Pulmonary hypertension in COPD: results from the ASPIRE registry. Eur Respir J. 2013;41(6):1292–1301. doi:10.1183/09031936.00079512
198. Vizza CD, Hoeper MM, Huscher D, et al. Pulmonary hypertension in patients with COPD: results from COMPERA. Chest. 2021;160(2):678–689. doi:10.1016/j.chest.2021.02.012
199. Vitulo P, Stanziola A, Confalonieri M, et al. Sildenafil in severe pulmonary hypertension associated with chronic obstructive pulmonary disease: a randomized controlled multicenter clinical trial. J Heart Lung Transplant. 2017;36(2):166–174. doi:10.1016/j.healun.2016.04.010
200. Goudie AR, Lipworth BJ, Hopkinson PJ, et al. Tadalafil in patients with chronic obstructive pulmonary disease: a randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2014;2(4):293–300. doi:10.1016/S2213-2600(14)70013-X
201. Stolz D, Rasch H, Linka A, et al. A randomized, controlled trial of bosentan in severe COPD. Eur Respir J. 2008;32(3):619–628. doi:10.1183/09031936.00011308
202. Nathan SD, Behr J, Collard HR, et al. Riociguat for idiopathic interstitial pneumonia-associated pulmonary hypertension (RISE-IIP): a randomized, placebo-controlled phase 2b study. Lancet Respir Med. 2019;7:780–790. doi:10.1016/S2213-2600(19)30250-4
203. Waxman A, Restrepo-Jaramillo R, Thenappan T, et al. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med. 2021;384(4):325–334. doi:10.1056/NEJMoa2008470
204. Bartolome S, Hoeper MM, Klepetko W. Advanced pulmonary arterial hypertension: mechanical support and lung transplantation. Eur Respir Rev. 2017;26(146):170089. doi:10.1183/16000617.0089-2017
205. Moser B, Jaksch P, Taghavi S, et al. Lung transplantation for idiopathic pulmonary arterial hypertension on intraoperative and postoperatively prolonged extracorporeal membrane oxygenation provide optimally controlled reperfusion and excellent outcome. Eur J Cardiothorac Surg. 2018;53:178–185. doi:10.1093/ejcts/ezx212
206. Kuntz CL, Hadjiliadis D, Ahya VN, et al. Risk factors for early primary graft dysfunction after lung transplantation: a registry study. Clin Transplant. 2009;23(6):819–830. doi:10.1111/j.1399-0012.2008.00951.x
207. Kurzyna M, Dabrowski M, Bielecki D, et al. Atrial septostomy in treatment of end-stage right heart failure in patients with pulmonary hypertension. Chest. 2007;131(4):977–983. doi:10.1378/chest.06-1227
208. Horn EM, Chakinala M, Oudiz R, et al. Could pulmonary arterial hypertension patients be at a lower risk from severe COVID-19? Pulm Circ. 2020;10(2):204589402092279.
209. Scuri P, Iacovoni A, Abete R, et al. An unexpected recovery of patients with pulmonary arterial hypertension and SARS-CoV-2 pneumonia: a case series. Pulm Circ. 2020;10(3):204589402095658. doi:10.1177/2045894020956581
210. Nuche J, Perez-Olivares C, Segura de la Cal T, et al. Clinical course of COVID-19 in pulmonary arterial hypertension patients. Rev Esp Cardiol. 2020;73(9):775–778. doi:10.1016/j.recesp.2020.05.028
211. Farmakis IT, Giannakoulas G. Management of COVID-19 in patients with pulmonary arterial hypertension. Heart Fail Clin. 2023;19:107–114. doi:10.1016/j.hfc.2022.07.003
212. Belge C, Quark R, Godinas L, et al. COVID-19 in pulmonary arterial hypertension and chronic Thromboembolic pulmonary hypertension: a reference center survey. ERJ Open Res. 2020;6:00520–2020. doi:10.1183/23120541.00520-2020
213. Lee JD, Burger CD, Delossantos GB, et al. A survey-based estimate of COVID-19 incidence and outcomes among patients with pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension and impact on the process of care. Ann Am Thorac Soc. 2020;17(12):1576–1582. doi:10.1513/AnnalsATS.202005-521OC
214. Montani D, Certain M-C, Weatherald J, et al. COVID-19 in patients with pulmonary hypertension: a national prospective cohort study. Am J Respir Crit Care Med. 2022;206(5):573–583. doi:10.1164/rccm.202112-2761OC
215. Ackerman M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi:10.1056/NEJMoa2015432
216. Hoffman M, Kleine-Weber H, Krueger N, et al. The novel coronavirus 2019 (2019-nCoV) uses SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. Cell. 2020. doi:10.1016/j.cell.2020.02.052
217. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi:10.1016/j.trsl.2020.04.007
218. Profidia A, Pola R. Venous thromboembolism in COVID-19 patients. J Thromb Haemost. 2020;18(6):1516–1517. doi:10.1111/jth.14842
219. Libby P, Luscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41(32):3038–3044. doi:10.1093/eurheartj/ehaa623
220. Torres F, Farber H, Ristic A, et al. Efficacy and safety of ralinepag, a novel oral IP agonist, in PAH patients on mono or dual background therapy: results from a phase 2 randomized, parallel group, placebo-controlled trial. Eur Respir J. 2019;54:1901030. doi:10.1183/13993003.01030-2019
221. Humbert M, McLaughlin V, Gibbs JSR, et al. Sotatercept for the treatment of pulmonary arterial hypertension. N Engl J Med. 2021;384(13):1204–1215. doi:10.1056/NEJMoa2024277
222. Ghofrani HA, Morrell NW, Hoeper MM, et al. Imatinib in pulmonary arterial hypertension patients with inadequate response to established therapy. Am J Respir Crit Care Med. 2010;182(9):1171–1177. doi:10.1164/rccm.201001-0123OC
223. Humbert M, Monti G, Fartoukh M, et al. Platelet-derived growth factor expression in primary pulmonary hypertension: comparison of HIV seropositive and HIV seronegative patients. Eur Respir J. 1998;11(3):554–559. doi:10.1183/09031936.98.11030554
224. Hoeper MM, Barst RJ, Bourge RC, et al. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the randomized IMPRES study. Circulation. 2013;127(10):1128–1138. doi:10.1161/CIRCULATIONAHA.112.000765
225. Frost AE, Barst RJ, Hoeper MM, et al. Long-term safety and efficacy of imatinib in pulmonary arterial hypertension. J Heart Lung Transplant. 2015;34(11):1366–1375. doi:10.1016/j.healun.2015.05.025
226. Kimura G, Kataoka M, Inami T, et al. Sorafenib as a potential strategy for refractory pulmonary arterial hypertension. Pulm Pharmacol Ther. 2017;44:46–49. doi:10.1016/j.pupt.2017.03.009
227. Keogh A, Dwyer N, Kotlyar E, et al. Acute hemodynamic improvement in chronic pulmonary arterial hypertension on dual therapy following RT234 inhalation. Chest. 2020;158(4):A2162–A2163. doi:10.1016/j.chest.2020.08.1860
228. Lazarus HM, Denning J, Wring S, et al. A trial design to maximize knowledge of the effects of rodatristat ethyl in the treatment of pulmonary arterial hypertension (ELEVATE 2). Pulm Circ. 2022;12:e12088. doi:10.1002/pul2.12088
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


