Back to Journals » Infection and Drug Resistance » Volume 15
The Epidemiological, Clinical Features and Outcomes of Imported Chinese COVID-19 Patients Following Inactivated Vaccines Injection
Authors Li J, Jiang N, Zeng QL, Zhang Y, He X, Chu Y, Jin W, Liu Y, Shi W, Yang M, He W, Han Q, Ma L, Xu Y, Guo Y, Zhang L , Ji F
Received 31 December 2021
Accepted for publication 7 April 2022
Published 22 April 2022 Volume 2022:15 Pages 2115—2125
DOI https://doi.org/10.2147/IDR.S356460
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Jianwu Li,1,2,* Na Jiang,1,2,* Qing-Lei Zeng,3,* Yue Zhang,4 Xinyuan He,4 Yao Chu,1,2 Wenni Jin,1,2 Yi Liu,4 Wan Shi,1,2 Miao Yang,1,2 Weihan He,1,2 Qing Han,5 Le Ma,4 You Xu,1,2 Yaling Guo,1,2 Lei Zhang,6– 9 Fanpu Ji2,4,10,11
1Isolation Ward for Covid-19, the Eighth Hospital of Xi’an City, Xi’an Jiaotong University, Xi’an, Shaanxi, People’s Republic of China; 2Shaanxi Provincial Clinical Medical Research Center of Infectious Diseases, Xi’an, Shaanxi, People’s Republic of China; 3Department of Infectious Diseases, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China; 4Department of Infectious Diseases, the Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, People’s Republic of China; 5Department of Integrated Traditional Chinese and Western Medicine, the Eighth Hospital of Xi’an City, Xi’an Jiaotong University, Xi’an, Shaanxi, People’s Republic of China; 6China-Australia Joint Research Centre for Infectious Diseases, School of Public Health, Xi’an Jiaotong University Health Science Centre, Xi’an, Shaanxi, People’s Republic of China; 7Artificial Intelligence and Modelling in Epidemiology Program, Melbourne Sexual Health Centre, Alfred Health, Melbourne, Australia; 8Central Clinical School, Faculty of Medicine, Monash University, Melbourne, Australia; 9Department of Epidemiology and Biostatistics, College of Public Health, Zhengzhou University, Zhengzhou, Henan, People’s Republic of China; 10National & Local Joint Engineering Research Center of Biodiagnosis and Biotherapy, the Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, People’s Republic of China; 11Key Laboratory of Environment and Genes Related to Diseases, Xi’an Jiaotong University, Ministry of Education of China, Xi’an, Shaanxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fanpu Ji, Department of Infectious Diseases, the Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, People’s Republic of China, Tel/Fax +862987678223, Email [email protected] Yaling Guo, Isolation ward for COVID-19, the Eighth Hospital of Xi’an City, Xi’an Jiaotong University, Xi’an, Shaanxi Province, 710061, People’s Republic of China, Tel/Fax +8629 85230200, Email [email protected]
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination had been demonstrated as an effective way to reduce the risk of coronavirus disease 2019 (COVID-19), and only a few vaccines suffered from SARS-CoV-2 infection. However, limited data concerning the clinical features of these vaccines infected with SARS-CoV-2 can be identified.
Methods: We retrospectively collected and analyzed epidemiological and clinical characteristics data of the imported COVID-19 cases who received Chinese inactivated vaccines abroad. Data were extracted from electronic medical records from a designated hospital in the Shaanxi Province of China between March 22 and May 17, 2021.
Results: Totally, 46 confirmed SARS-CoV-2 infection patients were enrolled. The mean age was 40.5 years (range 20– 61), 41 (89.1%) are male. Eighteen (39.1%) patients were from Pakistan. Fourteen (30.4%) patients had at least one comorbidity. Forty (87.0%) and 6 cases were fully vaccinated and partly vaccinated. The time interval between vaccination and infection was 88 days (IQR, 33– 123), 31 (67.4%) and 15 (32.6%) were asymptomatic and symptomatic cases, respectively. Fever (3/46, 6.5%) was the most common symptom; however, none had a body temperature higher than 38.0°C, and no severe case was observed. Notably, the rate of SARS-CoV-2 shedding discontinuation at 7 days after hospitalization in asymptomatic cases was higher than symptomatic one (93.5% vs 40%, P < 0.0001).
Conclusion: Individuals who received Chinese inactivated vaccines abroad remain to have the probability of being infected with SARS-CoV-2, but all the vaccines infected with SARS-CoV-2 were asymptomatic or had mild symptoms with favorable clinical outcomes.
Keywords: SARS-CoV-2, COVID-19, vaccination, reinfection, asymptomatic infection
Introduction
As of June 19, 2021, the coronavirus disease 2019 (COVID-19) pandemic had caused more than 179 million cases and 3.8 million deaths worldwide.1 Unfortunately, the numbers of both infected patients and fatalities are still growing. Although social distance, quarantine, isolation and lockdown restrictions were effective in limiting the infection and spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a short period, they were not the long-term solution. The absence of immunity in the population leaves them susceptible to further waves of SARS-CoV-2 infection. Effective and safe vaccines were urgently needed for the global epidemic control of COVID-19. There were several vaccine types, such as mRNA vaccines, inactivated vaccines, adenovirus-vectored vaccines, protein subunit vaccines, and virus-like particle vaccines, had been developed or approved to be used urgently.2–6 And these vaccines had shown favorable safety and tolerability. They induced high neutralizing antibodies levels against SARS-CoV-2 in the Phase 1/2 trials and were highly effective in the prevention of both asymptomatic and symptomatic SARS-CoV-2 infection in Phase 3 clinical trials and real-world study.7–15 However, the production of neutralizing antibodies cannot completely protect individuals from the SARS-CoV-2 infection, especially with the emergence of mutant virus strains, vaccine breakthrough infection had been reported after BNT162b2 or mRNA-1273 vaccination.16 Furthermore, vaccination population showed lower neutralizing sensitivity and protective efficacy for omicron variant, compared to other SARS-CoV-2 variants, especially for people with inactivated vaccines injection.17,18 However, different booster vaccines significantly increased neutralizing antibodies (NAbs) in patients with two doses of inactivated vaccines, including the omicron variant.18,19
Currently, China has three inactivated vaccines, which have been approved to carry out phase 1/2/3 clinical studies and are widely licensed in more than 80 countries worldwide. A recent double-blind, randomized, phase 3 study performed in four Middle East countries had revealed that two inactivated SARS-CoV-2 vaccination could reduce 72.8–78.1% the risk of symptomatic COVID-19 in adults.20 And despite the mild low protective efficacy, two doses of CoronaVac could still prevent as high as 65.3–65.9% of symptomatic infections.21,22
In the study by et al, a total of 47 symptomatic COVID-19 cases and 26 asymptomatic cases were identified among 25,440 participants who received two doses of vaccines. However, the detailed clinical characteristics of these symptomatic and asymptomatic COVID-19 cases after injection of inactivated vaccines are rare to date. The current study intends to fill this knowledge gap from a unique aspect of imported Chinese COVID-19 cases who injected the inactivated vaccines.
Materials and Methods
Study Design and Participants
The Chinese government stipulates that all passengers abroad entering China mainland, including Xi’an City, should conduct pharyngeal swab nucleic acid screening and should be centralized quarantined and (or) treated in a designated hospital, and The Eighth Hospital of Xi’an City was the only location in Shaanxi Province. This retrospective, single center, observational study included consecutive hospitalized patients who received at least one dose of vaccine and who were confirmed to have SARS-CoV-2 infection between March 22 and May 17, 2021, in The Eighth Hospital of Xi’an City, Shaanxi Province, China.
As the most important port of entry in Northwest China, Xi’an City has continued to have imported cases. Passengers with positive results of SARS-CoV-2 RNA in the first screening were sent to our hospital for isolation, and the second specimens were then sent to the Centers for Disease Control and Prevention of Shaanxi Province or Xi’an City for reconfirmation. SARS-CoV-2 infection was diagnosed based on two times positive SARS-CoV-2 in the throat swab specimens from the upper respiratory tract with or without positive SARS-CoV-2 IgM and/or IgG, according to the Diagnosis and Treatment Scheme for COVID-19 released by the National Health Commission of China (8th Edition).23 All patients received the third SARS-CoV-2 RNA tests 7 days after hospitalization. The diagnostic reagents of RT-PCR were provided by the four companies (Beijing Applied Biological Technology, Shanghai Zhijiang Biotechnology, Shanghai BioGerm Medical Biotechnology and Shanghai Huirui Biotechnology). The procedures of specimen pretreatment, RNA extraction, RT-PCR reaction conditions, and results interpretation were strictly followed the manufacturers’ instructions.
Severity Definition for the SARS-CoV-2 Infection
Asymptomatic infection was defined as an individual with laboratory-confirmed SARS-CoV-2 infection who reported no fever and no respiratory symptoms, as well as normal chest CT findings.20,23 Symptomatic infection was defined as an individual with laboratory-confirmed SARS-CoV-2 infection who reported symptoms or abnormal chest CT findings, and severity status was categorized as mild, moderate, severe, or critical, according to the Diagnosis and Treatment Scheme for COVID-19 released by the National Health Commission of China.23 Mild COVID-19 cases were defined as mild clinical symptoms and no sign of pneumonia on imaging; moderate COVID-19 cases were defined as showing fever and respiratory symptoms with radiological findings of pneumonia. Severe COVID-19 cases needed to meet any one of the following criteria including: 1) Respiratory distress (RR ≥ 30 breaths/min); 2) Oxygen saturation ≤93% at rest; 3) Arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤300 mmHg (1 mmHg = 0.133 kPa); 4) The clinical symptoms progressively worsened, and the chest imaging showed >50% obvious lesion progression within 24–48 hours. Critical COVID-19 cases were defined as meeting any one of the following criteria: 1) Respiratory failure and requiring mechanical ventilation; 2) Shock; 3) With other organ failure that requires ICU care; or 4) Death.23
Full vaccination was defined as an individual who had received the second dose of vaccine for more than 14 days. Patients, who only received one dose of the vaccine, received two doses of the vaccine at a time or completed the second dose of vaccine for less than 14 days were defined as partly vaccinated.20 The time interval of vaccination to infection was defined as the period between the last dose of vaccination and confirmed SARS-CoV-2 infection.
Data Collection
Data were extracted from the electronic medical records using a pre-designed case report form. We extracted the vaccination, demographic, epidemiological, clinical, laboratory, management, and outcome data from patients. We directly communicated to patients if data were missing from the records or if clarification was needed. All data were checked by two physicians.
Statistical Analysis
Data are presented as the mean and standard deviation or median (interquartile range, IQR) for continuous variables and as absolute and relative frequencies for categorical variables. The data in the two groups were compared using Student’s t-test, Mann–Whitney U-test, χ2 test, or Fisher’s exact probability method, as appropriate. Analyses were carried out using SPSS statistical software, version 18.0 (IBM, Chicago, IL, USA). A p-value of <0.05 was set as the threshold for statistical significance.
Ethical Approval and Considerations
The protocol of this retrospective study was in compliance with the Helsinki Declaration and was approved by the Institutional Review Board of The Eighth Hospital of Xi’an City, Xi’an Jiaotong University. The study was exempted from the requirement for informed consent because of the retrospective design and deidentified individuals.
Results
Demographics and Clinical Characteristics at Baseline
Totally, 46 consecutively vaccinated patients were included in this study. The mean age was 40.5 years, 41 (89.1%) are male. All patients have been exposed to COVID-19 family members or patients. Forty-five patients were imported cases, and the remaining one was a local case. Eighteen (39.1%) of them from Pakistan (Table 1). Fourteen (30.4%) patients had at least one comorbidity (Tables 1 and 2).
 |
Table 1 Baseline Characteristics of Patients with SARS-CoV-2 Infection After Vaccination |
 |
Table 2 Demographic and Clinical Characteristics of SARS-CoV-2 Patients with Fully and Partly Vaccinated |
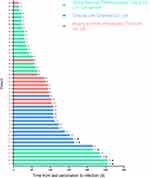
All patients received inactivated vaccines, 23 cases with SARS-CoV-2 Vaccine (Vero Cell) (China National Pharmaceutical Group, Beijing, China), 13 cases with CoronaVac (Sinovac Life Sciences, Beijing, China) and 10 with BIBP-CorV (Beijing Institute of Biological Products, Beijing, China). Forty (87.0%) patients were fully vaccinated, the remaining six patients were partly vaccinated, the detailed information of the latter is showed in Table S1. With the exception of the longer interval between vaccination and infection, no significant differences in the sex, age, BMI, smoking, the rate of symptomatic infection, laboratory testing, chest CT findings and SARS-CoV-2 shedding period were found between the fully and partly vaccinated patients (Table 2). The time interval between vaccination and infection was 88 days (IQR, 33–123), 50% patients infected SARS-CoV-2 more than 90 days after the vaccination, and six patients infected after more than 210 days (Figure 1).
Of the 46 patients, 31 (67.4%) and 15 (32.6%) were asymptomatic and symptomatic infection, respectively. In addition to the symptoms and chest CT findings, no difference was observed in demographic and clinical characteristics in both groups. Notably, the SARS-CoV-2 shedding period was shorter in asymptomatic cases (Table S2). Of the 15 patients with symptomatic infection, all had mild or moderate COVID-19. Fever (3/46) was the most common symptom, followed by cough, fatigue, myalgia, pharyngalgia and headache. No patient presented shortness of breath (Table 1).
Laboratory Characteristics
On admission, 6 and 11 patients had elevated levels of ALT and AST, respectively. Six (13.0%) patients had abnormal myocardial zymogram, including the elevations of LDH and creatine kinase in 4 and 3 patients, respectively. Eighteen (39.1%) and 6 (13.0%) patients had ESR and serum CRP exceeding the upper limit of the normal range, respectively. Only 2 patients had increased procalcitonin and no patients with increased D-dimer (Table 1).
Radiological Characteristics
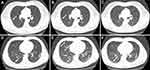
Fourteen (93.3%) of the 15 symptomatic infections had pneumonia manifestation on CT examination on admission. Of them, 6 (42.9%) cases had unilateral pneumonia, 8 (57.1%) had bilateral pneumonia, and 7 (50.0%) had multiple mottling and ground-glass opacity (Table 1). The median remission time of abnormal chest imaging was 7 days (IQR, 6–8). And the representative chest CT images of one mild and one “most severe” patients with moderate COVID-19 are presented in Figure 2.
Treatment and Prognosis
All received combination antiviral treatment with Arbidol Hydrochloride Tablets and interferon-α. For the 15 symptomatic infections, additional traditional Chinese herbal medicine Lianhua Qingwen granules were prescribed for 5–10 days. One patient received methylprednisolone at a dose of 40 mg for 7 days. Four (8.8%) patients received low-flow nasal cannula for 2–3 days; no patient required non-invasive ventilation or high-flow nasal cannula. No patients developed severe illnesses or needed ICU care, or died. And all patients had recovered uneventfully.
Discussion
Currently, the phase 3 trial concerning the efficacy of two Chinese inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults found that 72.8% (95% CI, 58.1%–82.4%) of vaccinees in WIV04 vaccine group and 78.1% (95% CI, 64.8%–86.3%) of vaccinees in HB02 vaccine group acquired immunity against COVID-19 during a median (range) follow-up duration of 77 (1–121) days.20 Two doses of CoronaVac also showed a 65.3–65.9% protection from symptomatic infections.21,22 However, no study reported detailed information on clinical manifestations and prognosis of Chinese patients after inactivated vaccination. The current study aimed to investigate Chinese imported COVID-19 patients after injection of inactivated vaccines. Our results showed that the vaccine protection could last at least 6–9 months. The vaccinated patients infected with SARS-CoV-2 experienced slighter clinical manifestations when historically compared with previous local COVID-19 cases in early 2020.24,25
In the current study, more than two-thirds of patients were asymptomatic infection, and for symptomatic infections, all of their symptoms were mild, no patient presented fever more than 38.0°C, shortness of breath or required high-flow nasal cannula. All symptomatic infections were diagnosed as mild or moderate COVID-19, and no cases were severe, required ICU care or death. Furthermore, the SARS-CoV-2 shedding period was short, and 76% of patients with negative SARS-CoV-2 on the seventh day of hospitalization, which was much shorter than the three weeks in mild or asymptomatic patients without vaccination.26 The chest CT abnormalities of symptomatic infection patients were also mild, 7 (46.7%) of them only unilateral lung involvement or no abnormalities. And the median time of imaging recovery was only 7 days.
Vaccinations have been shown to be highly effective in preventing SARS-CoV-2 infections, COVID-19-related hospitalizations, severe diseases, and death.10,15 The latest evidence indicated that no severe COVID-19 cases were identified after receiving the inactivated vaccines.20 In the current study, no severe COVID-19 cases were identified. Furthermore, the chronic comorbidity and high BMI did not present any risk to contribute the individuals to develop to symptomatic infection other than asymptomatic infection. Additionally, the SARS-CoV-2 shedding period was not different in patients with or without chronic comorbidity or high BMI. These results suggest that even people with a high risk of exacerbation before SARS-CoV-2 infection, such as chronic comorbidity or high BMI,25,27 may benefit more from vaccination. Since vaccines, especially inactivated vaccines, were safe and well tolerated, priority should be given to these high-risk populations.
The risk of SARS-CoV-2 infection after vaccination always has an overall higher probability of exposure to SARS-CoV-2, such as health care workers or individuals who are in close contact with COVID-19 patients.9,12 All the patients in our study were closely contacted with COVID-19 cases, suggesting that social distance and masks are still needed to prevent infection even after vaccination. More than two-thirds of the patients had asymptomatic infection, so it further emphasized the need for consecutive testing of the population who were fully vaccinated and exposed to confirmed COVID-19 patients. Furthermore, strengthening the management of these asymptomatic infections is also an important measure to prevent the spread of SARS-CoV-2 and then to control the COVID-19 pandemic.
The durability of the vaccine-induced immunity protection against infection remains unknown because too little time has elapsed since the start of the vaccination campaign. Studies showed that robust neutralizing antibodies against SARS-CoV-2 re-infection in previous COVID-19 patients could maintain up to 6–12 months.28–30 In the current study, the median interval between vaccination and infection was 88 days, and more than 210 days in 6 patients, including 4 partly vaccinated patients, a fully vaccinated individual who received inactivated vaccine may obtain longer-term immune protection. These results were similar or better than the previous reports that showed the protective effect of the vaccine might persist after 6 months of full vaccination.10,14 However, the emergence of “variants of concern” (VoC) of SARS-CoV-2 had reduced the effectiveness of different vaccines.17,18 NAbs to omicron dropped near the detection limit and waning memory T cell responses in three months after inactivated vaccines injection.17 Despite this, mRNA vaccines had been reported to delay the onset of breakthrough infections with less imaging abnormalities.31 Booster vaccines are needed to increase neutralizing antibodies in fully vaccinated individuals.18,19
Limitations
The current study has several limitations. First, small sample size, as only 46 cases were retrospectively included in this study, hampered us to observe potential severe COVID-19 cases among vaccinated individuals, although they had been all the cases of SARS-CoV-2 infection after vaccination and entering Xi’an City during the study period. Second, these early vaccines were all Chinese Han adults aged 18–59 years; our case series do not include the patients aged more than 60 or less than 18. Future studies are needed to assess the risk of post-vaccination SARS-CoV-2 infection and the disease severity in various populations, including older people and children, as well as ethnic and geographical diversity. Third, potential sources of bias may exist in this study since these imported cases from 8 countries distributed in Asia, Africa and Europe. Fourth, we did not perform the examination of the SARS-CoV-2 sequences and neutralizing antibody titers, thus we could not clarify the infection due to suboptimal immune responses to the vaccines or breakthrough infections with SARS-CoV-2 variants. Fifth, there were no omicron variants during the study period, and future research focused on the clinical features and outcomes of omicron breakthrough infections is needed, including patients with booster vaccines. Despite these limitations, our findings are important in understanding and filling the gap in the clinical characteristics of a significant number of Chinese COVID-19 patients who received Chinese inactivated vaccines.
Conclusions
Our study indicated a potential risk of SARS-CoV-2 infections even after successful vaccination with Chinese inactivated vaccines. More than two-thirds of patients presented with asymptomatic infection, and for symptomatic infection, all had mild or moderate COVID-19, along with a short SARS-CoV-2 shedding period. These observations in no way undermine the importance of the urgent efforts to vaccinate the population. Instead, it emphasizes the importance of vaccines in reducing the severity of the disease, and meanwhile, supports maintaining social distance and wearing masks to reduce the risk of reinfection even after vaccination in current circumstances. Furthermore, booster vaccines are needed to reduce the risk of infection and help control pandemics.
Abbreviations
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; NAbs, neutralizing antibody; IQR, interquartile range; BMI, body mass index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; ULN: upper limit of normal; VoC, variants of concern.
Acknowledgments
The study was supported by grants from the Fundamental Research Funds for the Central Universities for COVID-19 (xzy032020040). The funding body did not play any roles in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. Jianwu Li, Na Jiang, and Qing-Lei Zeng are co-first authors for this study.
Disclosure
All authors declared that they have no conflicts of interest related to this work.
References
1. Johns Hopkins University of Medicine Coronavirus Resource Center. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available from: https://coronavirus.jhu.edu/map.html.
2. Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854.
3. Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51.
4. Richmond P, Hatchuel L, Dong M, et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397:682–694.
5. Yang S, Li Y, Dai L, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021;21(8):1107–1119.
6. Funk CD, Laferrière C, Ardakani A. Target product profile analysis of COVID-19 vaccines in Phase III clinical trials and beyond: an early 2021 perspective. Viruses. 2021;13:418.
7. Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681.
8. Voysey M, Clemens SAC, Madhi SA, Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111.
9. Polack FP, Thomas SJ, Kitchin N, C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615.
10. Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829.
11. Chodick G, Tene L, Rotem RS, et al. The effectiveness of the TWO-DOSE BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2022;74(3):472–478.
12. Keehner J, Horton LE, Pfeffer MA, et al. SARS-CoV-2 infection after vaccination in health care workers in California. N Engl J Med. 2021;384:1774–1775.
13. Müller L, Andrée M, Moskorz W, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis. 2021;73(11):2065–2072.
14. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423.
15. Tande AJ, Pollock BD, Shah ND, et al. Impact of the COVID-19 vaccine on asymptomatic infection among patients undergoing pre-procedural COVID-19 molecular screening. Clin Infect Dis. 2022;74(1):59–65.
16. Hacisuleyman E, Hale C, Saito Y, et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;384:2212–2218.
17. Peng Q, Zhou R, Wang Y, et al. Waning immune responses against SARS-CoV-2 variants of concern among vaccinees in Hong Kong. EBioMedicine. 2022;77:103904.
18. Ai J, Zhang H, Zhang Y, et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect. 2022;11(1):337–343.
19. Assawakosri S, Kanokudom S, Suntronwong N, et al. Neutralizing activities against the Omicron variant after a heterologous booster in healthy adults receiving two doses of CoronaVac vaccination. J Infect Dis. 2022;1:jiac092. doi:10.1093/infdis/jiac092
20. Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326(1):35–45.
21. Fadlyana E, Rusmil K, Tarigan R, et al. A Phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18-59 years: an interim analysis in Indonesia. Vaccine. 2021;39(44):6520–6528.
22. Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):875–884.
23. National Health Commission of the People’s Republic of China. Chinese management guideline for COVID-19 (version 8.0). May 14, 202. http://www.nhc.gov.cn/jkj/s3577/202105/6f1e8ec6c4a540d99fafef52fc86d0f8/files/4a860a7e85d14d55a22fbab0bbe77cd9.pdf.
24. Hou W, Zhang W, Jin R, et al. Risk factors for disease progression in hospitalized patients with COVID-19: a retrospective cohort study. Infect Dis. 2020;52:498–505.
25. Zeng QL, Li GM, Ji FP, et al. Clinical course and treatment efficacy of COVID-19 near Hubei province, China: a multicentre, retrospective study. Transbound Emerg Dis. 2020;67:2971–2982.
26. Kim SM, Hwang YJ, Kwak Y. Prolonged SARS-CoV-2 detection and reversed RT-PCR results in mild or asymptomatic patients. Infect Dis. 2021;53:31–37.
27. Bhaskaran K, Bacon S, Evans SJ, et al. Factors associated with deaths due to COVID-19 versus other causes: population-based cohort analysis of UK primary care data and linked national death registrations within the Open SAFELY platform. Lancet Reg Health Eur. 2021;6:100109.
28. Hall VJ, Foulkes S, Charlett A, SIREN Study Group. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet. 2021;397:1459–1469.
29. Noh JY, Kwak JE, Yang JS, et al. Longitudinal assessment of anti-SARS-CoV-2 immune responses for six months based on the clinical severity of COVID-19. J Infect Dis. 2021;224(5):754–763.
30. Yao L, Wang GL, Shen Y, et al. Persistence of antibody and cellular immune responses in COVID-19 patients over nine months after infection. J Infect Dis. 2021;224(4):586–594.
31. Li X, Chan JM, Lam B, et al. COVID-19 mRNA vaccines delay the onset of breakthrough infections with less radiographic abnormalities. Clin Infect Dis. 2022;1:ciab1062.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.