Back to Journals » Journal of Blood Medicine » Volume 12
The Escalation of Osteosarcoma Stem Cells Apoptosis After the Co-Cultivation of Peripheral Blood Mononuclear Cells Sensitized with Mesenchymal Stem Cells Secretome and Colony Stimulating Factor-2 in vitro
Authors Mahyudin F, Prawiragara FA, Edward M, Utomo DN , Basuki MH, Bari YA, Nugraha AP, Rantam FA
Received 22 March 2021
Accepted for publication 22 June 2021
Published 8 July 2021 Volume 2021:12 Pages 601—611
DOI https://doi.org/10.2147/JBM.S305566
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Ferdiansyah Mahyudin,1 Fachrizal Arfani Prawiragara,1 Mouli Edward,1 Dwikora Novembri Utomo,1 Mohammad Hardian Basuki,1 Yunus Abdul Bari,1 Alexander Patera Nugraha,2 Fedik Abdul Rantam3
1Orthopedic and Traumatology Department, Faculty of Medicine, Dr Soetomo General Hospital, Airlangga University, Surabaya, Indonesia; 2Orthodontics Department, Faculty of Dental Medicine, Universitas Airlangga, Surabaya, Indonesia; 3Laboratory of Virology and Immunology, Microbiology Department, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia
Correspondence: Ferdiansyah Mahyudin Email [email protected]
Introduction: Peripheral blood mononuclear cells (PBMCs) sensitized with mesenchymal stem cells (MSCs) secretome and/or colony stimulating factor-2 (CSF-2) as an immunotherapy candidate may escalate osteosarcoma stem cells (OS-SCs) apoptosis. This study aimed to investigate the escalation of osteosarcoma stem cells’ apoptosis after the co-cultivation with PBMCs sensitized by MSCs secretome with/or CSF-2 and it was completed by analyzing the level of serum tumor necrosis factor-related apoptosis-inducing ligand (sTRAIL) and tumor necrosis factor-α (TNF-α) level, annexin V binding, caspase-3 and caspase-8 expression in vitro.
Methods: OS-SCs were derived from a single human osteosarcoma sample with its high grade and osteoblastic essential clinical characteristics obtained from a biopsy before the chemotherapy treatment. They were then isolated and cultured confirmed by the cluster of differentiation-133 (FITC) by applying immunofluorescence analysis with fluorescein isothiocyanate (FITC) labeled. MSCs secretome was obtained with cells extracted from the bone marrow of a healthy patient. Furthermore, enzyme linked immunosorbent assay (ELISA) was utilized to analyze sTRAIL and TNF-α level in each group. The expression of caspase-3, caspase-8, and annexin V assay in each group was examined by applying the immunofluorescence labeled with FITC. The comparison analysis between treatment groups and the control group was performed by utilizing the analysis of variance (ANOVA) and continued with Tukey Honest Significant Difference (HSD) (p< 0.05).
Results: There was a significant difference in the upregulation of sTRAIL and TNF-α level indicated by the increased annexin V, caspase-3, and caspase-8 expression binding between groups (p< 0.05).
Conclusion: MSCs Secretome and CSF-2 could significantly increase the activity of PBMCs through the improvement of sTRAIL and TNF-α levels which could lead to the escalation of OS-SCs apoptosis through an enhanced expression of caspase 3, caspase 8 and annexin V binding in vitro.
Keywords: cancer, caspase, osteosarcoma, peripheral blood mononuclear cells, mesenchymal stem cells, tumor necrosis factor
Introduction
As the most frequent primary bone tumor, osteosarcoma (OS) has the prevalence for approximately 4–5 out of 1.000.000 per year with higher prevalence in men than in women.1,2 Osteosarcoma is a neoplasm that can be diagnosed based on the histological examination of osteoid production associated with the aggressive malignant mesenchymal cells that tend to metastasize early and it has several types conventionally categorized by the cell types such as osteoblastic, chondroblastic and fibroblastic.3,4
In Indonesia, the incidence of bone tumors at Central Hospital Cipto Mangunkusumo, Jakarta was 1.2%, whereas the incidence of malignant bone tumors was 1.3%. The prevalence of osteosarcoma was one of the top five cancer cases found at the age group of 1–17 years old. The profile evaluation of bone tumors in children at Central Hospital Cipto Mangunkusumo in Jakarta during 1995–2004 showed that 73.7% of the cases were confirmed as osteosarcoma.5
In curing osteosarcoma, the existing therapy that has been used is the conventional therapies including surgery, chemotherapy, and radiotherapy.6 Prior investigation exhibited that homologous recombination fixes defects contained within the designed BRCAness genomic signature of high-grade osteosarcomas and apoptosis. There may be a link between caspase activation and the poly(ADP-ribose) polymerase (PARP). Furthermore, the diminished cell viability is uncovered by administrating Talazoparib on the MNNG/HOS cell line, but there is no sign in the study of apoptosis.7 The growing quality of life in the osteosarcoma patients has only been seen as the incremental changes in survival. In addition, the immune environment of the bone is highly specialized, and several immune signaling pathways have major contribution in bone homeostasis. Thus, the alternative therapy targeting deoxyribonucleate (DNA) that leads to DNA fragmentation known as apoptosis is still urgently needed.8
Moreover, apoptosis can occur through two different pathways, which are the extrinsic pathway and intrinsic pathway. The first pathway can be completed with an external stressor that initiates the apoptosis by activating Fas-associated death domain (FADD)/TNFRSF1A Associated Via Death Domain (TRADD) resulting in the pro-caspase activation.9,10 It is categorized into transduction pathways, oxygen reactive pathways, growth factor pathways, and cytokine pathways. Meanwhile, the intrinsic pathway is an apoptotic process involving mitochondrial dependent cytochrome C, caspase 3, caspase 9, apoptotic protease activating factor-1 (Apaf-1), Bcl-2, BAX, and BAK.11 The various cell death processes mentioned above are most likely becoming the option for osteosarcoma treatment by employing peripheral blood mononuclear cells (PBMCs) as an immunotherapy.
In addition, immunotherapy can be benefited for cancer therapy by applying cancer-specific immunoglobulins including monoclonal antibodies, cancer vaccines, and T-cell therapy. PBMCs consist of several cells such as lymphocytes like monocytes, natural Killer (NK) cells, dendritic cells (DC), T-helper cells (Th1, Th2, Th17), and macrophages which have different phenotypes and activations in the immune system.12 PBMCs significantly contribute in controlling the immune system through cytokine networks or interleukins, secretions and expressions such as fas ligand, perforin, granzyme B, and soluble tumor necrosis factor-related apoptosis-inducing ligand (sTRAIL) which can generate cancer cell necrosis through extrinsic and intrinsic routes.13 Although in many researches, the results revealed that the activation of PBMCs can be raised by administering antioxidants, lipopolysaccharides, or other types of herbs containing tannins.14 There is another compound which has not been widely studied to sensitize and activate PBMCs, that is mesenchymal stem cells (MSCs) secretome.
A secretome of MSCs is a combination of many secreted molecules which includes interleukins, growth factors and any of secreted extracellular vesicles like microvesicles, apoptotic bodies, ectosomes, and exosomes. MSCs secretome might contribute a significant capacity in the physiological activation of PBMCs both in vivo and in vitro.15 Additionally, another molecule that may stimulate and activate the PBMCs is the colony stimulating factor-2 (CSF-2). CSF-2 entails molecules that have the essential part in the transcription factors and immune cell proliferation consisting NK-cells, cytotoxic T lymphocyte (CTL), and monocytes that can expand the activity of mononuclear cells through the secretion of tumor necrosis factor-α (TNF-α), interleukin (IL)-2, and IL-6 with paracrine effect.16
Furthermore, TNF-α is one of the pro-inflammatory cytokines produced by peripheral blood mononuclear cells covering macrophages, T cells, eosinophils, mast cells, NK cells and some nerve cells. TNF-α also has a fundamental part in regulating the immune system which entails inducing the cell death through apoptosis.17 In addition, launched by several cell types, serum tumor necrosis factor-related apoptosis-inducing ligand (sTRAIL) is qualified in interlacing the transmembrane pro-apoptotic death receptors TRAIL-R1/DR4 and TRAIL-R2/DR5 in human. Therefore, the initial marker of pro-apoptotic induction molecule is the levels of TNF-α and TRAIL which reflect the activated extrinsic pathway. The sTRAIL level is influenced by the time, which means that the more it is secreted, the more it can affect the micro environment.18
Significantly, the enhancement of TNF-α and sTRAIL from PBMCs sensitized by CSF-2 and/or MSCs secretome may induce the apoptosis of cancer cells through extrinsic pathway. The biomarker which can be used to detect the apoptosis via extrinsic pathway along with annexin V, caspase-3 and caspase-8.19 Known as one of cellular proteins within the annexin group, annexin V is often employed to identified an apoptosis by tying up phosphatidylserine outside the plasma membrane. Annexin V which binds the uppermost layer of the cellular membrane’s phosphatidylserine could detect the process of cell damage or apoptosis.20 Meanwhile, caspase-3 is the part of protease enzyme family which contributes essentially and fundamentally in the apoptosis and caspase 8 is the initiator to cleave Atg3 during receptor-mediated cell death and induces apoptosis.19
As the cell targets, osteosarcoma stem cells (OS-SCs) can be generated from the patient’s osteosarcoma tissues. Besides that, not only that osteosarcoma tissues are the resource of cancer stem but these tissues can also produce the extracted osteosarcoma associated stromal cells which are non-tumoral mesenchymal stem cell-like cells.20,21 However, the isolated OS-SCs must be confirmed by utilizing the markers of CD133, SOX2 and CD44. The enhanced expression of SOX2 may directly contribute in the growth and initiation of tumor.22 Moreover, SOX2 expression in sarcoma patients is associated with the tumor grade, differentiation, invasive potential and lower patient survival.23 Meanwhile, in osteosarcoma, the features of CD117 and STRO-1 for the most lethal characteristics of the disease-metastasis and drug resistance, these markers offer candidates for the drug delivery of cancer stem cells aiming in eradicating the osteosarcoma.24
The combination of PBMCs with MSCs secretome, or PBMCs with CSF-2, or PBMCs with MSCs secretome and CSF-2 as mentioned above can lead to the discovery of immunotherapy for osteosarcoma treatment. Thus, this study’s main goal was to inquire the escalation of osteosarcoma stem cells (OS-SCs) apoptosis after the co-cultivation with PBMCs sensitized by MSCs secretome with/or colony stimulating factor-2 completed by analyzing sTRAIL and TNF-α level, annexin V binding, caspase-3 and caspase-8 expression in vitro.
Materials and Methods
Study Design
This scientific investigation was an in vitro study and a true experimental post-test only control group design. The size of this study sample was seven samples for each group. The sample was then selected blind-randomly and then assigned into each group. This study was carried out in Dr Soetomo General Hospital (RSUD. Dr Soetomo) and the Stem Cell Research and Development Center, Universitas Airlangga, Surabaya, Indonesia. Related to the study protocol, the ethical health committee of Dr Soetomo General Hospital, Surabaya, East Java, Indonesia, had granted the research ethics authorization for this study.
Isolation, Culture, Sub-Culture of Osteosarcoma Stem Cells
In this study, OS-SCs were derived from a single human osteosarcoma sample with its high-grade and osteoblastic essential clinical characteristics obtained from biopsy before the chemotherapy treatment. OS-SCs were isolated from osteosarcoma tissue with a 3–5 cm excision from the osteosarcoma patient treated at the department of Orthopedics and Traumatology in Dr Soetomo General Hospital, Surabaya, Indonesia. The excision was performed by surgeons after obtaining a written informed consent from the patient for the osteosarcoma tissue collection. The isolated osteosarcoma tissues were then cleaned by washing them with phosphate-buffered saline (PBS) (Sigma Aldrich, US) entailing 5% penicillin/streptomycin (Gibco, US). The specimens of the tissue were chopped in small pieces by applying a sterile scissor then placed into Erlenmeyer tube consisted of 5 mL Type I collagenase 0.075% within PBS containing 2% of penicillin/streptomycin. Twenty percent heat-inactivated fetal bovine serum (FBS) (Gibco, US) contained in 5 mL of α-minimal essential medium (Gibco, US) were blended into the tube to counterbalance the Type I collagenase activity. In addition, samples were then refined afterward by pouring them into the beaker glass wrapped with 3 plies of sterile gauze. The resulted supernatant of filtration was later centrifuged in 2000 rpm for 5 min, then disposed, and its pellet was resuspended by mixing it with PBS for pellet wash. The pellet was rewashed repeatedly and eventually resuspended with the complete medium. For the final step of this procedure, those suspensions were cultivated on the culture plate and then stored inside the incubator with 37°C and 5% CO2.25
Characterization of Osteosarcoma Stem Cells
To start this characterization, the filtration of digested OS-SCs was cultured in the sterile 10 cm petri dish and a complete 10 mL growth medium was added. Then, OS-SCs were incubated in a 5% CO2 incubator with temperature at 37°C for 5 days. The characterization of confluent OS-SCs was carried out by immunofluorescence staining with immunofluorescence isothiocyanate (FITC) labeled using the specific markers of OS-SCs such as cluster of differentiation (CD) CD133+, SOX2, and CD44+.22–25
Isolation and Sensitization of PBMC with BM-MSCs Secretome and/or CSF-2
The collection of peripheral blood sample from healthy volunteers was performed that had been examined for HIV and Hepatitis-B after obtaining the written informed consent from those volunteers. PBMCs isolation was done by employing the Ficoll system with a gradient of 1.077. A sample of 10 mL from the whole blood was mixed with EDTA, then washed with PBS and centrifugation at a speed of 1600 rpm. Next, the supernatant was removed and then the blood was taken with a micropipette and put into a 15-mL tube containing 5 mL Ficoll. Furthermore, 1600 rpm centrifugation was carried out at a temperature of 20°C. The buffy coat was slowly separated with a pipette and placed in sterile PBS. After centrifugation, the pellets were resuspended with a complete growing medium (RMPI 1640, 10% FBS) added with BM-MSCs secretomes or CSF-2 (Sigma Aldrich, US).
Moreover, bone marrow mesenchymal stem cells (BM-MSCs) secretome was generated from healthy volunteer, and the examination of HIV and Hepatitis-B was performed after obtaining the written informed consent from this volunteer. Two growth media were selected for producing mesenchymal stem cells conditioned medium (MSC-CM). Firstly, the chemically defined low glucose DMEM (DMEM-LG) (Sigma Aldrich, US) was selected as a conventionally used medium for MSC culture. Secondly, among the commercially available media designed to specifically support the growth of undifferentiated MSC, a defined, xeno-free, serum-free MSCs NutriStem medium was preferred for this process (Biological Industries, Israel). It was fundamental to note that only basal media without nutrimental supplements (FBS or NutriStem supplement) were used for MSC conditioning.26 BM-MSCs secretomes were generated from the Stem Cells Research and Development Center, Universitas Airlangga, Surabaya, Indonesia, and finally incubated at a 37°C incubator for 2 days.
Co-Cultivation of Osteosarcoma Stem Cells with Sensitized Peripheral Blood Mononuclear Cells
At this step, co-cultivation between the sensitized PBMCs and OS-SCs with a ratio of 5:1 was cultured in a 10 cm petri dish and then 10 mL MEM alpha medium with 20% FBS was administered. Finally, the sensitized PBMCs and OS-SCs were incubated in 5% CO2 incubator at a temperature of 37°C for 5 days.25
The Level of sTRAIL and TNF-α Analysis by Indirect Enzyme Linked Immunosorbent Assay
Enzyme linked immunosorbent assay (ELISA) was employed to analyze sTRAIL (E-EL-H1593, Elabscience, US) and TNF-α levels (E-EL-H0109, Elabscience, US) in each group. The first step was 100 μL supernatant medium coated in 96 well microplate by applying a coating buffer with a ratio of 1:10 for 24 h at 4°C. After an antigen coating, 3 times washing with 0.02% tween-X was performed, then continued with the blocking using 1% BSA. After the washing, each antibody was added in the ratio of 1:1500 and then incubated in an incubator at 37°C for 2 h. In the post-washing process, it was reacted with the secondary conjugate antibody labeled with alkaline phosphatase, combined with PNpp and incentivized in a dark room for 15 min, then the reaction was stopped with 1 N H2O2 or 1N HCl. Finally, the analysis was carried out with an ELISA reader with a 450 wavelength.25
Immunofluorescence Analysis on Annexin V, Caspase-3 and Caspase-8 Expression
After being co-cultivated with sensitized PBMCs after 5 days, OS-SCs were washed with sterile PBS, preserved with acetone at −20°C for 3–5 min, and then rewashed 3 times with PBS. In addition, blocking was performed with 1% serum. In addition, anti-caspase 8, anti-caspase 3, and anti-annexin V FITC-conjugated antibodies as much as 100 μL with a ratio of 1:500 were combined and incubated for 1 h at a 37°C. The comparison was done between normal cells and cells stained with 4,6-diamidino-2-phenylindole (DAPI). The sample was then observed under fluorescent inverted microscope with 20x magnification.25
Data Analysis
All data were collected, then processed, and statistically tested by using 20.0 version of Statistical Program for Social Science (SPSS) for Windows (IBM Corporation, Illinois, Chicago, US). The data was described as an average in each group and the normality data test (p>0.05) was carried out. The comparison analysis between the treatment groups and the control group was done by utilizing the analysis of variance (ANOVA) continued with Tukey Honest Significant Difference (HSD) (p<0.05). The analysis of statistics was employed to investigate the effect of times (2 days, 4 days, and 6 days) and the TNF-α and sTRAIL level, and the expression of caspase-3, caspase-8 and annexin V examination.
Results
OS-SCs were successfully isolated and cultured from a patient’s osteosarcoma tissue with the high grade and osteoblastic essential clinical characteristics showed the morphology of the OS-SCs (Figure 1A–D). The OS-SCs were sub-cultured until the 6th passage and then characterized with CD133+ and CD44+ markers (Figure 1E–H). Moreover, the morphology of PBMCs isolated from healthy people was further sensitized with MSCs secretome and CSF-2 for two days that can be seen in Figure 2A. As a result, the sensitized PBMCs co-cultivated with OS-SCs showed a reactivity that can be seen in Figure 2B–D.
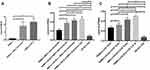
The highest TNF-α level was found in PBMCs + CSF-2 group as a significant difference was also discovered in TNF-α level in 2 days between PBMCs + CSF-2 group and PBMCs alone, and PBMCs + MSCs secretome and PBMCs alone (p<0.05). However, there was no significant difference in TNF-α level between PBMCs + CSF-2 group and PBMCs + MSCs secretome group in 2 days (p>0.05) (Figure 3A).
The highest sTRAIL level was expressed in PBMCs+OS-SCs+ MSCs secretome (6d) group. There was a significant difference located in sTRAIL level between OS-SCs group, PBMCs+MSCs secretome (2d) group, PBMCs+OS-SCs+MSCs secretome (2d) group and PBMCs+OS-SCs+MSCs secretome (6d) group (p<0.05). In addition, the significant difference was also shown in sTRAIL secretion between PBMCs+OS-SCs+MSCs secretome (6d) group and OS-SCs (6d) group (p<0.05) (Figure 3B). The highest sTRAIL level was found in PBMCs+OS-SCs+CSF2 group. A significant difference was also presented in sTRAIL level between OS-SCs (6d) group, PBMCs+CSF2 (2d) group, PBMCs+OS-SCs+CSF2 (2d) group, PBMCs+OS-SCs+CSF2 (4d) group and PBMCs+OS-SCs+CSF2 (6d) group (p<0.05). Moreover, a significant difference was also found between PBMCs+OS-SCs+CSF2 (6d) group and OS-SCs (6d) group (p<0.05) (Figure 3C). Annexin V binding was positively detected in OS-SCs of each group which can be seen in Figure 4A–C. Before and after the co-cultivation of OS-SCs and PBMCs sensitized with MSCs secretome can be seen in Figure 4A1 and A2. Before and after the co-cultivation of OS-SCs and PBMCs sensitized with CSF-2 can be seen in Figure 4B1 and B2. Before and after the co-cultivation of OS-SCs and PBMCs sensitized with MSCs secretome and CSF-2 can be seen in Figure 4C1 and C2. The highest Annexin V expression was uncovered in OS-SCs+PBMCs+MSCs Secretome+CSF2 group. Correspondingly, a significant difference was exhibited in Annexin V binding between groups (OS-SCs; OS-SCs+PBMCs; OS-SCs+PBMCs+Scrt; OS-SCs+PBMCs+Scrt+CSF) (p<0.05) (Figure 4D).
The expression of caspase-3 was exhibited positively in OS-SCs of each group, which can be seen in Figure 5A–C. Before and after the co-cultivation of OS-SCs and PBMCs sensitized with MSCs secretomes shown in Figure 5A1 and A2. Before and after the co-cultivation of OS-SCs and PBMCs and sensitized with CSF-2 can be seen in Figure 5B1 and B2. Before and after the co-cultivation of OS-SCs and PBMCs sensitized with MSCs secretomes and CSF-2 shown in Figure 5C1 and C2. The highest expression of caspase-3 was revealed in the OS-SCs+PBMCs+ MSCs secretome+CSF2 group. In a same manner, there was a significant difference was in caspase-3 expression between groups of OS-SCs, OS-SCs+PBMCs, OS-SCs+PBMCs+Scrt, OS-SCs+PBMCs+CSF, OS-SCs+PBMCs+Scrt+CSF (p<0.05). However, an insignificant difference was found between OS-SCs+PBMCs+Scrt group, OS-SCs+PBMCs+CSF group, and OS-SCs+PBMCs+Scrt+CSF group (p>0.05) (Figure 5D).
The expression of caspase-8 was revealed positively in OS-SCs of each group (Figure 6A–C). Before and after OS-SCs co-cultivated with PBMCs and sensitized with MSCs secretomes shown in Figure 6A1 and A2. Before and after OS-SCs co-cultivated with PBMCs and sensitized with CSF-2 shown in Figure 6B1 and B2. Before and after OS-SCs co-cultivated with PBMCs and sensitized with MSCs secretomes and CSF-2 Figure 6C1 and C2. The highest expression of caspase-8 was found in OS-SCs+PBMCs+Scrt+CSF group. Moreover, a significant difference was expressed in caspase-8 between groups (p<0.05) (Figure 6D).
Discussion
This investigation explored and closely examined the activity of sensitized PBMCs co-cultivated with OS-SCs. The sensitized PBMCs may secreted cytokine that is beneficial against osteosarcoma in vitro such as the production of memory T cells and regulatory T cells (CD25+) NK cells. The active initiation of NK cells is fundamental to induce OS-SCs apoptosis. The presence of activated CD25+ and NK cells can lead to the resistant type of T cells to the cell-mediated suppression of newly activated myeloid stem cells (MDSCs), and protect the body against tumor development and relapse.27
The sensitized PBMCs with MSCs secretome and/or CSF-2 indicate the presence of activated lymphocytes, namely CD25+, NK cells as well as CD4+ and CD8+ adaptive immune cells.25 The possibility that MSCs secretomes contain IL-2, IL-7 and IL-15 has been widely reported to support the homeostatic T-cell proliferation as well as the increased NK cell function, the maturation of IL-2 terminal NKT cells, and the expanded size of CD8+.28 Meanwhile, CSF-2 stimulates the activity of macrophages, NK cells, and cytotoxic T cells.16 Consequently, the sensitization of PBMCs by MSCs secretome and/or CSF-2 may enhance NK cells, CD4+ and CD8+.
Furthermore, TNF-α level in PBMCs improves after the sensitization of PBMCs through molecule signaling in macrophages. In this study, the result revealed that PBMCs that have been activated with MSCs secretomes or CSF-2 initiate the activation of macrophages to secrete TNF-α. The highest TNF-α level was expressed in PBMCs + CSF-2 group after 2 days. There was a significant difference exhibited in TNF-α level between groups. In addition, TNF-α is a molecule that contributes majorly in regulating the inflammatory factors and as a master in regulating cytokine production.17 In producing various kinds of cytokines and enzymes, macrophages can go through complex processes, which are both passive and active action. Macrophage activation comes from the signaling molecules lymphokine, chemokines, and pathogen associated molecular patterns (PAMPs) and then secretes the products like IL-1, IL-12, TNF-α, IL-10, nitric oxide (NO) and reactive oxidative stress (ROS), IL-18, and IL-17.29
In this study, the highest sTRAIL level was discovered in PBMCs + OS-SCs + MSCs secretome (6d) group. Consistently, a significant difference was demonstrated in sTRAIL levels between groups and in sTRAIL secretion between PBMCs + OS-SCs + MSCs secretome (6d) group and OS-SCs (6d) group. In addition, OS-SCs co-cultivated with PBMCs and sensitized with CSF-2 were significantly different in sTRAIL levels between groups. Furthermore, there was a display of significant difference between PBMCs + OS-SCs + CSF2 (6d) group and OS-SCs (6d) group. Therefore, 6 days was applied as a time frame reference in this study. In fact, TRAIL-3 and TRAIL-4 can be suppressed by receptor activator nuclear kappa beta ligand (RANKL) to induce apoptosis.30
Correspondingly, TNF-α and sTRAIL secreted by PBMCs are molecules that can be used as the indicators in PBMCs activation for osteosarcoma immunotherapy marked by the escalation of OS-SCs apoptosis. Furthermore, the signaling molecule from death-ligand-domain interaction can influence the prevalence of apoptosis via the gathering of death-inducing signaling complex (DISC). It is configurated by the FADD which enhances the autocatalytic caspase processing, caspase activation, and apoptosis. In line with this study, the ability of TRAIL to eliminate cancer cells was investigated by developing a recombinant form of TRAIL or TRAIL receptor agonist for cancer therapy such as receptor-specific monoclonal antibody.31
In this present study, the highest Annexin V binding in OS-SCs + PBMCs + MSCs Secretome + CSF2 was uncovered as a significant difference and found in Annexin V binding between groups. The addition of MSCs secretome and CSF-2 is possible to inhibit the secretion of IL-10; thus, the dominant secretion of pro-inflammatory cytokine occurs.32 The result of this study also revealed that the highest expression of caspase-8 was exhibited in OS-SCs + PBMCs + MSCs secretome + CSF group. Likewise, a significant difference was featured in caspase-8 expression between groups in this study.
Moreover, MSCs Secretome is a molecular complex that maintains the tissue’s microenvironment in a homeostasis condition. Meanwhile, CSF-2 is a pure molecule with the functions in increasing the activity of macrophages to response the imbalance condition by inducing, initiating, and signaling the macrophage’s activation. Subsequently, the activated macrophage will proliferate, differentiate and polarize the macrophage rapidly.33 Additionally, the activation of caspase-8 is stimulated the continuous signal from extrinsic factors to endogenous ligands.34 At the same time, the activation of macrophages that produces TNF-α and TRAIL which can activate endogenous ligands. The increased level of TNF-α and TRAIL depends on the time of production which can affect quickly or not in inducing the apoptosis. The extrinsic factor of apoptosis is influenced by the time and the possibility of inductor concentration against endogenous ligands. The extrinsic pathway of apoptosis is strongly influenced by the activity of caspase-3.35 In this present study, the highest expression of caspase-3 was found in the OS-SCs + PBMCs + MSCs secretome + CSF2 group. Accordingly, there was a significant difference demonstrated in caspase-3 expression between groups. This was in line with the previous study that investigated Shikonin from medical herbs that can enhance the osteosarcoma apoptosis through the up-regulation of caspase-3 and caspase-8.36 The presence of interactions between molecules is also influenced by the time, the concentration and the microenvironment which is very dominant in facilitating the level of TRAIL and TNF-α as the initial inductor that can trigger annexin V, caspase-8 and caspase-3 activation. The major limitation in this study is these cells that were derived only from a single human osteosarcoma tissue and a single healthy human BM-MSCs secretome.
Conclusion
Based on our study, MSCs secretome and CSF-2 can increase the activity of PBMCs through the enhancement of sTRAIL and TNF-α levels that can lead to the escalation of OS-SCs apoptosis via a raised expression of caspase 3 and caspase 8 which can be indicated by the increased annexin V binding in vitro. Further study is still needed to examine the apoptosis signaling pathway of OS-SCs co–cultivated by PBMCs sensitized with CSF-2 and/or MSCs secretome in vitro and in vivo.
Data Sharing Statement
Please contact the correspondence author with requests for data supporting reported results.
Institutional Review Board Statement
The study was performed accordingly based on the guidelines derived from the Declaration of Helsinki and the Ethics Committee of Dr Soetomo Regional General Hospital Ethical confirm and approve for these study protocols.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Acknowledgments
We would like to send our gratitude to the Faculty of Medicine, Airlangga University and Dr Soetomo Regional General Hospital for their tremendous support to our study.
Disclosure
The authors declare no conflicts of interest for this work.
References
1. Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F. WHO Classification of Tumours of Soft Tissue and Bone.
2. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13.
3. Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010;21(7):320–325. doi:10.1093/annonc/mdq276
4. Picci P, Manfrini M, Fabbri N, Gambarotti M, Vanel D, Atlas of Musculoskeletal Tumors and Tumorlike Lesions [Internet]. Cham: Springer International Publishing; 2014 [
5. Khan S, Barwar N, Kumar V. Surface osteosarcomas: diagnosis, treatment and outcome. Indian J Orthop. 2014;48(3):255. doi:10.4103/0019-5413.132503
6. Biazzo A, De Paolis M. Multidisciplinary approach to osteosarcoma. Acta Orthop Belg. 2016;82(4):690–698.
7. Kager L, Tamamyan G, Bielack S. Novel insights and therapeutic interventions for pediatric osteosarcoma. Future Oncol. 2017;13(4):357–368. doi:10.2217/fon-2016-0261
8. Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer. 2014;14(11):722–735.
9. Goldar S, Khaniani MS, Derakhshan SM, Baradaran B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac J Cancer Prev. 2015;16(6):2129–2144. doi:10.7314/APJCP.2015.16.6.2129
10. Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17(7):395–417.
11. Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7(12):1166–1173. doi:10.1038/sj.cdd.4400783
12. Fang F, Xiao W, Tian Z. Challenges of NK cell-based immunotherapy in the new era. Front Med. 2018;12(4):440–450. doi:10.1007/s11684-018-0653-9
13. Horinaka M. Application of molecular-targeting cancer prevention to tumor immunity. Nihon Eiseigaku Zasshi. 2014;69(1):8–14. doi:10.1265/jjh.69.8
14. Xie Y, Chen B, Wang H, Wang K, Li R. Advances in the research of peripheral blood mononuclear cell-derived multipotential cell. Zhonghua Shao Shang Za Zhi. 2016;32(12):762–764. doi:10.3760/cma.j.issn.1009-2587.2016.12.013
15. Lötvall J, Rajendran L, Gho YS, et al. The launch of Journal of Extracellular Vesicles (JEV), the official journal of the International Society for Extracellular Vesicles - about microvesicles, exosomes, ectosomes and other extracellular vesicles. J Extracell Vesicles. 2012;16;1. doi:10.1016/0006-2952(75)90016-7
16. Arellano M, Waller K. Granulocyte-macrophage-colony-stimulating factor and other cytokines: as adjuncts to cancer immunotherapy, stem cell transplantation, and vaccines. Curr Hematol Rep. 2004;3(6):424–431.
17. van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11(4):397–408. doi:10.1634/theoncologist.11-4-397
18. von Karstedt S, Montinaro A, Walczak H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat Rev Cancer. 2017;17(6):352–366.
19. Chaudhry GE, Jan R, Naveed Zafar M, Mohammad H, Muhammad TST. Vitex Rotundifolia Fractions Induced Apoptosis in Human Breast Cancer T-47D Cell Line via Activation of Extrinsic and Intrinsic Pathway. Asian Pac J Cancer Prev. 2019;20(12):3555–3562. doi:10.31557/APJCP.2019.20.12.3555
20. Brune JC, Tormin A, Johansson MC, et al. Mesenchymal stromal cells from primary osteosarcoma are non-malignant and strikingly similar to their bone marrow counterparts. Int J Cancer. 2011;129(2):319–330. doi:10.1002/ijc.25697
21. Le Nail LR, Brennan M, Rosset P, et al. Comparison of Tumor- and Bone Marrow-Derived Mesenchymal Stromal/Stem Cells from Patients with High-Grade Osteosarcoma. Int J Mol Sci. 2018;19(3):707. doi:10.3390/ijms19030707
22. Skoda J, Nunukova A, Loja T, et al. Cancer stem cell markers in pediatric sarcomas: sox2 is associated with tumorigenicity in immunodeficient mice. Tumour Biol. 2016;37(7):9535–9548. doi:10.1007/s13277-016-4837-0
23. Adhikari AS, Agarwal N, Wood BM, et al. CD117 and Stro-1 identify osteosarcoma tumor-initiating cells associated with metastasis and drug resistance. Cancer Res. 2010;70(11):4602–4612. doi:10.1158/0008-5472.CAN-09-3463
24. Menendez ST, Rey V, Martinez-Cruzado L, et al. SOX2 Expression and Transcriptional Activity Identifies a Subpopulation of Cancer Stem Cells in Sarcoma with Prognostic Implications. Cancers. 2020;12(4):964. doi:10.3390/cancers12040964
25. Mahyudin F, Yazid H, Edward M, Basuki MH, Bari YA, Rantam FA. The enhancement apoptosis of osteosarcoma mesenchymal stem cells co-cultivation with peripheral blood mononuclear cells sensitized by secretome and granulocyte macrophage colony-stimulating factor. J Adv Pharm Technol Res. 2020;11:213–219. doi:10.4103/japtr.JAPTR_52_20
26. Sagaradze G, Grigorieva O, Nimiritsky P, et al. Conditioned Medium from Human Mesenchymal Stromal Cells: towards the Clinical Translation. Int J Mol Sci. 2019;20(7):1656. doi:10.3390/ijms20071656
27. Anderson J, Toh ZQ, Reitsma A, Do LAH, Nathanielsz J, Licciardi PV. Effect of peripheral blood mononuclear cell cryopreservation on innate and adaptive immune responses. J Immunol Methods. 2019;465:61–66. doi:10.1016/j.jim.2018.11.006
28. Maumus M, Jorgensen C, Noël D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. Biochimie. 2013;95(12):2229–2234. doi:10.1016/j.biochi.2013.04.017
29. Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi:10.1002/jcp.26429
30. Secchiero P, Rimondi E, Agnoletto C. C-Reactive protein downregulates TRAIL expression in human peripheral monocytes via an Egr-1-dependent pathway. Clin Cancer Res. 2013;19(8):1949–1959. doi:10.1158/1078-0432.CCR-12-3027
31. Kamohara H, Matsuyama W, Shimozato O, Abe K, Galligan C, Yoshimura T. Regulation of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) and TRAIL receptor expression in human neutrophils. Immunology. 2004;111(2):186–194. doi:10.1111/j.0019-2805.2003.01794.x
32. Munoz LE, Franz S, Pausch F. The influence on the immunomodulatory effects of dying and dead cells of Annexin V. J Leukoc Biol. 2007;81(1):6–14. doi:10.1189/jlb.0306166
33. Zhou D, Huang C, Lin Z, et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26(2):192–197. doi:10.1016/j.cellsig.2013.11.004
34. Fritsch M, Günther SD, Schwarzer R, et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature. 2019;575(7784):683–687. doi:10.1038/s41586-019-1770-6
35. Wang W, Zhu M, Xu Z, et al. Ropivacaine promotes apoptosis of hepatocellular carcinoma cells through damaging mitochondria and activating caspase-3 activity. Biol Res. 2019;52(1):36. doi:10.1186/s40659-019-0242-7
36. Yang Q, Li S, Fu Z, et al. Shikonin promotes adriamycin-induced apoptosis by upregulating caspase-3 and caspase-8 in osteosarcoma. Mol Med Rep. 2017;16(2):1347–1352. doi:10.3892/mmr.2017.6729
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.