Back to Journals » Drug Design, Development and Therapy » Volume 18
The Emerging Roles of Nanocarrier Drug Delivery System in Treatment of Intervertebral Disc Degeneration-Current Knowledge, Hot Spots, Challenges and Future Perspectives
Authors Hu Y, Yang R, Liu S, Song Z, Wang H
Received 8 November 2023
Accepted for publication 9 March 2024
Published 29 March 2024 Volume 2024:18 Pages 1007—1022
DOI https://doi.org/10.2147/DDDT.S448807
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Yunxiang Hu,1,2,* Rui Yang,1,2,* Sanmao Liu,1,2,* Zefeng Song,3 Hong Wang1,2
1Department of Orthopedics, Central Hospital of Dalian University of Technology, Dalian City, Liaoning Province, People’s Republic of China; 2School of Graduates, Dalian Medical University, Dalian City, Liaoning Province, People’s Republic of China; 3School of Graduates, Dalian University of Technology, Dalian City, Liaoning Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hong Wang
Department of Orthopedics, Central Hospital of Dalian University of Technology, No. 826, Southwestern Road, Shahekou District, Dalian City, Liaoning Province, People’s Republic of China
, Email [email protected]
Abstract: Low back pain (LBP) is a common condition that has substantial consequences on individuals and society, both socially and economically. The primary contributor to LBP is often identified as intervertebral disc degeneration (IVDD), which worsens and leads to significant spinal problems. The conventional treatment approach for IVDD involves physiotherapy, drug therapy for pain management, and, in severe cases, surgery. However, none of these treatments address the underlying cause of the condition, meaning that they cannot fundamentally reverse IVDD or restore the mechanical function of the spine. Nanotechnology and regenerative medicine have made significant advancements in the field of healthcare, particularly in the area of nanodrug delivery systems (NDDSs). These approaches have demonstrated significant potential in enhancing the efficacy of IVDD treatments by providing benefits such as high biocompatibility, biodegradability, precise drug delivery to targeted areas, prolonged drug release, and improved therapeutic results. The advancements in different NDDSs designed for delivering various genes, cells, proteins and therapeutic drugs have opened up new opportunities for effectively addressing IVDD. This comprehensive review provides a consolidated overview of the recent advancements in the use of NDDSs for the treatment of IVDD. It emphasizes the potential of these systems in overcoming the challenges associated with this condition. Meanwhile, the insights and ideas presented in this review aim to contribute to the advancement of precise IVDD treatment using NDDSs.
Keywords: lumbar disc herniation, intervertebral disc degeneration, nanocarrier drug delivery system, targeted therapy, regenerative medicine
Introduction
Low back pain (LBP) is a widespread public health issue that results in profound and long-lasting disability, placing a substantial economic burden on both society and individuals affected by it.1 Based on recent research, it is estimated that over 1/4 of adults will encounter LBP at some stage during their lifetime. Furthermore, approximately 10% of individuals with LBP will develop persistent disabilities.2,3 The growing elderly population has led to a substantial rise in the economic impact of LBP. LBP has emerged as a prominent health issue exerting a substantial influence on public health and patients’ quality of life. The origins of LBP are multifaceted and involve a wide range of factors, including genetic predisposition, lifestyle choices, and the natural process of aging.4 While the origins of LBP are multifaceted, IVDD stands as the prevailing cause, characterized by evident structural alterations resulting from aging or mechanical strain.5,6 The intervertebral disc (IVD) is a type of fibrocartilage that acts as a connective tissue between neighboring vertebrae, providing flexibility and helping to distribute pressure. Over time, the IVD undergoes wear and tear due to ongoing mechanical stress during development and the aging process, leading to dysfunction and degeneration of the disc.7,8 Scientific research has provided evidence that the progressive degradation of the ECM, changes in the cellular properties of IVD cells, heightened cellular aging and mortality, and excessive inflammatory reactions are widely acknowledged as substantial factors in the initiation and advancement of IVDD. These factors exacerbate the disorder and disrupt the normal functioning of the IVD.9–11 Presently, the majority of patients rely on rest or conservative therapies as the primary approach to alleviate pain associated with IVDD. Additionally, various medications including non-steroidal anti-inflammatory drugs (NSAIDs), analgesics, and other blockers are commonly used. In cases where these treatments prove ineffective, surgical interventions are typically employed to relieve symptoms and improve the quality of life for patients.12,13 In summary, current interventions for IVDD do not effectively restore IVD function and are associated with drawbacks such as invasiveness, potential recurrence, and degeneration of adjacent IVDs. Considering the limitations of both non-surgical and surgical treatments, there is a pressing need to investigate more efficacious approaches for managing IVDD. A range of treatment strategies are under investigation, including the utilization of biomaterial substitutes, administration of therapeutic drugs via injection, gene therapy, and stem cell therapy.14–16 A significant focus in the management of IVDD is the identification of specific drug targets. Furthermore, the limited duration of action of bioactive substances utilized in IVD treatment poses a significant limitation, as it restricts the efficacy of the drugs. To overcome this challenge, emerging novel NDDSs hold promise in the development of innovative treatment strategies. NDDSs offer the advantage of incorporating multiple therapeutic agents, enabling tailored release kinetics and precise targeting capabilities.17,18 By concentrating and prolonging the residence of drugs within IVD tissues, NDDSs possess the capability to augment therapeutic outcomes and enhance the efficacy of treatment.19–23 This review provides a succinct overview of the underlying pathogenic mechanisms implicated in the progression of IVDD and delineates the current constraints associated with existing treatment modalities. Furthermore, it elucidates the notable advancements achieved in the field of NDDSs therapy for IVDD. The review culminates by presenting prospective directions aimed at advancing the utilization of NDDSs in the management of IVDD.
IVD: Anatomy and Pathophysiology
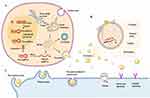
IVD is crucial cartilaginous structure situated between the vertebrae, serving a pivotal role in supporting and enabling proper spinal function. It maintains close association with the neighboring vertebrae and serves as a fundamental component for both mobility and shock absorption. Comprising three distinct elements, namely the nucleus pulposus (NP), the annulus fibrosus (AF), and the cartilaginous endplates (CEP) situated at the upper and lower ends of the disc, the IVD exhibits a complex anatomical composition.24,25 The NP is a hydrophilic structure enclosed by fibrous rings, and its primary function is to withstand pressure within the spine. Composed of water, inorganic salts, and NP cells, it forms an ECM along with aggregations of spinal cells.9 The ECM of the NP comprises type II collagen fibers, proteoglycans, and elastic fibers. Within the elastic fibers, there are negatively charged proteoglycan side chains that play a crucial role in maintaining the NP’s high hydration and permeability. These characteristics enable the NP to endure pressure and retain its shape. The primary function of the proteoglycans and collagen type II is to retain water and facilitate the distribution of compressive loads throughout the IVD via hydraulic mechanisms.26–28 Hence, the NP acts as a cushion and fulfills the role of a shock absorber, effectively preventing direct and excessive mechanical impact on the bones and cartilages. The AF is divided into inner and outer regions, primarily distinguished by their collagen composition. The inner annulus is composed of multiple layers of fibrocartilage, primarily consisting of collagen type II and a higher concentration of proteoglycans. On the other hand, the outer annulus is composed of fibrous tissue containing collagen type I fibers, which confer tensile strength and enable it to withstand pressure.29,30 In the vicinity of the outer AF, the content of collagen type I fibers increases, while the levels of collagen type II fibers and proteoglycans decrease. The primary function of the AF is to endure and support the osmotic pressure generated by the NP, providing inherent resistance against bending, torsion, and shear forces, especially during movements involving flexion, bending, and twisting of the IVD. Unlike the NP, the AF comprises fibroblasts as its predominant cell type, and their elongated cellular morphology facilitates the organization of an ECM rich in collagen type I. This arrangement of collagen type I serves as the foundational structure for lamellae within the AF.31 The CEP is a thin layer that envelops the upper and lower surfaces of the intervertebral disc (IVD). These structures are essential for facilitating the transport of fluid and solutes into and out of the IVD, thereby aiding in the provision of nutrients to the disc.32,33 The primary source of nutrient supply for the IVD is predominantly dependent on the infiltration of the CEP.34 Furthermore, the IVD has a limited capacity for internal healing, which makes it prone to degeneration. Moreover, the endplates, which have a cartilaginous composition, serve as a pliable and sturdy support system capable of bearing significant loads and evenly distributing pressure throughout the IVD and neighboring vertebrae.35 IVDD is a complex degenerative condition influenced by various factors such as aging, injuries, nutrition, biomechanics, genetics, and environmental elements. Regardless of the underlying causes, the primary characteristic of a degenerated IVD is an imbalance between the processes of synthesis and degradation, accompanied by a decline in the number and activity of NP cells. These changes can result in weakened tissue, alterations in cell morphology and function. From a clinical perspective, IVDD is a chronic and progressive disorder characterized by persistent pain in the lower back and legs, which can result in substantial disability. In more severe instances, IVDD can give rise to conditions such as disc herniation, nerve root impairments, compression of the spinal cord, spondylolisthesis, spinal stenosis, and even degenerative scoliosis. These conditions directly contribute to long-term disability.36,37 The painful symptoms arise as a result of molecular and cellular alterations within the tissue, which disrupt the structural integrity and mechanical characteristics of the IVD (Figure 1).
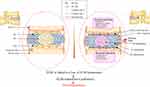 |
Figure 1 A diagram showing healthy IVD and the key pathogenic and anatomical alterations in IVDD. MMPs is Matrix metalloproteinases, ADAMTS is Metalloproteinase with Thrombospondin Motif. |
Diagnosis and Treatment of Lumbar Disc Herniation
The symptoms of intervertebral disc herniation vary depending on factors such as the patient’s age, the progression of the disease, the location and size of the protruded disc, the extent of nerve compression, and the presence of inflammatory response. Common symptoms include radiating radicular pain, muscle weakness, and sensory abnormalities that correspond to the affected nerve root. Patients may also experience acute or chronic lower back pain, limited range of motion in the lumbar region, compensatory scoliosis, hamstring tightness in children and adolescents with lumbar disc herniation, and in severe cases, cauda equina syndrome.38,39 Ancillary examinations, such as X-ray imaging, CT, MRI,40–42 myelography and CT myelography, selective nerve root injection and nerve root block procedure,43,44 nerve electrophysiological examinations are very helpful in diagnosing lumbar disc herniation.45 During the acute phase of LDH, conservative treatment is crucial. Physical therapy, exercise and change of health style, medicine, etc were proven to be useful.46–48 Surgical treatment includes minimally invasive surgery and open surgery. Percutaneous enzymatic or laser nucleolysis,49 ozone nucleolysis,50 microendoscopic discectomy (MED),51 percutaneous endoscopic lumbar discectomy (PELD) and percutaneous endoscopic interlaminar discectomy (PEID),52 the unilateral biportal endoscopic (UBE),53 lumbar interbody fusion,54–56 lumbar artificial disc replacement (ARD)57 were reported to be effective in treatment of LDH (Figure 2).
 |
Figure 2 Diagnostic methods and current treatments of LDH. |
NDDSs for the Treatment of IVDD
Presently, the majority of drugs or bioactive molecules employed for the treatment of IVDD are administered either systemically or through direct injection into the IVD. However, these treatment methods are associated with the drawback of limited therapeutic efficacy.58 Nanotechnology is leading the way in advancements related to medical diagnosis, imaging, and targeted drug delivery. It presents a promising opportunity to manipulate the size and properties of drugs and materials, granting precise control over their characteristics and behavior.59,60 Due to the remarkable progress and groundbreaking advancements in nanotechnology, numerous NDDSs have been created and integrated into the realm of IVDD treatment.61–63 The field of NDDSs in the treatment of IVDD is dynamic and continuously expanding. NDDSs offer several notable advantages that have been extensively validated in their ability to significantly enhance and repair IVDD. The field of NDDSs in the treatment of IVDD is dynamic and continuously expanding. NDDSs have been extensively validated for their ability to significantly enhance and repair IVDD, offering several notable advantages: 1. NDDSs exhibit non-toxicity, biodegradability, and excellent biocompatibility with the intervertebral disc, creating a favorable microenvironment that promotes IVDD regeneration. 2. Certain NDDSs can overcome cellular barriers and effectively reach the cytoplasmic space or activate specific transport mechanisms, thereby improving drug retention and efficacy. 3. The sustained release of drugs from NDDSs prolongs the presence of therapeutic drug levels, minimizing the requirement for frequent drug dosing, reducing treatment expenses, and at the same time improving patient adherence. 4.NDDSs that employ natural materials can provide additional benefits due to their composition resembling cartilage and other matrices relevant to the IVD. This similarity creates an enhanced cellular microenvironment for the treatment of IVDD.64–68 In summary, NDDSs demonstrate outstanding potential in IVDD treatment, offering advantages such as biocompatibility, enhanced drug delivery, prolonged drug release, and the utilization of natural materials that mimic the disc’s composition.
Major Categories of NDDS for IVDD
A wide range of NDDSs incorporating therapeutic agents have been employed to facilitate the restoration and rejuvenation of IVDs. Various approaches have been employed to exploit biocompatible, biodegradable and safe platforms for drug delivery, aiming to enhance therapeutic effectiveness. In this overview, we highlight several promising nanocarriers that demonstrate potential for managing IVDD. These encompass liposomes, inorganic nanoparticles, polymeric nanoparticles, nanofibers, polymer micelles, nanohydrogels, and exosomes (Table 1 and Figure 3).
 |
Table 1 Main Nano-Drug Delivery Systems for IVDD |
 |
Figure 3 NDDSs that are commonly used for IVDD therapy. |
Liposomes
Liposomes, the first nanoparticles to be successfully applied in clinical settings, are spherical structures at the nanoscale composed of lipid bilayers. Like cellular membranes, liposomes primarily consist of phospholipids and cholesterol. They possess a hydrophilic core and one or more hydrophobic compartments enclosed by lipid bilayers. This distinctive amphiphilic structure enables liposomes to encapsulate substances that are both hydrophobic and hydrophilic.85 In a study conducted by Banala et al,86 it indicated that the use of siRNA treatment as an intervention significantly reduced apoptosis within IVD. This suggests that delivering siRNA directly into the spinal discs holds potential for nonsurgical treatment of disc degeneration. Another study by Wang et al87 demonstrated that oxymatrine liposomes (OMT-LIP) notably increased the accumulation of OMT in degenerative discs. This resulted in the attenuation of apoptosis in nucleus pulposus cells, reduced expression of matrix metalloproteinases 3/9 and interleukin-6, and decreased degradation of type II collagen. In their in vivo study, X-ray imaging illustrated that OMT-LIP effectively inhibited IVDD.
Inorganic Nanoparticles
Inorganic nanoparticles are a class of nanoparticles derived from materials of inorganic nature. This category encompasses various types of nanoparticles such as carbon-based nanoparticles, metal nanoparticles, ceramic nanoparticles, calcium nanoparticles, and quantum dots.88 Zhou et al89 conducted a study demonstrating the potential of polydopamine-coated black phosphorus nanoparticles (PBNPs) in alleviating intracellular oxidative stress and enhancing the activity of antioxidant enzymes, specifically superoxide dismutase 1 (SOD1). The researchers observed that PBNPs had the ability to rescue degeneration of nucleus pulposus cells by upregulating mRNA and proteins associated with the oxidoreductase system, particularly by stabilizing SOD1 and preventing its degradation through the ubiquitination-proteasome pathway. This resulted in improvements in mitochondrial structure, enhanced antioxidation ability, and ultimately the rescue of IVD degeneration induced by reactive oxygen species (ROS) in a rat model. Therefore, PBNPs have the potential to serve as a contrast agent for discography with antioxidative properties. Similarly, Zhu et al63 discovered that manganese dioxide nanoparticles (MnO2 NPs) with a spherical and hollow structure can dissociate under low pH and hydrogen peroxide (H2O2) conditions, releasing loaded transforming growth factor-beta 3 (TGF-β3) molecules. In an oxidative stress environment, TGF-β3/MnO2 exhibited superior effects compared to TGF-β3 or MnO2 NPs alone, effectively suppressing matrix degradation, ROS production, and apoptosis in nucleus pulposus cells. When injected into the intervertebral discs of a rat model with IVDD, TGF-β3/MnO2 prevented disc degeneration and promoted self-regeneration. The study concluded that utilizing an MnO2 nanoplatform for controlled release of biological factors to regulate the IVDD microenvironment and facilitate endogenous repair could be an effective approach for treating IVDD.
Polymeric Nanoparticles (PNPs)
PNPs are commonly small, uniform, spherical structures with nanoscale dimensions, composed of biocompatible and biodegradable polymers. These nanoparticles have the ability to encapsulate drugs either within their core or attach them to their surface.90 In a study conducted by Lim et al,91 it was discovered that ABT263, a senolytic drug, can be loaded into poly (lactic-co-glycolic acid) nanoparticles (PLGA-ABT) and administered intradiscally in rat models with injury-induced IVDD. The single intradiscal injection of PLGA-ABT enables localized drug delivery to the avascular IVD, thereby avoiding potential systemic toxicity associated with systemic administration of senolytic drugs and reducing the morbidity caused by repetitive injections of free drugs into the IVD. This strategy leads to the selective elimination of senescent cells in the degenerative IVD, reduces the expression of pro-inflammatory cytokines and matrix proteases, inhibits the progression of IVDD, and even restores the structure of the IVDD. This study demonstrates, for the first time, the effective treatment of senescence-associated IVDD through local delivery of a senolytic drug. Furthermore, this approach holds promise for treating other types of degenerative diseases associated with cellular senescence. In a study conducted by Arul et al,92 they developed nanoparticles using cellulose acetate and polycaprolactone-polyethylene glycol. These nanoparticles were conjugated with a novel fluorescent dye called 1-oxo-1H-pyrido [2,1-b] [1,3] benzoxazole-3-carboxylic acid (PBC) using an oil-in-water emulsion technique. The study aimed to investigate the release patterns of two drugs: indomethacin (IND), which is not soluble in water, and 4-aminopyridine (4-AP), which is water-soluble. The researchers injected these nanoparticles into the IVDs of mouse tails in vivo. The findings demonstrated that the nanoparticles were confined to the NPCs and the injection site, present in both the NP and AF of the IVD. This suggest that these fluorescent nano-formulations hold promise as a versatile technology platform for delivering therapeutic agents specifically to IVD and other tissues that necessitate targeted drug injections.
Nanofibers
Nanofibers, being a significant biomaterial, exhibits distinct characteristics including a significant surface-area-to-volume ratio, low density and high porosity. The main components of most nanofibers are biodegradable synthetic polymers and natural polymers.93 In the realm of biomedical research and applications, nanofibers find extensive application as scaffolds in tissue engineering. In a study conducted by Yu et al,94 they developed a biocompatible scaffold made of polyurethane called F-PECUU. This scaffold was loaded with fucoidan, a polysaccharide derived from marine sources, with the aim of improving the microenvironment of IVDD and promoting disc regeneration. The F-PECUU scaffold demonstrated the ability to reduce inflammation and oxidative stress induced by lipopolysaccharide in AF cells. It also prevented the degradation of the ECM. In vivo experiments showed that the scaffold promoted the deposition of ECM, thereby maintaining disc height, water content, and mechanical properties. This study highlights the potential of functional scaffolds containing marine polysaccharides for treating IVDD. By improving the harsh microenvironment associated with disc degeneration, these scaffolds have the potential to contribute to the treatment and regeneration of intervertebral discs. In another study conducted by Tu et al,95 a combination of Micro-sol electrospinning technology and collagen type I (Col-I) self-assembly technique was utilized to fabricate a biomimetic scaffold consisting of layered micro/nanofibers. The scaffold was designed to release basic fibroblast growth factor (bFGF) in order to promote the repair and regeneration of the annulus AF. By incorporating Col-I, which can mimic the microenvironment of the ECM, the scaffold provided structural and biochemical cues that are conducive to AF tissue regeneration. The study suggests that these micro/nanofibrous scaffolds hold promise for clinical applications in treating AF deficiencies caused by IVDD.95
Polymeric Micelles
Polymeric micelles are self-assembled structures formed by amphiphilic block copolymers in the presence of water. These micelles possess a core-shell configuration, where the hydrophobic core is surrounded by a hydrophilic shell facing the aqueous environment. This distinct structure allows for the encapsulation of poorly soluble drugs within the hydrophobic core. The size of polymeric micelles is predominantly influenced by the ratio of hydrophilic to hydrophobic segments in amphiphilic molecules and can be tailored according to the specific properties of the drug being encapsulated.96 In a study performed by Yu et al,97 it was discovered that the PAKM micelle, which is the monomer of interest, exhibited enhanced bioactivities due to a synergistic effect. The PAKM micelle demonstrated improved viability, activation of autophagy markers (P62, LC3 II), upregulation of the ECM-related transcription factor SOX9, and maintenance of ECM components (Collagen II, Aggrecan) in human adipose-derived stem cells (ADSCs). Furthermore, when PAKM micelles were injected along with human ADSCs into rats, it resulted in increased disc height and water content compared to control groups. These findings indicate that PAKM micelles have the ability to promote cell survival and differentiation, suggesting their potential as a therapeutic agent for IVDD. In another study conducted by Chang et al98 they demonstrated that the use of polyplex nano-micelles as carriers for mRNA medicine, specifically for the anabolic factor Runx1, played a critical role in attenuating the advancement of IVDD. IVDD is characterized by a metabolic imbalance within the disc. The use of this platform, which involves the delivery of Runx1 through polyplex nano-micelles, holds promise as a potential strategy in the field of regenerative medicine. It offers a potential avenue for developing treatments aimed at addressing the imbalanced metabolic state associated with IVDD.
Nanohydrogels
Nanohydrogels are three-dimensional polymer networks formed through the chemical or physical cross-linking of hydrogels with nanomaterials. These unique structures exhibit a blend of characteristics found in both hydrogels and nanoparticles. Unlike traditional hydrogels like microgels that rely on intermolecular cross-linking, nanohydrogels are primarily defined by intramolecular cross-linking.99 Chang et al100 conducted a study that in a rat model of nutrient-restricted IVD, the local injection of psh-circSTC2-lipo@MS resulted in enhanced synthesis of the ECM and restoration of the NP tissue after 8 weeks. The researchers further confirmed that psh-circSTC2-lipo@MS, serving as a targeted gene delivery system, is safe and controllable. It demonstrated significant potential in regulating the balance of ECM metabolism within an abnormal microenvironment. Luo et al101 demonstrated effective sustained delivery of chondroitin sulfate (CS). This hydrogel was found to successfully inhibit the expression of inflammatory cytokines and maintain a balance between anabolic and catabolic processes in an inflammation-simulated environment. Importantly, the HA/CS hydrogel exhibited significant improvements in reducing degeneration in a rat model of IVDD induced by puncture. The self-antioxidant HA/CS hydrogel, developed in this study, holds great promise as a novel therapeutic platform for treating IVDD. Its ability to inhibit inflammation, maintain the anabolic/catabolic balance, and ameliorate degeneration highlights its potential as an innovative approach for the treatment of IVDD (Table 2).
 |
Table 2 Recently Representative Research of Nano-Drug Delivery Systems for Treatment of IVDD |
Exosomes
Exosomes are small extracellular vesicles that are released by various cell types, including mesenchymal stem cells (MSCs). They are composed of phospholipid bilayers, similar to the cell membrane, and have a diameter ranging from 50 to 100 nanometers. These nanoscale vesicles originate from intracellular multivesicular bodies and play a role in modulating the biological activities of recipient cells through their cargo.102 Exosome-based acellular therapy has gained attention in recent years due to the low immunogenicity and excellent stability of exosomes.103 They are considered miniature versions of the parent cells and carry a range of constituents. These constituents include approximately 4400 proteins, 194 lipids, 1639 mRNAs, and 764 microRNAs (miRNAs) in exosomes derived from various cell types. The composition of these molecules determines the biological functions and therapeutic potential of exosomes104 (Figure 4). Zhu et al105 demonstrated that exosomes derived from bone MSCs and containing miR-142-3p can alleviate injury to nucleus pulposus cells (NPCs). This effect is achieved by suppressing the MAPK signaling pathway through the targeting of MLK3 by miR-142-3p. The research emphasizes the therapeutic potential of MSCs in mitigating the progression of IVDD. Additionally, the study suggests that exosomes derived from bone marrow MSCs hold promise as a therapeutic strategy for IVDD. Sun et al106 clarified that miR-194-5p, derived from extracellular vesicles (EVs) released by MSCs, plays a protective role in IVD cells that have been intervened by TNF-α. This protective effect occurs by inhibiting the expression of TRAF6, which provides a potential therapeutic target for the treatment of IVDD. In another study conducted by Sun et al,107 it was discovered that small EVs derived from mesenchymal stem cells, specifically referred to as iMSC-sEVs, possess the ability to rejuvenate senescent nucleus pulposus cells (NPCs) and mitigate the progression of IVDD. The rejuvenating effects of iMSC-sEVs were achieved through the delivery of miR-105-5p to senescent NPCs and subsequent activation of the Sirt6 pathway. These findings indicate that iMSCs hold promise as a potential source for large-scale production of MSC-derived small EVs, offering a cell-free therapeutic approach for IVDD treatment that overcomes certain limitations associated with current MSC-based applications. Zhu et al108 observed that exosomes derived from bone marrow mesenchymal stem cells (BMSCs) possess the potential to suppress TNF-α-induced apoptosis, ECM degradation, and fibrosis deposition in NPCs. This beneficial effect is achieved through the delivery of miR-532-5p, which targets the RASSF5 gene. These findings present a promising therapeutic strategy for addressing the progression of IVDD. Cui et al109 found that the expression of miR-129-5p was significantly reduced in IVD tissues affected by IVDD. By delivering miR-129-5p to NPCs through EVs derived from MSCs, a decrease in NP cell apoptosis, degradation of the ECM, and polarization of macrophages towards the M1 phenotype were observed. Notably, miR-129-5p directly targeted the LRG1 gene, leading to the activation of the p38 MAPK signaling pathway and subsequent polarization of macrophages towards the M1 phenotype. Additionally, in vivo experiments demonstrated that MSC-derived EVs loaded with miR-129-5p alleviated IVDD by inhibiting the LRG1/p38 MAPK signaling pathway. Taken together, these discoveries indicate that EVs derived from MSCs containing miR-129-5p have the potential to safeguard against IVDD by specifically targeting LRG1 and inhibiting the p38 MAPK signaling pathway. This discovery introduces a novel theranostic marker for IVDD. In another study conducted by Sun et al,110 that valuable insights were gained into the anti-angiogenesis mechanism of notochordal cell-derived exosomes (NC-exos). These findings highlight the therapeutic potential of NC-exos for the treatment of IVDD. Zhou et al111 discovered that hypoxic pre-treatment of MSC-derived small extracellular vesicles (H-sEVs) containing miR-17-5p can effectively regulate the proliferation and synthesis of NPCs by modulating the TLR4/PI3K/AKT pathway. This suggests that hypoxic pre-treatment is a promising and efficient method to enhance the therapeutic effects of MSC-derived sEVs. Combining miRNA with MSC-derived sEVs holds potential as a promising therapeutic approach for IVDD. Chen et al112 discovered that exosomes derived from human amniotic mesenchymal stem cells (HASCs) and containing miR-155-5p had a beneficial effect in alleviating IVDD. This effect was achieved by targeting TGFβR2, which promoted autophagy (a cellular process that removes damaged components) and reduced pyroptosis (a type of programmed cell death associated with inflammation). These findings suggest that exosomal miR-155-5p from HASCs could serve as a new therapeutic target for the treatment of IVDD. Zhao et al113 discovered that exosomes derived from degenerated nucleus pulposus cells (dNPc-exo) have the ability to transport miR-27a-3p and target the PPARγ/NFκB/PI3K/AKT signaling pathway. This interaction influences the polarization of macrophages towards the M1 phenotype. Through experiments conducted on a rat model of IVDD, it indicated that the presence of exosomes containing miR-27a-3p led to the activation of M1 macrophages and worsened the degradation of the IVD (Table 3 and Figure 5).
 |
Table 3 Representative Exosome Transferred miRNAs in Treatment of IVDD |
Cargo loading engineering for exosomes involves enhancing the delivery efficiency through modifications of the exosome membrane surface. These modifications can be achieved through direct alterations of the exosome membrane surface or indirect modifications of the exosome-derived parental cells. By targeting and modifying specific structures on the exosome membrane surface, such as proteins or molecules involved in targeting and homing functions, the delivery capabilities of exosomes can be improved. These modifications aim to optimize the delivery of cargo within exosomes and enhance their therapeutic potential.114–117 The progress of nanotechnology has played a significant role in the advancement of engineered exosomes. The combination of liposomes and exosomes has given rise to hybrid exosomes formed through membrane fusion, which hold great potential as nanodrug delivery systems. An important factor influencing the uptake of target cells is the lipid charge. By selecting liposomes tailored to the specific properties of the target cells, they can be hybridized with exosomes to improve the efficiency of cellular uptake. This approach seeks to optimize the delivery efficiency of exosomes and improve their effectiveness as a personalized therapeutic tool.118 To address the issue of exosome clearance, the utilization of bioactive materials as carriers has emerged as a strategy to enhance exosome retention at the disease site and enable sustained release. Moreover, these bioactive materials can provide additional therapeutic benefits. In the fields of tissue repair and regenerative medicine, bioactive scaffolds have been widely employed for delivering therapeutic agents or nanocarriers. An ideal scaffold material should possess excellent biocompatibility, preserve the structural integrity and activity of exosomes, facilitate prolonged release of exosomes, and promote the migration of target cells to the scaffold for uptake of exosomes, thereby exerting therapeutic effects.119,120 At present, the utilization of multifunctional nano-delivery platforms based on exosomes as promising therapeutic strategies for treating IVDD is predominantly confined to preclinical studies conducted in animal models. To advance these approaches, it is crucial to conduct more preclinical studies to gather additional data and prepare for future clinical investigations. An effective approach involves the use of exosome delivery systems loaded onto bioactive scaffolds, which enable enhanced retention and sustained release of exosomes at the disease sites. These systems, such as hydrogel-loaded exosomes, have shown encouraging therapeutic effects and find extensive applications in the fields of tissue repair and regenerative medicine.121,122 Plant-derived exosomes have gained increasing attention in recent years due to their plentiful sources and lower immunogenicity when compared to exosomes derived from mammals. Another advantage of plant-derived exosomes is their absence of zoonotic or human pathogens. Plant-derived exosomes have demonstrated significant potential as nanocarriers and have been the subject of extensive investigation as delivery platforms in numerous studies. While multifunctional nano-delivery platforms based on exosomes show promise for the treatment of IVDD, further preclinical studies are necessary to gather additional data and advance our understanding. Additionally, the use of bioactive scaffolds and plant-derived exosomes present exciting avenues for research and development in this field.123,124 The progress in nanomaterials technology has opened up avenues for the creation of nanocomposite biomimetic scaffolds and nanohydrogel scaffolds. These innovative scaffolds combine the beneficial characteristics of both natural and synthetic materials. They hold immense potential for future exploration in the field of bio-scaffold-loaded exosome-based therapies, especially when utilized in conjunction with 3D printing technology.125,126 This approach represents a promising direction for further research and development, as it allows for the fabrication of intricate and customized scaffolds with enhanced properties by incorporating exosomes with therapeutics.
Conclusion and Future Perspectives
IVDD is a prevalent degenerative condition that results in lower back and leg pain. This disorder significantly impacts patients’ quality of life and places a substantial financial strain on individuals, families, and society. Current treatments for IVDD mainly concentrate on symptom management rather than targeting the underlying degenerative process. These treatments are highly invasive, usually, with a high risk of recurrence, and degeneration of adjacent IVD. Moreover, these treatments are inadequate in terms of halting disease progression or reversing the degenerative process of IVDD. Numerous potential medications, such as growth factors, MSCs, platelet-rich plasma, and gene-editing techniques, have undergone examination in preclinical and clinical trials over the past few decades, showing promise as treatments for IVDD. However, their efficacy has been underwhelming. The primary challenges in addressing IVDD repair are associated with its avascular structure, limited endogenous cell repair mechanisms, and an unfavorable microenvironment in the degenerated area. Overcoming these hurdles is a desirable approach for treating IVDD, but implementing it in clinical practice remains a significant challenge. Fortunately, the rapid advancements in regenerative medicine and NDDSs have brought new hope to IVDD treatment. These developments hold the potential to address the limitations of current therapies and provide innovative solutions for IVDD management.
Ideal NDDSs targeting the IVD should possess several crucial properties. These include low or negligible toxicity, minimal invasiveness, high drug entrapment efficiency, controlled release capability, continuous delivery, and easy applicability in clinical settings. Nevertheless, existing technologies face challenges in fulfilling all these requirements simultaneously. Moreover, the controlled release properties and reproducibility of current NDDSs need to be thoroughly evaluated through large-scale studies. Another challenging aspect is ensuring the high biological stability and activity of the nanodrug, preventing its degradation by enzymes in the degenerated IVD. Additionally, studies on NDDSs as a treatment for IVDDs are still in their early stages, with limited studies primarily carried out on animal models such as rats and rabbits. Nonetheless, it is important to recognize that there are anatomical variations between animal models and humans, which could hinder their suitability for accurately representing IVDD. The pathogenesis of IVDD is multifaceted, and treatments that solely target a single factor or signaling pathway often yield ineffective results. Therefore, future research efforts may concentrate on the development of NDDSs that employ multitarget synergistic therapies to address the intricate nature of IVDD.
Data Sharing Statement
All data analyzed were included in this paper; further requests can be consulted and data can be obtained from the correspondent author.
Acknowledgments
Yunxiang Hu, Rui Yang and Saomao Liu contributed equally to this work and shared the co-first authorship.
Funding
There is no funding to report.
Disclosure
The authors declare no competing interests in this work.
References
1. Knezevic NN, Candido KD, Vlaeyen JWS, Van Zundert J, Cohen SP. Low back pain. Lancet. 2021;398(10294):78–92. doi:10.1016/S0140-6736(21)00733-9
2. Yu P, Mao F, Chen J, et al. Characteristics and mechanisms of resorption in lumbar disc herniation. Arthritis Res Ther. 2022;24(1):205. doi:10.1186/s13075-022-02894-8
3. Ma K, Chen S, Li Z, et al. Mechanisms of endogenous repair failure during intervertebral disc degeneration. Osteoarthritis Cartilage. 2019;27(1):41–48. doi:10.1016/j.joca.2018.08.021
4. Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet. 2017;389(10070):736–747. doi:10.1016/S0140-6736(16)30970-9
5. Kirnaz S, Capadona C, Wong T, et al. Fundamentals of Intervertebral Disc Degeneration. World Neurosurg. 2022;157:264–273. doi:10.1016/j.wneu.2021.09.066
6. Francisco V, Pino J, González-Gay M, et al. A new immunometabolic perspective of intervertebral disc degeneration. Nat Rev Rheumatol. 2022;18(1):47–60. doi:10.1038/s41584-021-00713-z
7. Wang F, Cai F, Shi R, Wang XH, Wu XT. Aging and age related stresses: a senescence mechanism of intervertebral disc degeneration. Osteoarthritis Cartilage. 2016;24(3):398–408. doi:10.1016/j.joca.2015.09.019
8. Wu PH, Kim HS, Jang IT. Intervertebral disc diseases PART 2: a review of the current diagnostic and treatment strategies for intervertebral disc disease. Int J Mol Sci. 2020;21:6.
9. Krut Z, Pelled G, Gazit D, Gazit Z. Stem cells and exosomes: new therapies for intervertebral disc degeneration. Cells. 2021;10(9):2241. doi:10.3390/cells10092241
10. Xin J, Wang Y, Zheng Z, Wang S, Na S, Zhang S. Treatment of Intervertebral Disc Degeneration. Orthop Surg. 2022;14(7):1271–1280. doi:10.1111/os.13254
11. Liang H, Luo R, Li G, Zhang W, Song Y, Yang C. The proteolysis of ECM in intervertebral disc degeneration. Int J Mol Sci. 2022;23:3.
12. Alrwaily M, Timko M, Schneider M, et al. Treatment-based classification system for low back pain: revision and update. Phys Ther. 2016;96(7):1057–1066. doi:10.2522/ptj.20150345
13. Corp N, Mansell G, Stynes S, et al. Evidence-based treatment recommendations for neck and low back pain across Europe: a systematic review of guidelines. Eur J Pain. 2021;25(2):275–295. doi:10.1002/ejp.1679
14. Flotte TR. Epigenome editing strategies for low back pain. Hum Gene Ther. 2019;30(9):1037–1038. doi:10.1089/hum.2019.29094.trf
15. Richardson SM, Kalamegam G, Pushparaj PN, et al. Mesenchymal stem cells in regenerative medicine: focus on articular cartilage and intervertebral disc regeneration. Methods. 2016;99:69–80. doi:10.1016/j.ymeth.2015.09.015
16. Urits I, Capuco A, Sharma M, et al. Stem cell therapies for treatment of discogenic low back pain: a comprehensive review. Curr Pain Headache Rep. 2019;23(9):65. doi:10.1007/s11916-019-0804-y
17. Shi P, Cheng Z, Zhao K, et al. Active targeting schemes for nano-drug delivery systems in osteosarcoma therapeutics. J Nanobiotechnology. 2023;21(1):103. doi:10.1186/s12951-023-01826-1
18. Lv Y, Li W, Liao W, et al. Nano-drug delivery systems based on natural products. Int J Nanomed. 2024;19:541–569. doi:10.2147/IJN.S443692
19. Das UN. Bioactive lipids in intervertebral disc degeneration and its therapeutic implications. Biosci Rep. 2019;39(10). doi:10.1042/BSR20192117
20. Sun J, Yang F, Wang L, et al. Delivery of coenzyme Q10 loaded micelle targets mitochondrial ROS and enhances efficiency of mesenchymal stem cell therapy in intervertebral disc degeneration. Bioact Mater. 2023;23:247–260. doi:10.1016/j.bioactmat.2022.10.019
21. Li J, Duan W, Chai S, et al. Wogonin, a bioactive ingredient from huangqi guizhi formula, alleviates discogenic low back pain via suppressing the overexpressed NGF in intervertebral discs. Mediators Inflamm. 2023;2023:4436587. doi:10.1155/2023/4436587
22. Guo T, Zhang X, Hu Y, et al. New hope for treating intervertebral disc degeneration: microsphere-based delivery system. Front Bioeng Biotechnol. 2022;10:933901. doi:10.3389/fbioe.2022.933901
23. Wu R, Huang L, Xia Q, et al. Injectable mesoporous bioactive glass/sodium alginate hydrogel loaded with melatonin for intervertebral disc regeneration. Mater Today Bio. 2023;22:100731. doi:10.1016/j.mtbio.2023.100731
24. Vergroesen PP, Kingma I, Emanuel KS, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage. 2015;23(7):1057–1070. doi:10.1016/j.joca.2015.03.028
25. Iatridis JC, Nicoll SB, Michalek AJ, Walter BA, Gupta MS. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013;13(3):243–262. doi:10.1016/j.spinee.2012.12.002
26. Calderon L, Collin E, Velasco-Bayon D, Murphy M, O’Halloran D, Pandit A. Type II collagen-hyaluronan hydrogel--a step towards a scaffold for intervertebral disc tissue engineering. Eur Cell Mater. 2010;20:134–148. doi:10.22203/eCM.v020a12
27. Zhou X, Shen N, Tao Y, et al. Nucleus pulposus cell-derived efficient microcarrier for intervertebral disc tissue engineering. Biofabrication. 2023;15(2):025008. doi:10.1088/1758-5090/acb572
28. Brissenden AJ, Amsden BG. In situ forming macroporous biohybrid hydrogel for nucleus pulposus cell delivery. Acta Biomater. 2023;170:169–184. doi:10.1016/j.actbio.2023.08.029
29. Liang T, Zhang LL, Xia W, Yang HL, Luo ZP. Individual collagen fibril thickening and stiffening of annulus fibrosus in degenerative intervertebral disc. Spine. 2017;42(19):E1104–E1111. doi:10.1097/BRS.0000000000002085
30. Tromp IN, Foolen J, van Doeselaar M, et al. Comparison of annulus fibrosus cell collagen remodeling rates in a microtissue system. J Orthop Res. 2021;39(9):1955–1964. doi:10.1002/jor.24921
31. Moriguchi Y, Borde B, Berlin C, et al. In vivo annular repair using high-density collagen gel seeded with annulus fibrosus cells. Acta Biomater. 2018;79:230–238. doi:10.1016/j.actbio.2018.07.008
32. Wang Y, Videman T, Battié MC. Lumbar vertebral endplate lesions: prevalence, classification, and association with age. Spine. 2012;37(17):1432–1439. doi:10.1097/BRS.0b013e31824dd20a
33. Fontana G, See E, Pandit A. Current trends in biologics delivery to restore intervertebral disc anabolism. Adv Drug Deliv Rev. 2015;84:146–158. doi:10.1016/j.addr.2014.08.008
34. Boyd LM, Carter AJ. Injectable biomaterials and vertebral endplate treatment for repair and regeneration of the intervertebral disc. Eur Spine J. 2006;15(Suppl 3):S414–421. doi:10.1007/s00586-006-0172-2
35. Sun Q, Tian FM, Liu F, et al. Denosumab alleviates intervertebral disc degeneration adjacent to lumbar fusion by inhibiting endplate osteochondral remodeling and vertebral osteoporosis in ovariectomized rats. Arthritis Res Ther. 2021;23(1):152. doi:10.1186/s13075-021-02525-8
36. Ding WY, Yang DL, Cao LZ, et al. Intervertebral disc degeneration and bone density in degenerative lumbar scoliosis: a comparative study between patients with degenerative lumbar scoliosis and patients with lumbar stenosis. Chin Med J. 2011;124(23):3875–3878.
37. Roh EJ, Darai A, Kyung JW, et al. Genetic therapy for intervertebral disc degeneration. Int J Mol Sci. 2021;22(4):1579. doi:10.3390/ijms22041579
38. Kreiner DS, Hwang SW, Easa JE, et al. An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J. 2014;14(1):180–191. doi:10.1016/j.spinee.2013.08.003
39. van der Windt DA, Simons E, Riphagen II, et al. Physical examination for lumbar radiculopathy due to disc herniation in patients with low-back pain. Cochrane Database Syst Rev. 2010;2:Cd007431.
40. Tao S, Jin L, Hou Z, Zhang W, Chen T, Zhang Y. A New radiographic feature of lower lumbar disc herniation in young patients. Int Orthop. 2018;42(3):583–586. doi:10.1007/s00264-017-3723-8
41. Kim JH, van Rijn RM, van Tulder MW, et al. Diagnostic accuracy of diagnostic imaging for lumbar disc herniation in adults with low back pain or sciatica is unknown; a systematic review. Chiropr Man Therap. 2018;26:37. doi:10.1186/s12998-018-0207-x
42. Zheng K, Wen Z, Li D. The clinical diagnostic value of lumbar intervertebral disc herniation based on MRI images. J Healthc Eng. 2021;2021:5594920. doi:10.1155/2021/5594920
43. Jackson RP, Becker GJ, Jacobs RR, Montesano PX, Cooper BR, McManus GE. The neuroradiographic diagnosis of lumbar herniated nucleus pulposus: i. A comparison of computed tomography (CT), myelography, CT-myelography, discography, and CT-discography. Spine. 1989;14(12):1356–1361. doi:10.1097/00007632-198912000-00012
44. Pinto M, Mehbod AA, Swanberg BA, Dawson JM, Schellhas K. Provocative discography: diagnostic efficacy and safety in symptomatic degenerative disk disease. Clin Spine Surg. 2022;35(7):E571–E575. doi:10.1097/BSD.0000000000001329
45. Morita M, Miyauchi A, Okuda S, Oda T, Iwasaki M. Electrophysiological study for nerve root entrapment in patients with isthmic spondylolisthesis. Clin Spine Surg. 2017;30(3):E198–E204. doi:10.1097/BSD.0000000000000047
46. Yildirim P, Gultekin A. The effect of a stretch and strength-based yoga exercise program on patients with neuropathic pain due to lumbar disc herniation. Spine. 2022;47(10):711–719. doi:10.1097/BRS.0000000000004316
47. Zhang B, Xu H, Wang J, Liu B, Sun G. A narrative review of non-operative treatment, especially traditional Chinese medicine therapy, for lumbar intervertebral disc herniation. Biosci Trends. 2017;11(4):406–417. doi:10.5582/bst.2017.01199
48. Awadalla AM, Aljulayfi AS, Alrowaili AR, et al. Management of lumbar disc herniation: a systematic review. Cureus. 2023;15:10.
49. Nerubay J, Caspi I, Levinkopf M, Tadmor A, Bubis JJ. Percutaneous laser nucleolysis of the intervertebral lumbar disc. An experimental study. Clin Orthop Relat Res. 1997;337:42–44. doi:10.1097/00003086-199704000-00005
50. Grangeat AM, Erario MLA. The use of medical ozone in chronic intervertebral disc degeneration can be an etiological and conservative treatment. Int J Mol Sci. 2023;24(7):6538. doi:10.3390/ijms24076538
51. He J, Xiao S, Wu Z, Yuan Z. Microendoscopic discectomy versus open discectomy for lumbar disc herniation: a meta-analysis. Eur Spine J. 2016;25(5):1373–1381. doi:10.1007/s00586-016-4523-3
52. Pan M, Li Q, Li S, et al. Percutaneous Endoscopic Lumbar Discectomy: indications and Complications. Pain Physician. 2020;23(1):49–56.
53. Chu PL, Wang T, Zheng JL, et al. Global and current research trends of unilateral biportal endoscopy/biportal endoscopic spinal surgery in the treatment of lumbar degenerative diseases: a bibliometric and visualization study. Orthop Surg. 2022;14(4):635–643. doi:10.1111/os.13216
54. Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg. 2015;1(1):2–18. doi:10.3978/j.issn.2414-469X.2015.10.05
55. Lamartina C, Berjano P. Prone single-position extreme lateral interbody fusion (Pro-XLIF): preliminary results. Eur Spine J. 2020;29(Suppl 1):6–13. doi:10.1007/s00586-020-06303-z
56. Meyer SA, Mummaneni PV. Axial interbody fusion. J Neurosurg Spine. 2011;15(3):271. doi:10.3171/2011.1.SPINE10913
57. Franco D, Largoza G, Montenegro TS, Gonzalez GA, Hines K, Harrop J. Lumbar total disc replacement: current usage. Neurosurg Clin N Am. 2021;32(4):511–519. doi:10.1016/j.nec.2021.05.010
58. Colella F, Garcia JP, Sorbona M, et al. Drug delivery in intervertebral disc degeneration and osteoarthritis: selecting the optimal platform for the delivery of disease-modifying agents. J Control Release. 2020;328:985–999. doi:10.1016/j.jconrel.2020.08.041
59. Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71. doi:10.1186/s12951-018-0392-8
60. Liu W, Ma Z, Wang Y, Yang J. Multiple nano-drug delivery systems for intervertebral disc degeneration: current status and future perspectives. Bioact Mater. 2023;23:274–299. doi:10.1016/j.bioactmat.2022.11.006
61. Liang C, Li H, Li C, et al. Fabrication of a layered microstructured polymeric microspheres as a cell carrier for nucleus pulposus regeneration. J Biomater Sci Polym Ed. 2012;23(18):2287–2302. doi:10.1163/156856211X614789
62. Frapin L, Clouet J, Delplace V, Fusellier M, Guicheux J, Le Visage C. Lessons learned from intervertebral disc pathophysiology to guide rational design of sequential delivery systems for therapeutic biological factors. Adv Drug Deliv Rev. 2019;149-150:49–71. doi:10.1016/j.addr.2019.08.007
63. Zhu L, Yang Y, Yan Z, et al. Controlled release of TGF-β3 for effective local endogenous repair in IDD using rat model. Int J Nanomed. 2022;17:2079–2096. doi:10.2147/IJN.S358396
64. Adepu S, Ramakrishna S. Controlled drug delivery systems: current status and future directions. Molecules. 2021;26(19):5905. doi:10.3390/molecules26195905
65. Zhang J, Jiao J, Niu M, et al. Ten years of knowledge of nano-carrier based drug delivery systems in ophthalmology: current evidence, challenges, and future prospective. Int J Nanomed. 2021;16:6497–6530. doi:10.2147/IJN.S329831
66. Zhou D, Zhou F, Sheng S, Wei Y, Chen X, Su J. Intra-articular nanodrug delivery strategies for treating osteoarthritis. Drug Discov Today. 2023;28(3):103482. doi:10.1016/j.drudis.2022.103482
67. Hang Y, Liu Y, Teng Z, Cao X, Zhu H. Mesoporous nanodrug delivery system: a powerful tool for a new paradigm of remodeling of the tumor microenvironment. J Nanobiotechnology. 2023;21(1):101. doi:10.1186/s12951-023-01841-2
68. Li B, Shao H, Gao L, Li H, Sheng H, Zhu L. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: a review. Drug Deliv. 2022;29(1):2130–2161. doi:10.1080/10717544.2022.2094498
69. Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. doi:10.1016/j.addr.2012.09.037
70. Sarfraz M, Afzal A, Yang T, et al. Development of dual drug loaded nanosized liposomal formulation by a reengineered ethanolic injection method and its pre-clinical pharmacokinetic studies. Pharmaceutics. 2018;10(3):151. doi:10.3390/pharmaceutics10030151
71. Manshian BB, Jiménez J, Himmelreich U, Soenen SJ. Personalized medicine and follow-up of therapeutic delivery through exploitation of quantum dot toxicity. Biomaterials. 2017;127:1–12. doi:10.1016/j.biomaterials.2017.02.039
72. Arias LS, Pessan JP, Vieira APM, Lima TMT, Delbem ACB, Monteiro DR. Iron oxide nanoparticles for biomedical applications: a perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics. 2018;7(2). doi:10.3390/antibiotics7020046
73. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124. doi:10.1038/s41573-020-0090-8
74. Zhang H, Yu S, Zhao X, Mao Z, Gao C. Stromal cell-derived factor-1α-encapsulated albumin/heparin nanoparticles for induced stem cell migration and intervertebral disc regeneration in vivo. Acta Biomater. 2018;72:217–227. doi:10.1016/j.actbio.2018.03.032
75. Ahlawat J, Henriquez G, Narayan M. Enhancing the delivery of chemotherapeutics: role of biodegradable polymeric nanoparticles. Molecules. 2018;23(9):2157. doi:10.3390/molecules23092157
76. Singh A, Rath G, Singh R, Goyal AK. Nanofibers: an effective tool for controlled and sustained drug delivery. Curr Drug Deliv. 2018;15(2):155–166. doi:10.2174/1567201814666171002115230
77. Haidar MK, Eroglu H. Nanofibers: new Insights for Drug Delivery and Tissue Engineering. Curr Top Med Chem. 2017;17(13):1564–1579. doi:10.2174/1568026616666161222102641
78. Aranda-Lara L, García BEO, Isaac-Olivé K, Ferro-Flores G, Meléndez-Alafort L, Morales-Avila E. Drug delivery systems-based dendrimers and polymer micelles for nuclear diagnosis and therapy. Macromol Biosci. 2021;21(3):e2000362. doi:10.1002/mabi.202000362
79. de Prinse M, Qi R, Amsden BG. Polymer micelles for the protection and delivery of specialized pro-resolving mediators. Eur J Pharm Biopharm. 2023;184:159–169. doi:10.1016/j.ejpb.2023.01.020
80. Vinogradov SV. Nanogels in the race for drug delivery. Nanomedicine. 2010;5(2):165–168. doi:10.2217/nnm.09.103
81. Pan Y, Li Y, Dong W, Jiang B, Yu Y, Chen Y. Role of nano-hydrogels coated exosomes in bone tissue repair. Front Bioeng Biotechnol. 2023;11:1167012. doi:10.3389/fbioe.2023.1167012
82. Zou J, Yang W, Cui W, et al. Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon-bone healing. J Nanobiotechnology. 2023;21(1):14. doi:10.1186/s12951-023-01778-6
83. Shahabipour F, Banach M, Sahebkar A. Exosomes as nanocarriers for siRNA delivery: paradigms and challenges. Arch Med Sci. 2016;12(6):1324–1326. doi:10.5114/aoms.2016.62911
84. Mondal J, Pillarisetti S, Junnuthula V, et al. Hybrid exosomes, exosome-like nanovesicles and engineered exosomes for therapeutic applications. J Control Release. 2023;353:1127–1149. doi:10.1016/j.jconrel.2022.12.027
85. Li T, Takeoka S. A novel application of maleimide for advanced drug delivery: in vitro and in vivo evaluation of maleimide-modified pH-sensitive liposomes. Int J Nanomed. 2013;8:3855–3866. doi:10.2147/IJN.S47749
86. Banala RR, Vemuri SK, Dar GH, et al. Efficiency of dual siRNA-mediated gene therapy for intervertebral disc degeneration (IVDD). Spine J. 2019;19(5):896–904. doi:10.1016/j.spinee.2018.10.016
87. Wang H, Ding Y, Zhang W, et al. Oxymatrine liposomes for intervertebral disc treatment: formulation, in vitro and vivo assessments. Drug Des Devel Ther. 2020;14:921–931. doi:10.2147/DDDT.S242493
88. Ma X, Luan Z, Li J. Inorganic nanoparticles-based systems in biomedical applications of stem cells: opportunities and challenges. Int J Nanomed. 2023;18:143–182. doi:10.2147/IJN.S384343
89. Zhou T, Yang X, Chen Z, et al. Prussian blue nanoparticles stabilize SOD1 from ubiquitination-proteasome degradation to rescue intervertebral disc degeneration. Adv Sci. 2022;9(10):e2105466.
90. Tng DJ, Song P, Lin G, et al. Synthesis and characterization of multifunctional hybrid-polymeric nanoparticles for drug delivery and multimodal imaging of cancer. Int J Nanomed. 2015;10:5771–5786. doi:10.2147/IJN.S86468
91. Lim S, An SB, Jung M, et al. Local delivery of senolytic drug inhibits intervertebral disc degeneration and restores intervertebral disc structure. Adv Healthc Mater. 2022;11(2):e2101483. doi:10.1002/adhm.202101483
92. Arul MR, Zhang C, Alahmadi I, et al. Novel injectable fluorescent polymeric nanocarriers for intervertebral disc application. J Funct Biomater. 2023;14(2):52. doi:10.3390/jfb14020052
93. Kralovic M, Vjaclovsky M, Tonar Z, et al. Nanofiber fractionalization stimulates healing of large intestine anastomoses in rabbits. Int J Nanomed. 2022;17:6335–6345. doi:10.2147/IJN.S364888
94. Yu Q, Han F, Yuan Z, et al. Fucoidan-loaded nanofibrous scaffolds promote annulus fibrosus repair by ameliorating the inflammatory and oxidative microenvironments in degenerative intervertebral discs. Acta Biomater. 2022;148:73–89. doi:10.1016/j.actbio.2022.05.054
95. Tu Z, Han F, Zhu Z, et al. Sustained release of basic fibroblast growth factor in micro/nanofibrous scaffolds promotes annulus fibrosus regeneration. Acta Biomater. 2023;166:241–253. doi:10.1016/j.actbio.2023.05.034
96. Yang Y, Li Y, Chen K, et al. Dual Receptor-targeted and redox-sensitive polymeric micelles self-assembled from a folic acid-hyaluronic Acid-SS-Vitamin E Succinate polymer for precise cancer therapy. Int J Nanomed. 2020;15:2885–2902. doi:10.2147/IJN.S249205
97. Yu C, Li D, Wang C, et al. Injectable kartogenin and apocynin loaded micelle enhances the alleviation of intervertebral disc degeneration by adipose-derived stem cell. Bioact Mater. 2021;6(10):3568–3579. doi:10.1016/j.bioactmat.2021.03.018
98. Chang CC, Tsou HK, Chang HH, et al. Runx1 messenger RNA delivered by polyplex nanomicelles alleviate spinal disc hydration loss in a rat disc degeneration model. Int J Mol Sci. 2022;23(1).
99. Quazi MZ, Park N. Nanohydrogels: advanced polymeric nanomaterials in the era of nanotechnology for robust functionalization and cumulative applications. Int J Mol Sci. 2022;23(4):1943. doi:10.3390/ijms23041943
100. Chang H, Cai F, Zhang Y, et al. Silencing gene-engineered injectable hydrogel microsphere for regulation of extracellular matrix metabolism balance. Small Methods. 2022;6:4.
101. Luo H, Wang Z, He Z, et al. Injectable chondroitin sulfate-grafted self-antioxidant hydrogels ameliorate nucleus pulposus degeneration against overactive inflammation. Biomater Sci. 2023;11(10):3629–3644. doi:10.1039/D3BM00359K
102. Gurunathan S, Kang MH, Kim JH. A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. Int J Nanomed. 2021;16:1281–1312. doi:10.2147/IJN.S291956
103. Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183–3195. doi:10.7150/thno.52570
104. Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. doi:10.1186/s13578-019-0282-2
105. Zhu L, Shi Y, Liu L, Wang H, Shen P, Yang H. Mesenchymal stem cells-derived exosomes ameliorate nucleus pulposus cells apoptosis via delivering miR-142-3p: therapeutic potential for intervertebral disc degenerative diseases. Cell Cycle. 2020;19(14):1727–1739. doi:10.1080/15384101.2020.1769301
106. Sun Z, Tang X, Li Q, Wang H, Sun H, Tian J. Mesenchymal stem cell extracellular vesicles-derived microRNA-194-5p delays the development of intervertebral disc degeneration by targeting TRAF6. Regen Ther. 2022;19:88–96. doi:10.1016/j.reth.2021.12.001
107. Sun Y, Zhang W, Li X. Induced pluripotent stem cell-derived mesenchymal stem cells deliver exogenous miR-105-5p via small extracellular vesicles to rejuvenate senescent nucleus pulposus cells and attenuate intervertebral disc degeneration. Stem Cell Res Ther. 2021;12(1):286. doi:10.1186/s13287-021-02362-1
108. Zhu G, Yang X, Peng C, Yu L, Hao Y. Exosomal miR-532-5p from bone marrow mesenchymal stem cells reduce intervertebral disc degeneration by targeting RASSF5. Exp Cell Res. 2020;393(2):112109. doi:10.1016/j.yexcr.2020.112109
109. Cui S, Zhang L. microRNA-129-5p shuttled by mesenchymal stem cell-derived extracellular vesicles alleviates intervertebral disc degeneration via blockade of LRG1-mediated p38 MAPK activation. J Tissue Eng. 2021;12:20417314211021679. doi:10.1177/20417314211021679
110. Sun Z, Liu B, Liu ZH, et al. Notochordal-cell-derived exosomes induced by compressive load inhibit angiogenesis via the miR-140-5p/Wnt/β-Catenin Axis. Mol Ther Nucleic Acids. 2020;22:1092–1106. doi:10.1016/j.omtn.2020.10.021
111. Zhou ZM, Bao JP, Peng X, et al. Small extracellular vesicles from hypoxic mesenchymal stem cells alleviate intervertebral disc degeneration by delivering miR-17-5p. Acta Biomater. 2022;140:641–658. doi:10.1016/j.actbio.2021.11.044
112. Chen D, Jiang X, Zou H. hASCs-derived exosomal miR-155-5p targeting TGFβR2 promotes autophagy and reduces pyroptosis to alleviate intervertebral disc degeneration. J Orthop Translat. 2023;39:163–176. doi:10.1016/j.jot.2023.02.004
113. Zhao X, Sun Z, Xu B, et al. Degenerated nucleus pulposus cells derived exosome carrying miR-27a-3p aggravates intervertebral disc degeneration by inducing M1 polarization of macrophages. J Nanobiotechnology. 2023;21(1):317. doi:10.1186/s12951-023-02075-y
114. Zhang H, Wu J, Wu J, et al. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J Nanobiotechnology. 2019;17(1):29. doi:10.1186/s12951-019-0461-7
115. Jia G, Han Y, An Y, et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302–316. doi:10.1016/j.biomaterials.2018.06.029
116. Kooijmans SA, Aleza CG, Roffler SR, van Solinge WW, Vader P, Schiffelers RM. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J Extracell Vesicles. 2016;5:31053. doi:10.3402/jev.v5.31053
117. Tian T, Cao L, He C, et al. Targeted delivery of neural progenitor cell-derived extracellular vesicles for anti-inflammation after cerebral ischemia. Theranostics. 2021;11(13):6507–6521. doi:10.7150/thno.56367
118. Villata S, Canta M, Cauda V. EVs and Bioengineering: from Cellular Products to Engineered Nanomachines. Int J Mol Sci. 2020;21(17). doi:10.3390/ijms21176048
119. Poongodi R, Chen YL, Yang TH, et al. Bio-scaffolds as cell or exosome carriers for nerve injury repair. Int J Mol Sci. 2021;22(24). doi:10.3390/ijms222413347
120. Zou Z, Li H, Xu G, Hu Y, Zhang W, Tian K. Current knowledge and future perspectives of exosomes as nanocarriers in diagnosis and treatment of diseases. Int J Nanomed. 2023;18:4751–4778. doi:10.2147/IJN.S417422
121. Zhang Y, Huo M, Wang Y, et al. A tailored bioactive 3D porous poly(lactic-acid)-exosome scaffold with osteo-immunomodulatory and osteogenic differentiation properties. J Biol Eng. 2022;16(1):22. doi:10.1186/s13036-022-00301-z
122. Mi S, Chang Z, Wang X, et al. Bioactive spinal cord scaffold releasing neurotrophic exosomes to promote in situ centralis neuroplasticity. ACS Appl Mater Interfaces. 2023;15(13):16355–16368. doi:10.1021/acsami.2c19607
123. Zhang M, Xiao B, Wang H, et al. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol Ther. 2016;24(10):1783–1796. doi:10.1038/mt.2016.159
124. Liu B, Lu Y, Chen X, et al. Protective role of shiitake mushroom-derived exosome-like nanoparticles in D-galactosamine and lipopolysaccharide-induced acute liver injury in mice. Nutrients. 2020;12:2.
125. Liu H, Gu R, Li W, et al. Engineering 3D-printed strontium-titanium scaffold-integrated highly bioactive serum exosomes for critical bone defects by osteogenesis and angiogenesis. ACS Appl Mater Interfaces. 2023;15(23):27486–27501. doi:10.1021/acsami.3c00898
126. Li Q, Yu H, Zhao F, et al. 3D printing of microenvironment-specific bioinspired and exosome-reinforced hydrogel scaffolds for efficient cartilage and subchondral bone regeneration. Adv Sci. 2023;10(26):e2303650. doi:10.1002/advs.202303650
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.