Back to Journals » Drug Design, Development and Therapy » Volume 16
The Emerging Potential of Parthenolide Nanoformulations in Tumor Therapy
Authors An T , Yin H, Lu Y, Liu F
Received 21 December 2021
Accepted for publication 5 April 2022
Published 29 April 2022 Volume 2022:16 Pages 1255—1272
DOI https://doi.org/10.2147/DDDT.S355059
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Tao An,1 Huanhuan Yin,1 Yanting Lu,2 Feng Liu1,3
1School of Pharmaceutical Sciences, Qilu University of Technology (Shandong Academy of Sciences), Jinan, Shandong Province, People’s Republic of China; 2College of TCM, Shandong University of Traditional Chinese Medicine, Jinan, Shandong Province, People’s Republic of China; 3Key Laboratory for Applied Technology of Sophisticated Analytical Instruments of Shandong Province, Shandong Analysis and Test Center (SDATC), Qilu University of Technology (Shandong Academy of Sciences), Jinan, Shandong Province, People’s Republic of China
Correspondence: Feng Liu, Key Laboratory for Applied Technology of Sophisticated Analytical Instruments of Shandong Province, Shandong Analysis and Test Center (SDATC), Qilu University of Technology (Shandong Academy of Sciences), 19th Keyuan Road, Jinan, 250014, Shandong Province, People’s Republic of China, Tel +86 531-82605319, Fax +86 531-82964889, Email [email protected]
Abstract: Plant-derived sesquiterpene lactones are promising natural sources for the discovery of anti-cancer drugs. As an extensively studied sesquiterpene lactone, the tumor suppression effect of parthenolide (PTL) has been clarified by targeting a number of prominent signaling pathways and key protein regulators in carcinogenesis. Notably, PTL was also the first small molecule reported to eradicate cancer stem cells. Nevertheless, the clinical application of PTL as an antitumor agent remains limited, owing to some disadvantages such as low water solubility and poor bioavailability. Thus, nanomedicine has attracted much interest because of its great potential for transporting poorly soluble drugs to desired body sites. In view of the significant advantages over their free small-molecule counterparts, nanoparticle delivery systems appear to be a potential solution for addressing the delivery of hydrophobic drugs, including PTL. In this review, we summarized the key anticancer mechanisms underlined by PTL as well as engineered PTL nanoparticles synthesized to date. Therefore, PTL nanoformulations could be an alternative strategy to maximize the therapeutic value of PTL.
Keywords: parthenolide, signaling pathways, cellular processes, nanomedicine, cancer therapy
Graphical Abstract:
Introduction
As a medical herb, Feverfew (Tanacetum parthenium) is conventionally used in Europe to treat fever, inflammation, migraines, rheumatoid arthritis, and menstrual irregularities. Parthenolide (PTL, Figure 1) is a sesquiterpene lactone found in feverfew, which is currently considered to be responsible for the herb’s therapeutical potential.1 Initially, conventional extraction methods using chloroform and petroleum ether were performed to extract PTL; various extraction methods have been developed, such as high-performance liquid chromatography (HPLC), Soxhlet extraction, supercritical fluid extraction (SFE) and microwave-assisted extraction (MAE).1,2
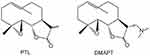 |
Figure 1 Chemical structure of PTL and DMAPT. |
The anti-cancer property of PTL was firstly validated in 1973. Furthermore, its patent application for tumor suppression was approved in 2005.3 Additionally, the in vitro and in vivo antitumor potential of PTL in multiple cancer types has been confirmed by numerous researches, which mainly resulted from its cytotoxicity to the bulk population of cancer cells as well as from selectively targeting cancer stem cells (CSCs); it is a subpopulation currently believed to be responsible for chemotherapy resistance and tumor relapse.3–10 Further studies revealed a series of direct PTL targets [p65, IκB kinase (IKK), focal adhesion kinase 1 (FAK1), and others] that indirectly affect signaling pathways, which account for cell cycle arrest, apoptosis induction, metastasis suppression, redox imbalance, and epigenetic regulation involved in PTL’s antitumor properties.3,4,11,12 The potential utility of PTL as radio-sensitization agent and complementary therapy against various cancers has also been widely studied and summarized.13–15 As reviewed by Malgorzata et al, PTL has been combined with various anticancer agents, such as tubulin-directed agents, anthracyclines, antimetabolites, histone deacetylase inhibitors, mTOR inhibitors, and inducers of reactive oxygen species (ROS).14
Despite its deciphered anticancer potential and mechanisms of action in pre-clinical experiments, the clinical application of PTL remains hindered because of some disadvantages, including weak aqueous solubility, low oral bioavailability, and relative instability under chemical and physiological conditions.16,17 As a result, various methods for synthesizing PTL derivatives to yield compounds with better hydrophilicity and improved potency have been proposed.4,6,18 Dimethylamino parthenolide (DMAPT, Figure 1), a representative among hydrophilic PTL analogues, showed improved water solubility and oral bioavailability. Thus, it has advanced into the first phase of a clinical trial for the treatment of acute myeloid leukemia (AML).19
Nanomedicine is a rapidly developing field that exploits nanoparticles (NPs) to facilitate the diagnosis and treatment of a wide range of diseases. Nanoparticles applied in nanomedicine generally refer to a type of colloidal drug delivery system, which comprises particles with a size range from 10 to 200 nm in diameter.20 By far, diverse types of nanoparticles have been developed as drug carriers, including but not limited to liposomes, polymeric micelles, carbon nanotubes, mesoporous silica nanoparticles, metal-based nanoparticles, and dendrimers. Moreover, these can be made of diverse materials, including lipids, phospholipids, polymers, proteins, and inorganic materials.21,22
Compared with free drug counterparts, nanoparticles entrapment has displayed distinct advantages, such as improved bioavailability, prolonged circulation time, and ease of functionalization by surface modification. Furthermore, the enhanced permeability and retention (EPR) effect caused by the large amount of leaky vascularization and impaired lymphatic drainage at the tumor site enables non-targeted nanoparticles to accumulate in tumor tissues.23 Some of these nanoparticles have been approved as cancer therapeutics by the Food and Drug Administration.21 Besides the chemical modification of PTL for property improvement, the development of nanoscale drug delivery systems offers another promising strategy to overcome the poor water solubility and bioavailability of PTL as well as to determine its efficient and selective delivery to tumor tissues; the latter of which has not been summarized as compared to the extensively reviewed bioactivities and combination treatments of PTL. Therefore, we focused on the key antitumor mechanisms of PTL as well as its efficiency in being formulated as a nanoparticle delivery system.
Antitumor Mechanisms of PTL
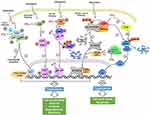
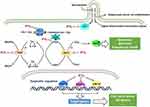
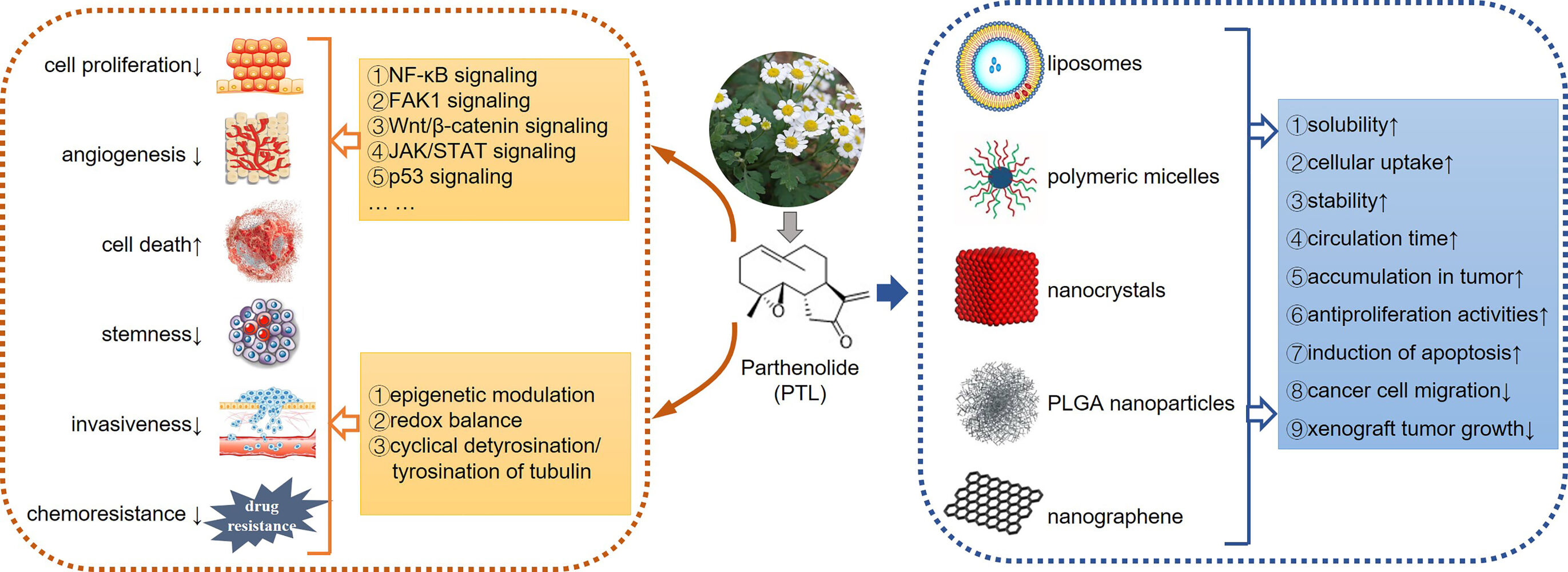
Current evidence demonstrates that the antitumor mechanism of PTL is multifactorial and complex, due to the high electrophilic reactivity of α-methylene-γ-lactone present in PTL, thereby resulting in the alkylation of various proteins. A number of PTL targets have thus been identified and summarized.4 Furthermore, newer potential targets of PTL, such as USP7 and EGFR, continue to be reported,24–27 thereby giving rise to the regulatory effect of PTL on various signaling pathways (Figure 2) and cellular processes (Figure 3) responsible for proliferation, cell cycle regulation, stemness, cell death, angiogenesis and metastasis.3,4 Thus, it is not surprising that PTL displays diverse anticancer effects, including abrogated cell viability and angiogenesis, cell cycle arrest, cell death induction, and decrease in stemness, invasiveness, and chemoresistance (Table 1).
 |  |  |  |
Table 1 Pharmacological Activities of PTL in Tumors (Since 2019) |
Signaling Pathways Affected by PTL
As shown in Figure 2, several signaling pathways closely related to tumorigenesis and progression were suppressed by PTL; among these, the prominent NF-κB signaling pathway was the first to be inhibited. Mechanism investigation illustrated that PTL inhibited NF-κB signaling by alkylating cysteine 38 in p65 and cysteine 179 in IKK.28,29 Additionally, a later study predicted that tumor necrosis factor receptor-associated factor 6 (TRAF6) might have been a novel target involved in the PTL-associated inhibition of NF-κB.30 Moreover, the role of PTL in preventing NF-κB activation contributed to the suppression of hypoxia-inducible factor-1α (HIF-1α) signaling.31 Recent studies have elucidated that PTL inhibited Wnt/β-catenin signaling by targeting ubiquitin specific peptidase 7 (USP7) and ribosomal protein L10 (RPL10), a deubiquitination enzyme stabilizing β-catenin and a ribosomal protein related to the synthesis of the transcriptional regulator 4/lymphoid enhancer binding factor 1 (TCF4/LEF1), respectively.24,32 Furthermore, FAK1 and janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling were found to be impaired by PTL by covalently modifying specific cysteine molecules.33,34
Apart from inhibiting the aforementioned signaling pathways, PTL also activated p53 functions by promoting the ubiquitination of E3 ligase murine double minute 2 (MDM2) in an ataxia-telangiectasia mutated (ATM)-dependent manner, thus leading to either cell cycle arrest or apoptosis.35 Specially, USP7 was reported to interact with and stabilize MDM2 and p65.36,37 Thus, the inhibitory effect of PTL on USP7 might be a further step for activating p53 and inhibiting NF-κB. Additionally, the activation of c-Jun N-terminal kinase (JNK) by PTL enhanced the sensitivity of human cancer cells to tumor necrosis factor-α (TNF-α) or tumor necrosis factor-related apoptosis-inducing ligand (TRAIL).38,39
Cellular Processes Influenced by PTL
To date, PTL has been found to exert antitumor effects on several cellular processes, including redox balance and epigenetic modulation; the targets involved in these two processes have also been revealed (Figure 3). Several studies have indicated that PTL disrupted intracellular redox homeostasis by depleting glutathione (GSH) and inhibiting its metabolic enzymes, including glutamate-cysteine ligase catalytic subunit (GCLC), thioredoxin reductase 1/2 (TrxR1/2), and GSH peroxidase 1 (GPX1), thus leading to increased ROS level.40–42 Moreover, ROS enhancement by PTL seemed to elicit different forms of cell death, such as necrosis, apoptosis, and autophagic cell death, depending on the tumor cell type.40,41,43 Meanwhile, the action of PTL on ROS partially accounts for its distinctive ability to selectively induce cell death in cancer cells, while sparing the equivalent normal cells. This is due to the fact that oxidative stress in cancer cells is more frequently elevated than that in normal cells. Thus, additional ROS produced by PTL may promote tumor cell death, whereas normal cells may maintain redox homeostasis by adaptive antioxidant responses.44 Moreover, the epigenetic targets of PTL consist of various enzymes, including histone deacetylase 1 (HDAC1), DNA methyltransferase 1 (DNMT1), and lysine methyltransferase 5C (KMT5C); these regulate the transcription of various genes, such as p21 and high in normal-1 (HIN-1).45–47 In addition, PTL disrupted the cyclical detyrosination/tyrosination of tubulin by inhibiting tubulin carboxypeptidase (TCP), which led to a reduced frequency of microtentacles and suppressed tumor cell reattachment to endothelial layers (Figure 2).48,49
Interactions between signaling pathways and cellular processes may be affected by PTL. For instance, the stimulated generation of ROS by PTL was able to induce autophagy and cause NF-kB downregulation.40,43 Furthermore, NF-κB inhibition contributed to epigenetic regulation,47 thereby further complicating the antitumor mechanism of PTL.
Pharmacological Effects of PTL
To understand the pharmacological effects of PTL, in vitro studies on its efficacy, pharmacological activities, and potential molecular mechanisms in a variety of cancer cells as well as available in vivo models since 2019 are tabulated in Table 1, thus further extending and qualifying existing reviews of its biological activities.3,4,50 As shown in Table 1, PTL modulation toward the aforementioned signaling pathways and cellular processes exerts multiple pharmacological effects against a myriad of tumor cell types.
The antiproliferative activity of PTL was detected in almost all studies; the results indicated that PTL exhibited half-maximal inhibitory concentration (IC50) between the range of 2.5–25 μM for most tumor cells listed, thus showcasing its cytotoxicity to different cancer cells.51–59 Cell cycle arrest, induction of cell death, and changes in related proteins were further detected, thus further supporting the cytotoxic potential of PTL. Overall, PTL was shown to induce different effects depending on the cell type, which can be illustrated by its ability to induce cell cycle arrest at different phases as well as several types of cell death in various cancer cells. For example, PTL arrested uveal melanoma cells in the G1 phase by upregulating p21 and downregulating cyclin D1, which are two G1 phase cell cycle regulatory proteins.60 On the other hand, PTL decreased the expression of survivin promoting G2/M cell cycle transition, thereby triggering G2/M cell cycle arrest in glioblastoma cells.61 Apoptosis, necrosis, and autophagy are the three forms of cell death caused by PTL;62 among which, apoptosis is the most studied. It is well known that apoptosis is elicited by two distinct pathways, the extrinsic and mitochondria-mediated intrinsic pathways; it culminates in the activation of caspases, which function as the main apoptotic effectors.63
Numerous studies demonstrated that PTL treatment could induce extrinsic or intrinsic apoptosis in tumor cells by inhibiting the activities of the NF-κB, STAT3, Wnt and JNK signaling pathways, activating the p53 signaling pathway, regulating the Bcl-2 family members, and generating ROS.24,62,64–71 Furthermore, PTL-induced cell death of breast cancer and multiple myeloma cells was dramatically attenuated by co-treatment with the pan-caspase inhibitor, Q-VD-OPh or Z-VAD-fmk, thus indicating that caspases are involved in PTL-induced apoptotic cell death; it also concurs the presence of other forms of cell death.33,72 Indeed, a large number of studies reported that PTL was capable of inducing autophagic or necrotic cell death.50,62 For instance, PTL mediated cell death through ROS-mediated autophagy in human osteosarcoma (Saos-2 and MG-63) and triple-negative breast cancer (MDA-MB-231) cells.40,43 In particular, PTL was capable of simultaneously inducing mixed forms of cell death, as evidenced by observations of PTL-induced apoptosis and autophagy in Hela and HepG2 cells as well as apoptosis and necrosis in HL60 cells.73–75 Additionally, the role of autophagy in cell death involves the fact that its inhibition significantly blocked PTL-induced apoptosis in pancreatic cancer cells,76 but potentiated PTL-induced apoptosis in human breast cancer cells.77
Although previous studies have elucidated the selective targeting effect of PTL on CSCs from primary or sensitive cancer cells,3 a recent study by Yi et al suggested that PTL also effectively eliminated leukemia stem cells (LSCs) from adriamycin (ADM)-resistant K562 cells (K562/ADM) by suppressing aberrantly activated NF-κB.64 In addition, NF-κB inactivation by PTL sensitized gastric tumor and esophageal squamous cell carcinoma (ESCC) cells to chemotherapeutic drugs including ADM and cisplatin.78,79 PTL exerted its anti-angiogenic effects by inhibiting the NF-кB-mediated VEGF expression in ESCC cells.79 Moreover, several studies have pointed out that PTL inhibits migration, invasion, and metastasis, which benefits from its abilities to regulate epithelial-to-mesenchymal transition (EMT) and to inhibit FAK1, TCP, and STAT3.33,80–83
In short, the ultimate outcomes resulting from the affected signaling pathways and cellular processes by PTL include, but are not limited to, impaired cell proliferation and angiogenesis, induction of cell death, and reduced stemness, invasiveness, and chemoresistance. This has been confirmed by a large body of research, thus providing a sufficient basis for studies investigating and developing a wide range of PTL nanoformulations for various cancer therapies.
Nanoformulations of PTL for Tumor Therapy
To the best of our knowledge, several types of PTL nanocarriers, including liposomes,84–89 polymeric micelles,90–96 nanocrystals,97 PLGA nanoparticles,98 and nanographene,99 have been synthesized to deliver PTL and ameliorate its anti-cancer efficacy. Whether PTL is co-encapsulated into nanoparticles with other drugs or small molecules and whether the nanoparticles are modified for targeted therapy, these studies can be classified into three types. Furthermore, the types, materials, and properties of nanocarriers mentioned in these studies, as well as the in vitro and in vivo models employed to evaluate the anticancer effects of nanoformulations, are separately tabulated in Tables 2–4.
 |
Table 2 Unmodified Nanoparticles Solely Incorporated with PTL |
 |
Table 3 Undecorated Nanovectors Encapsulated PTL and Other Agents |
 |
Table 4 Targeted Nanocarriers Encapsulated PTL with or without Other Agents |
PTL is Solely Incorporated into Unmodified Nanoparticles
Baranello et al synthesized different types of micelles formed from amphiphilic diblock copolymers of PSMA-b-PS or PSMA-b-PBA; PTL was successfully loaded into these micelles. However, PTL exhibited the greatest loading efficiency and capacity in PSMA100-b-PS258 micelles, thereby indicating that the composition and hydrophobic core chemistry of micelles were significant parameters for optimization.90 Although subsequent studies suggested that PTL-loaded PSMA100-b-PS258 micelles did not exhibit better cytotoxic ability toward MV4-11 cells than free PTL, it protected sequestered PTL from damage by both cells and deactivating chemicals, such as GSH.91 Similarly, the application of stealthy liposomes and micelles fabricated by F127 or biodegradable PTL-PTMC as nanocarriers of PTL did not appear to efficiently increase its cytotoxicity against MCF-7, MDA-MB-231, and patient T-lineage acute lymphoblastic leukemia (T-ALL) or B-cell precursor acute lymphoblastic leukemia (BCP-ALL) cells.89,92,93 However, the combination of stealthy liposomal PTL slightly sensitized the antitumor effects of stealthy liposomes loaded with vinorelbine.89
Unlike the nanocarriers mentioned above, the carboxyl-functionalized nanographene (fGn) as well as the PTL-nanocrystal delivery system showed improved antiproliferation activities in comparison with individual PTL. The IC50 of PTL and PTL-fGn for Panc-1 cells were 39 and 9.5 μM respectively, whereas the IC50 of PTL for HepG2 cells decreased from 50.891 μM to 33.618 μM when delivered with nanocrystals.97,99 Interestingly, there was no significant difference in cytotoxic activity between DMAPT and DMAPT-fGn; this inefficacy may be due to the lack of a hydrophobic interaction between DMAPT and fGn.99 Thus, whether incorporation of DMAPT into other nanovectors could enhance its cytotoxicity is worth exploring. Furthermore, the in vitro and in vivo combination of PTL-nanocrystals and sorafenib achieved remarkable synergistic anti-cancer effects, as reflected by the MTT, wound-healing and HepG2 xenograft assays.97
Co-Delivery of PTL and Other Agents in Undecorated Nanovectors
Given the molecular complexity of cancer, combination therapy has attracted tremendous attention because of its ability to increase drug efficacy, improve drug resistance, and reduce systemic toxicity. Kanwaldeep et al constructed a paclitaxel and PTL co-delivery system of PEG2000-DSPE/vitamin E-TPGS mixed micelles, which retained a high encapsulation efficiency (>95%) and chemical stability over a storage period of 45 days. Furthermore, co-encapsulation of these two drugs significantly suppressed the viability of sensitive and taxol-resistant A549 cells compared to their free drug counterparts in solutions and single drug-loaded micelles.94 In addition, as a component of these mixed micelles, it is noteworthy that vitamin E-TPGS aided in not only maintaining high encapsulation efficiency due to its bulky structure and large surface area, but also enhanced chemosensitization by inhibiting P-glycoprotein (P-gp) efflux.94,100 Recently, a liposome system loaded with betulinic acid, PTL, honokiol and ginsenoside Rh2 displayed in vitro and in vivo antitumor activity comparable to cisplatin, the first-line therapy for lung cancer. In addition, this cocktail liposome therapy circumvented obvious kidney damage induced by cisplatin, as revealed by hematoxylin and eosin (H&E) staining. It also did not cause any significant damage to other major organs, including the heart, liver, spleen, and lungs, thereby indicating that this cocktail is a safer alternative for lung cancer treatment.84
To exploit the advances in photothermal therapy, Jin Xin and co-workers constructed thermosensitive liposomes (TSLs), in which PTL and the photosensitizer indocyanine green (ICG) were co-loaded. ICG converts light energy into heat energy upon near-infrared light irradiation. Compared to groups treated with paclitaxel, a combination of free drugs with or without laser, or PTL-ICG TSLs without laser, groups treated with PTL-ICG TSLs with laser exhibited lower cell viability, higher ROS induction and apoptosis, and better in vivo anti-cancer effects. According to these results, the two benefits of heat energy released by ICG under near-infrared radiation at the tumor location were validated. On the one hand, the heat-promoted phase transition of these liposomes enhanced their fluidity and permeability, thus allowing loaded drugs to effectively diffuse to tumor tissues at high concentrations. On the other hand, the maximum temperature of tumor tissues treated with PTL-ICG TSLs with laser reached 47.4°C± 2.68°C, which led to irreversible damage and further synergized with the oxidative stress of PTL.85
Nanocarriers Encapsulated with PTL Were Modified to Enhance Their Targeting Ability
To further improve drug delivery and selective targeting toward cancer cells, targeted nano-encapsulation of PTL with or without other agents was developed according to the characteristics of specific types of tumor cells and their microenvironment or the suction of the magnetic field. CD44 is a pivotal receptor involved in myelopoiesis; its specific variant isoforms have been reported to be overexpressed in AML cells, thus indicating that CD44 can serve as a promising receptor for targeted delivery of anti-AML drugs.101,102 As a result, an intervention was developed by encapsulating PTL into PLGA nanoparticles conjugated with antiCD44 with higher tumor targeting efficiency than PLGA-PTL nanoparticles. Although the cytotoxic abilities of PLGA-antiCD44-PTL and PLGA-PTL nanoparticles were not compared, PLGA-antiCD44-PTL nanoparticles exhibited stronger cytotoxicity than free PTL.98
Leukemia stem cells (LSCs) within the bone marrow (BM) microenvironment are thought to be the primary mediators of relapse and chemotherapy resistance in AML. Furthermore, E-selectin expressed in the BM endothelium provides a feasible approach for targeted BM delivery. In view of the remarkable capability of PTL to eradicate cancer stem cells including LSCs, a multistage vector system (MSV) was developed by entrapping PTL in mPEG-PLA micelles coated with protective degradable porous silicon particles and an E-selectin thioaptamer. In contrast to the negligible therapeutic efficacy of PTL-loaded micelles, MSV-PTL significantly reduced the tumor burden of patient-derived AML xenografts, concurrent with the inhibition of NF-κB and activation of γ-H2AX; this supports the effectiveness of the MSV system for targeted BM delivery. Moreover, the decreased level of secondary xenotransplants implied that the directed delivery of PTL to the BM using the MSV system led to the elimination of LSCs.95
The tLyP-1 peptide has been verified to possess cell-penetrating ability and tumor-targeting capacity, which are derived from its C-terminal structure and affinity to the neuropilin-1 receptor overexpressed in several kinds of cancer cells (eg glioma and lung cancer).103 In which tLyP-1-modified liposomes entrapped in PTL and ginsenoside compound K (CK) were synthesized, the level of ROS and induced apoptosis of A549 cells in vitro significantly increased. Besides, PTL/CK tLyP-1 liposomes exhibited greater anticancer efficacy than the combined administration of these two compounds in A549 tumor-bearing mice.87 In addition, Ran et al developed PTL-loaded PEG-PLA micelles decorated with a “Y”-shaped DWVAP peptide, which could guide micelles across multiple biological barriers and ultimately target glioma and its associated stem cells. Moreover, combined therapy of PTL-loaded, DWVAP-modified PEG-PLA micelles with temozolomide or DWVAP-modified PEG-PLA micelles loaded with paclitaxel achieved outstanding anti-glioma efficacy, according to the Kaplan–Meier survival analysis.96
Recently, by virtue of the magnetic field, magnetic nanoparticles (MNPs) were chosen to modify the surface of liposomes (lips) loaded with PTL by Gao and co-workers.86,88 In one study, PTL-ICG-Lips, similar to PTL-ICG TSLs mentioned above, were coated with MNPs. In terms of in vitro heating efficiency and drug release, there was no significant difference between the PTL-ICG-Lips@MNPs group treated only with laser and PTL-ICG-Lips@MNPs group treated with laser plus magnet, which were stronger than non-laser irradiation treated groups. These results suggested the dominant role of photothermal conversion mediated by ICG in these processes. However, the presence of the magnetic field increased the heating rate and percentage of drug release at an earlier stage. In addition, cells treated with ICG-C6-Lips@MNPs (magnet plus laser) exhibited the highest cellular uptake. Consistently, in vivo studies also showed that the PTL-ICG-Lips@MNPs (magnet plus laser) group displayed the fastest heating rate, highest temperature, and highest intratumoral PTL concentration.86 These findings indicated that the magnetic field could enrich the magnetic liposomes in the irradiated area, thus further enhancing the efficiency of photothermal conversion and facilitating the release and uptake of PTL.
Another multifunctional delivery system generated by Gao et al was the encapsulation of PTL and glucose oxidase (GOD) into nanomagnetic liposomes coated with chitosan, named GOD-PTL-Lips@MNPs. The addition of chitosan endowed GOD-PTL-Lips@MNPs with the capability to release drug at a slightly acidic pH, which was characteristic of the tumor microenvironment. As a result, this increased the targeting ability of the system together with the magnetic field. By consuming glucose, GOD in this system lowers the pH and generates H2O2 as well as starves the cancer cells to death. A lower pH further promotes drug release; H2O2 can be subsequently catalyzed by iron ions in MNPs to produce hydroxyl radicals (•OH), a noxious ROS. Meanwhile, PTL protects •OH from scavenging by depleting GSH,40 which amplifies the intracellular oxidative stress and thus leads to cell apoptosis. Under an extra magnetic field, the GOD-PTL-Lips@MNPs demonstrated prominent antitumor effects in vitro and in vivo through the integration of chemo-, chemodynamic, starvation and magnetic-targeting therapies.88
As discussed above, the incorporation of PTL into nanocarriers results in increased solubility, cellular uptake and stability, prolonged circulation time, and enhanced accumulation at tumor sites. Therefore, the majority of nanocarriers encapsulated with PTL, especially when combined with other agents such as photosensitizers, anticancer drugs, and MNPs, demonstrated higher anticancer efficiency than free PTL. These mechanisms are reflected by better antiproliferative activities, more effective induction of apoptosis, higher suppression rate of migration, and xenograft tumor growth. Histological examination demonstrated the low toxicity of this novel therapeutic agent.
In addition, a variety of naturally occurring sesquiterpene lactones structurally related to PTL with anticancer efficiency, as represented by micheliolide (MCL, Figure 4), melampomagnolide B (MMB, Figure 4), and costunolide (COS, Figure 4) have been reported.104–106 Similar with PTL, most of current researches focus on structural modification of these compounds to improve their antitumor effects, stability, and sustainable release.107–111 Several studies started to investigate the incorporation of MCL analogs and COS into nanoparticles.112,113 Bone-targeted PSMA-b-PS NPs entrapped with triazole MCL analog exhibited excellent serum stability and significantly reduced LSC burden in leukemic mice.112 Another study demonstrated that COS and COS-NPs, in combination with doxorubicin (DOX), stimulated the activity of caspase-3 and induced apoptosis of HCT116 and MDA-MB-231-Luc. They also suppressed the tumor growth of HCT116 and MDA-MB-231-Luc implants in nude mice. There was no significant difference in the anti-tumor activity of COS and COS-NPs; the authors deemed that this may have been due to the dose-selective approach for determining the optimal anti-cancer activity for both COS and Nano-COS. Thus, dose-response relationship will be investigated in future studies.113 In addition, Niu et al constructed pH-responsive mesoporous silica nanoparticles (MSNs) loaded with COS, which increased stability and enhanced anti-fibrotic effect of pure COS.114 In short, above-mentioned results implied that nanoparticles entrapped with sesquiterpene lactones show great promise in the treatment of cancer and other diseases.
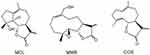 |
Figure 4 Chemical structure of MCL, MMB and COS. |
Conclusion
Studies from the past decades have validated the great potential of PTL as an anticancer agent with extremely intricate bioactivities. However, poor aqueous solubility, instability, low bioavailability, and drug-targeting property of PTL limit its in vivo anticancer efficacy and clinical application.86 As such, the development of nanoparticle-based platforms has been utilized in multiple biomedical fields, including hydrophobic drug delivery, which undoubtedly provides a promising strategic improvement.115 Indeed, several natural product-derived anticancer nanodrugs, including nanoparticle albumin–bound (NAB)-paclitaxel and liposomal vincristine, have been used in clinical practice.21 However, no clinical trials have been reported for nanocomposites of PTL and its structurally related sesquiterpene lactones. Furthermore, despite multiple in vitro and in vivo experiments that have reported the benefits of nanoparticle-based formulations of other natural anti-cancer drugs in the treatment of various cancer types, including quercetin, curcumin, resveratrol, and andrographolide, only a few clinical trials have been performed, thus, suggesting that the investigation of these nanocarriers is still in its relative infancy.116–118 Further optimization can be performed because the efficiency of nanoparticles can be influenced by many parameters, such as nanocarrier types, compositions (eg materials, ligand modification, co-encapsulated agents), and physical properties (eg size, shape, surface charge).21 As such, PTL and other natural product-based nanoformulations with improved properties will undoubtedly emerge; clinical trials need to be encouraged to further validate the security and therapeutic efficiency of these nanoparticles for cancer. Finally, PTL has been recently predicted to be a possible agent for the treatment of other diseases, such as Hutchinson-Gilford Progeria syndrome and hypertrophic cardiomyopathy.119,120 Thus, the therapeutic value of PTL nanoparticles for these diseases deserves further study.
Acknowledgments
The authors acknowledge funding from the Young Scientists Fund of National Science Foundation of China (No. 81803587), the Project of Science and Technology of Yunnan Province (2019FD054), PhD Start-up Fund for Tao An from Qilu University of Technology (No. 81110573), and Industry-University Cooperation Collaborative Education Project of Ministry of Education (202102403007).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Végh K, Alberti Á, Riethmüller E, Tóth A, Béni S, Kéry Á. Supercritical fluid extraction and convergence chromatographic determination of parthenolide in Tanacetum parthenium L.: experimental design, modeling and optimization. J Supercrit Fluids. 2014;95:84–91. doi:10.1016/j.supflu.2014.07.029
2. Alam P, Siddiqui NA, Rehman MT, et al. Box-Behnken Design (BBD)-Based optimization of microwave-assisted extraction of parthenolide from the stems of tarconanthus camphoratus and cytotoxic analysis. Molecules. 2021;26(7):1876. doi:10.3390/molecules26071876
3. Ghantous A, Sinjab A, Herceg Z, Darwiche N. Parthenolide: from plant shoots to cancer roots. Drug Discov Today. 2013;18(17–18):894–905. doi:10.1016/j.drudis.2013.05.005
4. Freund RRA, Gobrecht P, Fischer D, Arndt HD. Advances in chemistry and bioactivity of parthenolide. Nat Prod Rep. 2020;37(4):541–565. doi:10.1039/C9NP00049F
5. Siveen KS, Uddin S, Mohammad RM. Targeting acute myeloid leukemia stem cell signaling by natural products. Mol Cancer. 2017;16(1):13. doi:10.1186/s12943-016-0571-x
6. Araujo TG, Vecchi L, Lima P, et al. Parthenolide and its analogues: a new potential strategy for the treatment of triple-negative breast tumors. Curr Med Chem. 2020;27(39):6628–6642. doi:10.2174/0929867326666190816230121
7. Dandawate PR, Subramaniam D, Jensen RA, Anant S. Targeting cancer stem cells and signaling pathways by phytochemicals: novel approach for breast cancer therapy. Semin Cancer Biol. 2016;40–41:192–208. doi:10.1016/j.semcancer.2016.09.001
8. Ren Y, Yu J, Kinghorn AD. Development of anticancer agents from plant-derived sesquiterpene lactones. Curr Med Chem. 2016;23(23):2397–2420. doi:10.2174/0929867323666160510123255
9. Mathema VB, Koh YS, Thakuri BC, Sillanpaa M. Parthenolide, a sesquiterpene lactone, expresses multiple anti-cancer and anti-inflammatory activities. Inflammation. 2012;35(2):560–565. doi:10.1007/s10753-011-9346-0
10. Ghantous A, Gali-Muhtasib H, Vuorela H, Saliba NA, Darwiche N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov Today. 2010;15(15–16):668–678. doi:10.1016/j.drudis.2010.06.002
11. Koprowska K, Czyz M. [Molecular mechanisms of parthenolide’s action: old drug with a new face]. Postepy Hig Med Dosw. 2010;64:100–114. Norwegian.
12. Kreuger MR, Grootjans S, Biavatti MW, Vandenabeele P, D’Herde K. Sesquiterpene lactones as drugs with multiple targets in cancer treatment: focus on parthenolide. Anticancer Drugs. 2012;23(9):883–896. doi:10.1097/CAD.0b013e328356cad9
13. Wyrebska A, Gach K, Janecka A. Combined effect of parthenolide and various anti-cancer drugs or anticancer candidate substances on malignant cells in vitro and in vivo. Mini Rev Med Chem. 2014;14(3):222–228. doi:10.2174/1389557514666140219113509
14. Sztiller-Sikorska M, Czyz M. Parthenolide as cooperating agent for anti-cancer treatment of various malignancies. Pharmaceuticals. 2020;13(8):194. doi:10.3390/ph13080194
15. Pordanjani SM, Hosseinimehr SJ. The role of NF-kB inhibitors in cell response to radiation. Curr Med Chem. 2016;23(34):3951–3963. doi:10.2174/0929867323666160824162718
16. Lesiak K, Koprowska K, Zalesna I, Nejc D, Duchler M, Czyz M. Parthenolide, a sesquiterpene lactone from the medical herb feverfew, shows anticancer activity against human melanoma cells in vitro. Melanoma Res. 2010;20(1):21–34. doi:10.1097/CMR.0b013e328333bbe4
17. Nasim S, Crooks PA. Antileukemic activity of aminoparthenolide analogs. Bioorg Med Chem Lett. 2008;18(14):3870–3873. doi:10.1016/j.bmcl.2008.06.050
18. Ren Y, Kinghorn AD. Development of potential antitumor agents from the scaffolds of plant-derived terpenoid lactones. J Med Chem. 2020;63(24):15410–15448. doi:10.1021/acs.jmedchem.0c01449
19. Guzman ML, Rossi RM, Neelakantan S, et al. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood. 2007;110(13):4427–4435. doi:10.1182/blood-2007-05-090621
20. Wu D, Si M, Xue HY, Wong HL. Nanomedicine applications in the treatment of breast cancer: current state of the art. Int J Nanomedicine. 2017;12:5879–5892. doi:10.2147/IJN.S123437
21. Zhang J, Tang H, Liu Z, Chen B. Effects of major parameters of nanoparticles on their physical and chemical properties and recent application of nanodrug delivery system in targeted chemotherapy. Int J Nanomedicine. 2017;12:8483–8493. doi:10.2147/IJN.S148359
22. Jasinski DL, Li H, Guo P. The effect of size and shape of RNA nanoparticles on biodistribution. Mol Ther. 2018;26(3):784–792. doi:10.1016/j.ymthe.2017.12.018
23. Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011;63(3):131–135. doi:10.1016/j.addr.2010.03.011
24. Li X, Kong L, Yang Q, et al. Parthenolide inhibits ubiquitin-specific peptidase 7 (USP7), Wnt signaling, and colorectal cancer cell growth. J Biol Chem. 2020;295(11):3576–3589. doi:10.1074/jbc.RA119.011396
25. Zhang S, Ju X, Yang Q, et al. USP47 maintains the stemness of colorectal cancer cells and is inhibited by parthenolide. Biochem Biophys Res Commun. 2021;562:21–28. doi:10.1016/j.bbrc.2021.05.017
26. Ai XY, Zhang H, Gao SY, et al. Sesquiterpene binding Gly-Leu-Ser/Lys-”co-adaptation pocket” to inhibit lung cancer cell epithelial-mesenchymal transition. Oncotarget. 2017;8(41):70192–70203. doi:10.18632/oncotarget.19599
27. Li X, Huang R, Li M, et al. Parthenolide inhibits the growth of non-small cell lung cancer by targeting epidermal growth factor receptor. Cancer Cell Int. 2020;20(1):561. doi:10.1186/s12935-020-01658-1
28. Garcia-Pineres AJ, Castro V, Mora G, et al. Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J Biol Chem. 2001;276(43):39713–39720. doi:10.1074/jbc.M101985200
29. Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol. 2001;8(8):759–766. doi:10.1016/S1074-5521(01)00049-7
30. Kong FC, Zhang JQ, Zeng C, et al. Inhibitory effects of parthenolide on the activity of NF-kappaB in multiple myeloma via targeting TRAF6. J Huazhong Univ Sci Technolog Med Sci. 2015;35(3):343–349. doi:10.1007/s11596-015-1435-0
31. Kim SL, Park YR, Lee ST, Kim SW. Parthenolide suppresses hypoxia-inducible factor-1alpha signaling and hypoxia induced epithelial-mesenchymal transition in colorectal cancer. Int J Oncol. 2017;51(6):1809–1820. doi:10.3892/ijo.2017.4166
32. Zhu X, Yuan C, Tian C, et al. The plant sesquiterpene lactone parthenolide inhibits Wnt/beta-catenin signaling by blocking synthesis of the transcriptional regulators TCF4/LEF1. J Biol Chem. 2018;293(14):5335–5344. doi:10.1074/jbc.M117.819300
33. Berdan CA, Ho R, Lehtola HS, et al. Parthenolide covalently targets and inhibits focal adhesion kinase in breast cancer cells. Cell Chem Biol. 2019;26(7):1027–1035 e1022. doi:10.1016/j.chembiol.2019.03.016
34. Liu M, Xiao C, Sun M, Tan M, Hu L, Yu Q. Parthenolide inhibits STAT3 signaling by covalently targeting janus kinases. Molecules. 2018;23(6):1478.
35. Gopal YN, Chanchorn E, Van Dyke MW. Parthenolide promotes the ubiquitination of MDM2 and activates p53 cellular functions. Mol Cancer Ther. 2009;8(3):552–562. doi:10.1158/1535-7163.MCT-08-0661
36. Tavana O, Gu W. Modulation of the p53/MDM2 interplay by HAUSP inhibitors. J Mol Cell Biol. 2017;9(1):45–52. doi:10.1093/jmcb/mjw049
37. Colleran A, Collins PE, O’Carroll C, et al. Deubiquitination of NF-kappaB by Ubiquitin-Specific Protease-7 promotes transcription. Proc Natl Acad Sci U S A. 2013;110(2):618–623. doi:10.1073/pnas.1208446110
38. Zhang S, Lin ZN, Yang CF, Shi X, Ong CN, Shen HM. Suppressed NF-kappaB and sustained JNK activation contribute to the sensitization effect of parthenolide to TNF-alpha-induced apoptosis in human cancer cells. Carcinogenesis. 2004;25(11):2191–2199. doi:10.1093/carcin/bgh234
39. Nakshatri H, Rice SE, Bhat-Nakshatri P. Antitumor agent parthenolide reverses resistance of breast cancer cells to tumor necrosis factor-related apoptosis-inducing ligand through sustained activation of c-Jun N-terminal kinase. Oncogene. 2004;23(44):7330–7344. doi:10.1038/sj.onc.1207995
40. D’Anneo A, Carlisi D, Lauricella M, et al. Parthenolide generates reactive oxygen species and autophagy in MDA-MB231 cells. A soluble parthenolide analogue inhibits tumour growth and metastasis in a xenograft model of breast cancer. Cell Death Dis. 2013;4:e891. doi:10.1038/cddis.2013.415
41. Duan D, Zhang J, Yao J, Liu Y, Fang J. Targeting thioredoxin reductase by parthenolide contributes to inducing apoptosis of hela cells. J Biol Chem. 2016;291(19):10021–10031. doi:10.1074/jbc.M115.700591
42. Pei S, Minhajuddin M, Callahan KP, et al. Targeting aberrant glutathione metabolism to eradicate human acute myelogenous leukemia cells. J Biol Chem. 2013;288(47):33542–33558. doi:10.1074/jbc.M113.511170
43. Yang C, Yang QO, Kong QJ, Yuan W, Ou Yang YP. Parthenolide induces reactive oxygen species-mediated autophagic cell death in human osteosarcoma cells. Cell Physiol Biochem. 2016;40(1–2):146–154. doi:10.1159/000452532
44. Xu Y, Fang F, Miriyala S, et al. KEAP1 is a redox sensitive target that arbitrates the opposing radiosensitive effects of parthenolide in normal and cancer cells. Cancer Res. 2013;73(14):4406–4417. doi:10.1158/0008-5472.CAN-12-4297
45. Liu Z, Liu S, Xie Z, et al. Modulation of DNA methylation by a sesquiterpene lactone parthenolide. J Pharmacol Exp Ther. 2009;329(2):505–514. doi:10.1124/jpet.108.147934
46. Gopal YN, Arora TS, Van Dyke MW. Parthenolide specifically depletes histone deacetylase 1 protein and induces cell death through ataxia telangiectasia mutated. Chem Biol. 2007;14(7):813–823. doi:10.1016/j.chembiol.2007.06.007
47. Nakshatri H, Appaiah HN, Anjanappa M, et al. NF-kappaB-dependent and -independent epigenetic modulation using the novel anti-cancer agent DMAPT. Cell Death Dis. 2015;6:e1608. doi:10.1038/cddis.2014.569
48. Fonrose X, Ausseil F, Soleilhac E, et al. Parthenolide inhibits tubulin carboxypeptidase activity. Cancer Res. 2007;67(7):3371–3378. doi:10.1158/0008-5472.CAN-06-3732
49. Whipple RA, Vitolo MI, Boggs AE, Charpentier MS, Thompson K, Martin SS. Parthenolide and costunolide reduce microtentacles and tumor cell attachment by selectively targeting detyrosinated tubulin independent from NF-kappaB inhibition. Breast Cancer Res. 2013;15(5):R83. doi:10.1186/bcr3477
50. Carlisi D, Lauricella M, D’Anneo A, et al. Parthenolide and its soluble analogues: multitasking compounds with antitumor properties. Biomedicines. 2022;10(2):514. doi:10.3390/biomedicines10020514
51. Marino S, Bishop RT, Carrasco G, Logan JG, Li B, Idris AI. Pharmacological inhibition of NFkappaB reduces prostate cancer related osteoclastogenesis in vitro and osteolysis ex vivo. Calcif Tissue Int. 2019;105(2):193–204. doi:10.1007/s00223-019-00538-9
52. Ge W, Liu Z, Sun Y, et al. Design and synthesis of parthenolide-SAHA hybrids for intervention of drug-resistant acute myeloid leukemia. Bioorg Chem. 2019;87:699–713. doi:10.1016/j.bioorg.2019.03.056
53. Mehri S, Mohammadi S, Nikbakht M, Sahmani M, Zahedpanah M. Osteopontin siRNA does not confer resistance to toxic effects of parthenolide in Jurkat cells. Exp Oncol. 2020;42(3):188–191. doi:10.32471/exp-oncology.2312-8852.vol-42-no-3.15180
54. Ge W, Hao X, Han F, et al. Synthesis and structure-activity relationship studies of parthenolide derivatives as potential anti-triple negative breast cancer agents. Eur J Med Chem. 2019;166:445–469. doi:10.1016/j.ejmech.2019.01.058
55. De Blasio A, Di Fiore R, Pratelli G, et al. A loop involving NRF2, miR-29b-1-5p and AKT, regulates cell fate of MDA-MB-231 triple-negative breast cancer cells. J Cell Physiol. 2020;235(2):629–637. doi:10.1002/jcp.29062
56. Dawood M, Ooko E, Efferth T. Collateral sensitivity of parthenolide via NF-kappaB and HIF-alpha inhibition and epigenetic changes in drug-resistant cancer cell lines. Front Pharmacol. 2019;10:542. doi:10.3389/fphar.2019.00542
57. Ding Y, Li S, Ge W, et al. Design and synthesis of parthenolide and 5-fluorouracil conjugates as potential anticancer agents against drug resistant hepatocellular carcinoma. Eur J Med Chem. 2019;183:111706. doi:10.1016/j.ejmech.2019.111706
58. Sun L, Yuan W, Wen G, et al. Parthenolide inhibits human lung cancer cell growth by modulating the IGF1R/PI3K/Akt signaling pathway. Oncol Rep. 2020;44(3):1184–1193. doi:10.3892/or.2020.7649
59. Luo Q, Wu X, Chang W, et al. ARID1A hypermethylation disrupts transcriptional homeostasis to promote squamous cell carcinoma progression. Cancer Res. 2020;80(3):406–417. doi:10.1158/0008-5472.CAN-18-2446
60. Che ST, Bie L, Li X, Qi H, Yu P, Zuo L. Parthenolide inhibits the proliferation and induces the apoptosis of human uveal melanoma cells. Int J Ophthalmol. 2019;12(10):1531–1538. doi:10.18240/ijo.2019.10.03
61. Tang TK, Chiu SC, Lin CW, Su MJ, Liao MH. Induction of survivin inhibition, G(2)/M cell cycle arrest and autophagic on cell death in human malignant glioblastoma cells. Chin J Physiol. 2015;58(2):95–103. doi:10.4077/CJP.2015.BAC267
62. Gali-Muhtasib H, Hmadi R, Kareh M, Tohme R, Darwiche N. Cell death mechanisms of plant-derived anticancer drugs: beyond apoptosis. Apoptosis. 2015;20(12):1531–1562. doi:10.1007/s10495-015-1169-2
63. Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20(3):175–193. doi:10.1038/s41580-018-0089-8
64. Yi J, Wang L, Wang XY, et al. Suppression of aberrant activation of NF-kappaB pathway in drug-resistant leukemia stem cells contributes to parthenolide-potentiated reversal of drug resistance in leukemia. J Cancer. 2021;12(18):5519–5529. doi:10.7150/jca.52641
65. Kwak SW, Park ES, Lee CS. Parthenolide induces apoptosis by activating the mitochondrial and death receptor pathways and inhibits FAK-mediated cell invasion. Mol Cell Biochem. 2014;385(1–2):133–144. doi:10.1007/s11010-013-1822-4
66. Carlisi D, D’Anneo A, Angileri L, et al. Parthenolide sensitizes hepatocellular carcinoma cells to TRAIL by inducing the expression of death receptors through inhibition of STAT3 activation. J Cell Physiol. 2011;226(6):1632–1641. doi:10.1002/jcp.22494
67. Dai Y, Guzman ML, Chen S, et al. The NF (Nuclear factor)-kappaB inhibitor parthenolide interacts with histone deacetylase inhibitors to induce MKK7/JNK1-dependent apoptosis in human acute myeloid leukaemia cells. Br J Haematol. 2010;151(1):70–83. doi:10.1111/j.1365-2141.2010.08319.x
68. Talib WH, Al Kury LT. Parthenolide inhibits tumor-promoting effects of nicotine in lung cancer by inducing P53 - dependent apoptosis and inhibiting VEGF expression. Biomed Pharmacother. 2018;107:1488–1495. doi:10.1016/j.biopha.2018.08.139
69. Li H, Lu H, Lv M, Wang Q, Sun Y. Parthenolide facilitates apoptosis and reverses drug-resistance of human gastric carcinoma cells by inhibiting the STAT3 signaling pathway. Oncol Lett. 2018;15(3):3572–3579. doi:10.3892/ol.2018.7739
70. Yuan L, Wang Z, Zhang D, Wang J. Metabonomic study of the intervention effects of Parthenolide on anti-thyroid cancer activity. J Chromatogr B Analyt Technol Biomed Life Sci. 2020;1150:122179. doi:10.1016/j.jchromb.2020.122179
71. Provance OK, Geanes ES, Lui AJ, et al. Disrupting interferon-alpha and NF-kappaB crosstalk suppresses IFITM1 expression attenuating triple-negative breast cancer progression. Cancer Lett. 2021;514:12–29. doi:10.1016/j.canlet.2021.05.006
72. Suvannasankha A, Crean CD, Shanmugam R, et al. Antimyeloma effects of a sesquiterpene lactone parthenolide. Clin Cancer Res. 2008;14(6):1814–1822. doi:10.1158/1078-0432.CCR-07-1359
73. Jeyamohan S, Moorthy RK, Kannan MK, Arockiam AJ. Parthenolide induces apoptosis and autophagy through the suppression of PI3K/Akt signaling pathway in cervical cancer. Biotechnol Lett. 2016;38(8):1251–1260. doi:10.1007/s10529-016-2102-7
74. Sun J, Zhang C, Bao YL, et al. Parthenolide-induced apoptosis, autophagy and suppression of proliferation in HepG2 cells. Asian Pac J Cancer Prev. 2014;15(12):4897–4902. doi:10.7314/APJCP.2014.15.12.4897
75. Pozarowski P, Halicka DH, Darzynkiewicz Z. Cell cycle effects and caspase-dependent and independent death of HL-60 and Jurkat cells treated with the inhibitor of NF-kappaB parthenolide. Cell Cycle. 2003;2(4):377–383. doi:10.4161/cc.2.4.420
76. Liu W, Wang X, Sun J, Yang Y, Li W, Song J. Parthenolide suppresses pancreatic cell growth by autophagy-mediated apoptosis. Onco Targets Ther. 2017;10:453–461. doi:10.2147/OTT.S117250
77. Lu C, Wang W, Jia Y, Liu X, Tong Z, Li B. Inhibition of AMPK/autophagy potentiates parthenolide-induced apoptosis in human breast cancer cells. J Cell Biochem. 2014;115(8):1458–1466. doi:10.1002/jcb.24808
78. Liu M, Yang Y, Liu D, Cao Y, Li Y. Parthenolide increases the sensitivity of gastric cancer cells to chemotherapy. J Tradit Chin Med. 2020;40(6):908–916. doi:10.19852/j.cnki.jtcm.2020.06.002
79. Tian B, Xiao Y, Ma J, et al. Parthenolide inhibits angiogenesis in esophageal squamous cell carcinoma through suppression of VEGF. Onco Targets Ther. 2020;13:7447–7458. doi:10.2147/OTT.S256291
80. Liu D, Han Y, Liu L, et al. Parthenolide inhibits the tumor characteristics of renal cell carcinoma. Int J Oncol. 2021;58(1):100–110. doi:10.3892/ijo.2020.5148
81. Zhu SM, Park YR, Seo SY, Kim IH, Lee ST, Kim SW. Parthenolide inhibits transforming growth factor beta1-induced epithelial-mesenchymal transition in colorectal cancer cells. Intest Res. 2019;17(4):527–536. doi:10.5217/ir.2019.00031
82. Iida-Norita R, Kawamura M, Suzuki Y, et al. Vasohibin-2 plays an essential role in metastasis of pancreatic ductal adenocarcinoma. Cancer Sci. 2019;110(7):2296–2308. doi:10.1111/cas.14041
83. Wu SY, Xing F, Sharma S, et al. Nicotine promotes brain metastasis by polarizing microglia and suppressing innate immune function. J Exp Med. 2020;217(8). doi:10.1084/jem.20191131
84. Jin X, Yang Q, Cai N, Zhang Z. A cocktail of betulinic acid, parthenolide, honokiol and ginsenoside Rh2 in liposome systems for lung cancer treatment. Nanomedicine. 2020;15(1):41–54. doi:10.2217/nnm-2018-0479
85. Jin X, Lu X, Zhang Z, Lv H. Indocyanine green-parthenolide thermosensitive liposome combination treatment for triple-negative breast cancer. Int J Nanomedicine. 2020;15:3193–3206. doi:10.2147/IJN.S245289
86. Gao W, Li L, Zhang X, et al. Nanomagnetic liposome-encapsulated parthenolide and indocyanine green for targeting and chemo-photothermal antitumor therapy. Nanomedicine. 2020;15(9):871–890. doi:10.2217/nnm-2019-0038
87. Jin X, Zhou J, Zhang Z, Lv H. The combined administration of parthenolide and ginsenoside CK in long circulation liposomes with targeted tLyp-1 ligand induce mitochondria-mediated lung cancer apoptosis. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S931–S942. doi:10.1080/21691401.2018.1518913
88. Gao W, Wei S, Li Z, et al. Nano magnetic liposomes-encapsulated parthenolide and glucose oxidase for ultra-efficient synergistic antitumor therapy. Nanotechnology. 2020;31(35):355104. doi:10.1088/1361-6528/ab92c8
89. Liu Y, Lu WL, Guo J, et al. A potential target associated with both cancer and cancer stem cells: a combination therapy for eradication of breast cancer using vinorelbine stealthy liposomes plus parthenolide stealthy liposomes. J Control Release. 2008;129(1):18–25. doi:10.1016/j.jconrel.2008.03.022
90. Baranello MP, Bauer L, Benoit DS. Poly(styrene-alt-maleic anhydride)-based diblock copolymer micelles exhibit versatile hydrophobic drug loading, drug-dependent release, and internalization by multidrug resistant ovarian cancer cells. Biomacromolecules. 2014;15(7):2629–2641. doi:10.1021/bm500468d
91. Baranello MP, Bauer L, Jordan CT, Benoit DSW. Micelle delivery of parthenolide to acute myeloid leukemia cells. Cell Mol Bioeng. 2015;8(3):455–470. doi:10.1007/s12195-015-0391-x
92. Ridolfo R, Ede BC, Diamanti P, et al. Biodegradable, drug-loaded nanovectors via direct hydration as a new platform for cancer therapeutics. Small. 2018;14(32):e1703774. doi:10.1002/smll.201703774
93. Deller RC, Diamanti P, Morrison G, et al. Functionalized triblock copolymer vectors for the treatment of acute lymphoblastic leukemia. Mol Pharm. 2017;14(3):722–732. doi:10.1021/acs.molpharmaceut.6b01008
94. Gill KK, Kaddoumi A, Nazzal S. Mixed micelles of PEG(2000)-DSPE and vitamin-E TPGS for concurrent delivery of paclitaxel and parthenolide: enhanced chemosenstization and antitumor efficacy against non-small cell lung cancer (NSCLC) cell lines. Eur j Pharm Sci. 2012;46(1–2):64–71. doi:10.1016/j.ejps.2012.02.010
95. Zong H, Sen S, Zhang G, et al. In vivo targeting of leukemia stem cells by directing parthenolide-loaded nanoparticles to the bone marrow niche. Leukemia. 2016;30(7):1582–1586. doi:10.1038/leu.2015.343
96. Ran D, Zhou J, Chai Z, et al. All-stage precisional glioma targeted therapy enabled by a well-designed D-peptide. Theranostics. 2020;10(9):4073–4087. doi:10.7150/thno.41382
97. Liang P, Wu H, Zhang Z, Jiang S, Lv H. Preparation and characterization of parthenolide nanocrystals for enhancing therapeutic effects of sorafenib against advanced hepatocellular carcinoma. Int J Pharm. 2020;583:119375. doi:10.1016/j.ijpharm.2020.119375
98. Darwish NHE, Sudha T, Godugu K, et al. Novel targeted nano-parthenolide molecule against NF-kB in acute myeloid leukemia. Molecules. 2019;24(11):2103. doi:10.3390/molecules24112103
99. Karmakar A, Xu Y, Mustafa T, et al. Nanodelivery of parthenolide using functionalized nanographene enhances its anticancer activity. Rsc Adv. 2015;5(4):2411–2420. doi:10.1039/C4RA10871J
100. Collnot EM, Baldes C, Schaefer UF, et al. TPGS P-glycoprotein inhibition mechanism: influence on conformational flexibility, intracellular ATP levels, and role of time and site of access. Mol Pharm. 2010;7(3):642–651. doi:10.1021/mp900191s
101. Legras S, Gunthert U, Stauder R, et al. A strong expression of CD44-6v correlates with shorter survival of patients with acute myeloid leukemia. Blood. 1998;91(9):3401–3413. doi:10.1182/blood.V91.9.3401
102. Sun D, Zhou JK, Zhao L, et al. Novel curcumin liposome modified with hyaluronan targeting CD44 plays an anti-leukemic role in acute myeloid leukemia in vitro and in vivo. ACS Appl Mater Interfaces. 2017;9(20):16857–16868. doi:10.1021/acsami.7b02863
103. Wang W, Li M, Zhang Z, et al. Design, synthesis and evaluation of multi-functional tLyP-1-hyaluronic acid-paclitaxel conjugate endowed with broad anticancer scope. Carbohydr Polym. 2017;156:97–107. doi:10.1016/j.carbpol.2016.08.100
104. Viennois E, Xiao B, Ayyadurai S, et al. Micheliolide, a new sesquiterpene lactone that inhibits intestinal inflammation and colitis-associated cancer. Lab Invest. 2014;94(9):950–965. doi:10.1038/labinvest.2014.89
105. Penthala NR, Balasubramaniam M, Dachavaram SS, et al. Antitumor properties of novel sesquiterpene lactone analogs as NFkappaB inhibitors that bind to the IKKbeta ubiquitin-like domain (ULD). Eur J Med Chem. 2021;224:113675. doi:10.1016/j.ejmech.2021.113675
106. Huang H, Park S, Zhang H, et al. Targeting AKT with costunolide suppresses the growth of colorectal cancer cells and induces apoptosis in vitro and in vivo. J Exp Clin Cancer Res. 2021;40(1):114. doi:10.1186/s13046-021-01895-w
107. Zeng B, Cheng Y, Zheng K, et al. Design, synthesis and in vivo anticancer activity of novel parthenolide and micheliolide derivatives as NF-kappaB and STAT3 inhibitors. Bioorg Chem. 2021;111:104973. doi:10.1016/j.bioorg.2021.104973
108. Ma WW, Shi QQ, Ding YH, Long J, Zhang Q, Chen Y. Synthesis of micheliolide derivatives and their activities against AML progenitor cells. Molecules. 2013;18(5):5980–5992. doi:10.3390/molecules18055980
109. Ding Y, Guo H, Ge W, et al. Copper(I) oxide nanoparticles catalyzed click chemistry based synthesis of melampomagnolide B-triazole conjugates and their anti-cancer activities. Eur J Med Chem. 2018;156:216–229. doi:10.1016/j.ejmech.2018.06.058
110. Janganati V, Ponder J, Thakkar S, Jordan CT, Crooks PA. Succinamide derivatives of melampomagnolide B and their anti-cancer activities. Bioorg Med Chem. 2017;25(14):3694–3705. doi:10.1016/j.bmc.2017.05.008
111. Srivastava SK, Abraham A, Bhat B, et al. Synthesis of 13-amino costunolide derivatives as anticancer agents. Bioorg Med Chem Lett. 2006;16(16):4195–4199. doi:10.1016/j.bmcl.2006.05.083
112. Ackun-Farmmer MA, Alwaseem H, Counts M, et al. Nanoparticle-mediated delivery of micheliolide analogs to eliminate leukemic stem cells in the bone marrow. Adv Ther. 2022;5(1). doi:10.1002/adtp.202100100
113. El-Far AH, Godugu K, Salaheldin TA, Darwish NHE, Saddiq AA, Mousa SA. Nanonutraceuticals: anti-cancer activity and improved safety of chemotherapy by costunolide and its nanoformulation against colon and breast cancer. Biomedicines. 2021;9(8):990. doi:10.3390/biomedicines9080990
114. Niu X, Wang X, Niu B, et al. Costunolide loaded in pH-responsive mesoporous silica nanoparticles for increased stability and an enhanced anti-fibrotic effect. Pharmaceuticals. 2021;14(10):951. doi:10.3390/ph14100951
115. Caruthers SD, Wickline SA, Lanza GM. Nanotechnological applications in medicine. Curr Opin Biotechnol. 2007;18(1):26–30. doi:10.1016/j.copbio.2007.01.006
116. Khan H, Ullah H, Martorell M, et al. Flavonoids nanoparticles in cancer: treatment, prevention and clinical prospects. Semin Cancer Biol. 2021;69:200–211. doi:10.1016/j.semcancer.2019.07.023
117. Rahaiee S, Assadpour E, Faridi Esfanjani A, Silva AS, Jafari SM. Application of nano/microencapsulated phenolic compounds against cancer. Adv Colloid Interface Sci. 2020;279:102153. doi:10.1016/j.cis.2020.102153
118. Paul S, Roy D, Pati S, Sa G. The adroitness of andrographolide as a natural weapon against colorectal cancer. Front Pharmacol. 2021;12:731492. doi:10.3389/fphar.2021.731492
119. Schuldt M, Pei J, Harakalova M, et al. Proteomic and functional studies reveal detyrosinated tubulin as treatment target in sarcomere mutation-induced hypertrophic cardiomyopathy. Circ Heart Fail. 2021;14(1):e007022. doi:10.1161/CIRCHEARTFAILURE.120.007022
120. Wang D, Liu S, Xu S. Identification of hub genes, key pathways, and therapeutic agents in Hutchinson-Gilford Progeria syndrome using bioinformatics analysis. Medicine. 2020;99(7):e19022. doi:10.1097/MD.0000000000019022
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.