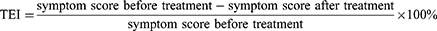Back to Journals » Drug Design, Development and Therapy » Volume 18
The Efficacy of Chaihu-Guizhi-Ganjiang Decoction on Chronic Non-Atrophic Gastritis with Gallbladder Heat and Spleen Cold Syndrome and Its Metabolomic Analysis: An Observational Controlled Before-After Clinical Trial
Authors Wen T, Liu X, Pang T, Li M, Jiao G, Fan X, Tang J, Zhang C, Wang Z, Yue X, Chen W , Zhang F
Received 6 November 2023
Accepted for publication 9 March 2024
Published 21 March 2024 Volume 2024:18 Pages 881—897
DOI https://doi.org/10.2147/DDDT.S446336
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Tao Wen,1,* Xuan Liu,2,* Tao Pang,1,* Mingming Li,1 Guangyang Jiao,3 Xiangcheng Fan,1 Jigui Tang,4 Ci’an Zhang,4 Zhipeng Wang,1 Xiaoqiang Yue,4,* Wansheng Chen,1,3,5 Feng Zhang1,5
1Department of Pharmacy, Changzheng Hospital, Naval Medical University, Shanghai, People’s Republic of China; 2Oncology-Department, Shanghai Guanghua Hospital of Integrative Medicine, Shanghai, People’s Republic of China; 3The SATCM Key Laboratory for New Resources & Quality Evaluation of Chinese Medicine, Institute of Chinese Materia Medica, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 4Department of Traditional Chinese Medicine, Changzheng Hospital, Naval Medical University, Shanghai, People’s Republic of China; 5Shanghai Key Laboratory for Pharmaceutical Metabolite Research, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Feng Zhang; Wansheng Chen, Changzheng Hospital, Naval Medical University, No. 415, Fengyang Road, Shanghai, 200003, People’s Republic of China, Email [email protected]; [email protected]
Purpose: The aim of this study was to verify the effectiveness and explore the mechanism of Chaihu-Guizhi-Ganjiang decoction (CGGD) in the treatment of chronic non-atrophic gastritis (CNAG) with gallbladder heat and spleen cold syndrome (GHSC) by metabolomics based on UHPLC-Q-TOF/MS.
Patients and Methods: An observational controlled before-after study was conducted to verify the effectiveness of CGGD in the treatment of CNAG with GHSC from January to June 2023, enrolling 27 patients, who took CGGD for 28 days. 30 healthy volunteers were enrolled as the controls. The efficacy was evaluated by comparing the traditional Chinese medicine (TCM) syndrome and CNAG scores, and clinical parameters before and after treatment. The plasma levels of hormones related to gastrointestinal function were collected by ELISA. The mechanisms of CGGD in the treatment of CNAG with GHSC were explored using a metabolomic approach based on UHPLC-Q-TOF/MS.
Results: Patients treated with CGGD experienced a statistically significant improvement in TCM syndrome and CNAG scores (p < 0.01). CGGD treatment evoked the concentration alteration of 15 biomarkers, which were enriched in the glycerophospholipid metabolism, and branched-chain amino acids biosynthesis pathways. Moreover, CGGD treatment attenuated the abnormalities of the gastrointestinal hormone levels and significantly increased the pepsinogen level.
Conclusion: It was the first time that this clinical trial presented detailed data on the clinical parameters that demonstrated the effectiveness of CGGD in the treatment of CNAG with GHSC patients. This study also provided supportive evidence that CNAG with GHSC patients were associated with disturbed branched-chain amino acid metabolism and glycerophospholipid levels, suggesting that CNAG treatment based on TCM syndrome scores was reasonable and also provided a potential pharmacological mechanism of action of CGGD.
Keywords: traditional Chinese medicine, chronic gastritis, metabolomics, branched-chain amino acid, glycerophospholipid
Graphical Abstract:
Introduction
Chronic gastritis is a common disorder with a prevalence of approximately 50% worldwide.1 Chronic non-atrophic gastritis (CNAG), a subtype of chronic gastritis, is associated with nonspecific and atypical clinical symptoms such as discomfort, early satiation, epigastric pain, abdominal bloating, and postprandial fullness. It could develop into chronic atrophic gastritis and even gastric cancer, if without any drug invention.2 With the acceleration of the pace of modern life, people’s mental stress, such as anxiety and depression, caused an increase in CNAG incidence. Therefore, early treatment should be considered for CNAG patients.
Nonpharmacological treatment of CNAG includes diet and lifestyle changes, such as avoiding alcohol, coffee, and spicy food, which can alleviate the symptoms of CNAG, especially when it is not serious. Current pharmacological treatment primarily relies on proton pump inhibitors, which are indicated, especially when the symptoms are severe.2,3 Interestingly, traditional Chinese medicine (TCM), which provides personalized medical treatment based on the theory of TCM characterized by the holistic concept and differentiation treatment, has played an important role in CNAG treatment.4,5 According to TCM theory, among the series of symptoms presented in CNAG, epigastric distension and belching is the main manifestation as “gallbladder-heat syndrome”, and anorexia is the main manifestation as “spleen-cold syndrome”. “Gallbladder-heat syndrome and spleen-cold syndrome” are typical and predominant syndrome patterns of CNAG and can coexist and synergistically increase symptom intensity.2,6
Chaihu Guizhi Ganjiang Decoction (CGGD) was first officially recorded in Article 147 of Treatise on Febrile Diseases: Differentiation and Treatment of Pulse Syndrome of Taiyang Disease in 200–210 AD. It consists of Bupleuri Radix, Cinnamomi Ramulus, Zingiberis Rhizoma, Trichosanthis Radix, Scutellariae Radix, Ostreae Concha, and Glycyrrhizae Radix Et Rhizoma Praeparata Cum Melle. Since then, CGGD has been used to treat CNAG patients with gallbladder-heat syndrome and spleen-cold syndrome, showing effectiveness in alleviating the typical common symptoms.7 Therefore, physicians seem to only focus on its clinical practice. However, it is important to gain a better understanding of the basic clinical data for its efficacy, as well as its mechanism of action. Moreover, information regarding gastrointestinal hormone levels, which have been demonstrated to be pharmacological indices related to digestive function and gastrointestinal motility, is also limited.8–10
Metabolomics, as a part of systems biology, is an approach that involves the comprehensive analysis of all metabolites during a specific physiological period. It emphasizes studying the function of organisms from a holistic perspective, aligning with the concept of a “holistic view” of TCM which is characterized by multiple components, multiple pathways, and multiple targets. By investigating the overall changes in the systemic metabolic network, metabolomics provides a novel method and theoretical perspective for deciphering the complex mechanisms and scientific connotations of TCM. Therefore, many scholars have applied metabolomics to study the pharmacodynamic material basis, identify biomarkers of diseases, and explore the mechanisms of action of TCM.11,12 In the past decade, the technical platform of LC-MS-based metabolomics has enjoyed growing popularity due to its high throughput, versatility, and sensitivity in metabolite analysis.13
Therefore, an LC-MS-based metabolomics study was adopted for the investigations of the therapeutic efficacy of CGGD, by using plasma samples from CNAG with GHSC patients. Besides, a potential link was also elucidated between the therapeutic markers by metabolomics analysis with both the traditional TCM syndrome score and the plasma pharmacological index. Additionally, the gastrointestinal hormone levels were also analyzed, providing a supplement for the efficacy evaluation of the CGGD treatment. To our knowledge, this is the first report to evaluate the therapeutic mechanism of CGGD in the treatment of CNAG with GHSC, and to explain its therapeutic efficacy in scientific language on the basis of TCM syndromes. Our study offers a new research strategy for TCM efficacy and provides a scientific reference for the etiology and pathogenesis of gallbladder-heat syndrome and spleen-cold syndrome.
Materials and Methods
Study Design and Human Subjects
Approved by the Biomedical Research Ethics Committee of Shanghai Changzheng Hospital (Ethical Approval No. 2019SL033), the protocol of this study was registered at the Chinese Clinical Trial Registry (ChiCTR2200066224) and conducted in compliance with the Declaration of Helsinki. The period of patient recruitment was from January to June 2023. Written informed consent was signed by all the study participants. The subjects were required to meet the diagnosis of CNAG, which was based on the consensus on chronic gastritis in China by the Chinese Society of Gastroenterology in 2022.14 The representative TCM “gallbladder-heat syndrome and spleen-cold syndrome” was determined according to the consensus on diagnosis and treatment of CNAG by integrated traditional Chinese and Western medicines.15
The inclusion criteria were as follows: 1) between 18 and 75 years old; 2) meeting the diagnostic clinical symptoms; and 3) signing the informed consent. The following are the criteria for exclusion: 1) combined with Helicobacter pylori (Hp) infection; 2) combined with other gastrointestinal diseases; 3) accompanied by other chronic diseases or serious structural diseases (heart diseases, renal failure, etc.); 4) having a mental illness that makes normal doctor-patient communication impossible; and 5) taking other drugs that may affect this study. The withdrawal criteria were as follows: 1) not treated as planned; 2) taking medications other than those designed; and 3) refusing to continue to participate. As a result, a total of 27 CNAG with GHSC patients were eligible and enrolled for participation in this trial.
The flow chart of this clinical study is shown in Figure 1. In addition, the healthy controls were made up of age- and gender-matched healthy volunteers recruited from the Health Examination Center of the Shanghai Changzheng Hospital. Every subject with CNAG took a CGGD prescription twice a day, 102 g at a time, for 28 consecutive days.
 |
Figure 1 Flow chart of the clinical study. |
The clinical efficacy of CGGD before and after administration was based on clinicians’ evaluations. The primary index was the improvement rate of TCM syndrome scores after 28 days of administration, while the secondary index was that of the CNAG scores in Western medicine. Scores for patients’ TCM syndrome and CNAG scores are listed in Tables S1 and S2 (see Additional File).15 The Nimodipine method,16 the currently clinically accepted scoring method, was used to calculate the TCM therapeutic effect index (TEI) according to the following formula.
Preparation of Study Medication
The CGGD used in this study, a light brown granule, was prepared by Guangzhou Yifang Pharmaceutical Co. Ltd. (Foshan, Guangdong, China) (batch number: 1051843). The CGGD (102 g) consisted of Bupleuri Radix (24.00 g), Scutellariae Radix (9.00 g), Cinnamomi Ramulus (9.00 g), Pinelliae Rhizoma Praeparatum Cum Alumine (6.00 g), Zingiberis Rhizoma (6.00 g), Trichosanthis Radix (12.00 g), Ostreae Concha (30.00 g), and Glycyrrhizae Radix Et Rhizoma Praeparata Cum Melle (6.00 g). A total of 107 compounds were identified from the CGGD by the UHPLC-Q-TOF/MS method (Additional File: Figure S1 and Table S3). According to previous reports, the quality of CGGD was assessed using UHPLC-Q-TOF/MS, and four chemical compounds including baicalin, wogonoside, glycyrrhizinic acid, and saikosaponin A were identified as chemical markers for quality monitoring (Figure 2).17
Plasma Sample Collection
Preprandial venous blood (2 mL) was taken from healthy controls and patients in the morning at baseline and after 28 days of CGGD treatment, respectively. Then it was placed in ethylenediaminetetraacetic acid (EDTA) test tubes and centrifuged at 3000 rpm for 15 min at 4°C. Plasma samples were stored at −80°C in the biological sample bank of Shanghai Changzheng Hospital prior to analysis.
Clinical Index Detection
Plasma levels of secretin, cholecystokinin, pepsinogen, motilin, ghrelin, and gastrin were measured by human enzyme-linked immunosorbent assay (ELISA) kits (Hengyuan Biological Technology, Shanghai, China). All operations were carried out in accordance with the protocols of the kits.
Plasma Sample Preparation
Frozen plasma samples stored at −80°C were thawed at 4°C before analysis. All operations were carried out on ice. 100 μL of plasma sample was transferred to a 1.5 mL Eppendorf (EP) tube, and then 400 μL of cold methanol was added. The mixture was vortexed for 2 minutes and centrifuged at 14,000 rpm for 15 minutes at 4°C. Then, 200 μL of each supernatant was transferred into a new EP tube and lyophilized under vacuum. Dried samples were redissolved with a mixture of methanol/water (20:80 v/v, 100 μL), vortexed for 2 minutes, and centrifuged at 14,000 rpm for 15 minutes at 4°C, and finally each supernatant was prepared for MS analysis.
Plasma Metabolomics Analysis
LC-MS-based metabolomics analysis data were acquired on an ultra-high performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometer (UHPLC-Q-TOF/MS, purchased from Agilent, USA, including 1290 Infinity UHPLC and 6530 Q-TOF/MS system). An ACQUITY Premier Peptide HSS T3 Column (1.8 μm, 2.1×100 mm; Waters, USA) was used with the temperature maintained at 50°C. The injection volume for each sample was 5 µL and the flow rate was 0.35 mL/min. Mobile phase A was 0.1% formic acid in water, while mobile phase B was acetonitrile with 0.1% formic acid. The gradient elution program was set as follows: 0–1 min, 1% B; 1–5 min, 5% B; 5–15 min, 95% B; 15–17 min, 95% B. Mass spectrometry data were collected in positive and negative ion mode with an electrospray ion source (ESI). The data acquisition ranged from m/z 100 to 1700. The ion source temperature was set at 350°C, capillary voltage at 3.5 kV (ESI +) and 4.0 kV (ESI-), nebulizer gas pressure 45 Psi, dry gas flow rate at 11 L/min, sheath gas flow rate at 11 L/min, sheath gas temperature at 350°C, and fragment voltage 140 V.
5 μL from each sample in this study was pooled to make a quality control sample (QC), with one QC sample added repeatedly after every 10 runs. QC samples were used to assess instrument stability and data quality throughout the study.
Data Processing and Statistical Analysis
The raw data of UHPLC-Q-TOF/MS were converted into an abf format file by Analysis Base File Converter software, and then the converted data were processed by MS-DIAL version 4.70 (Yokohama City, Kanagawa, Japan) software. This software looks for components with repeatable differences in multiple sample groups and then tabulates the resulting data matrix, including retention time (RT), mass-to-charge ratio (m/z), normalized peak intensity, etc. The variables with a relative standard deviation (RSD) greater than 20% in QC samples and not meeting the 80% rule were filtered out. Subsequently, the remaining normalized data were imported into Metaboanalyst 5.0 (https://www.metaboanalyst.ca/) for multivariate statistical analysis, including T-test, fold change (FC), principal component analysis (PCA), and orthogonal partial least squares discriminant analysis (OPLS-DA), which facilitated the identification of differential metabolites.
Potential biomarkers were screened according to variable importance projection (VIP) ≥1.5, p values <0.05, and FC > 1.5. Based on the accurate mass and MS/MS spectrum information of the features, the identification of the compounds was assisted by combining a self-built library of standards, MS-FINDER version 3.52 (Yokohama City, Kanagawa, Japan), Human Metabolome Database (HMDB, www.hmdb.ca), Kyoto Encyclopedia of Genes and Genomes (KEGG, https://www.kegg.jp), PubChem (https://pubchem.ncbi.nlm.nih.gov/), Massbank (http://www.massbank.jp/) and Competitive Fragmentation Modeling for Metabolite Identification (CFM-ID, https://cfmid.wishartlab.com/). Then, Metaboanalyst 5.0 was utilized for pathway enrichment analysis and visualization of the biomarkers.
SPSS 26.0 (Chicago, IL, USA) was used for statistical analysis. All data were tested for normality before analysis. Data with a normal distribution were statistically analyzed by t-test and described as the mean ± standard deviation. Otherwise, the Wilcoxon nonparametric test was used for statistical analysis and data were described as the median (third quartile - first quartile). All statistical tests were two-sided, and p value less than 0.05 was considered statistically significant. JASP 0.16.3 (Amsterdam, The Netherlands) was used for correlation analysis.
Results
Clinical Characteristics of Patients
The clinical characteristics of the CNAG with GHSC patients before and after CGGD treatment and healthy controls are described in Table 1. A total of 27 patients had a mean age of 51.2 (ranging from 24 to 72) years and a BMI of 22.1 (ranging from 17.6 to 28.1), which were not significantly different from those of the healthy control group.
 |
Table 1 Demographic Details and Clinical Index of Participants |
CGGD Ameliorated the Primary and Secondary Indexes of Patients
The average scores of the TCM syndrome (primary index) and CNAG scores (secondary index) at baseline were 14.1 ± 3.3 and 20.8 ± 3.8, respectively, which represented a moderate severity of CNAG. After 28 days of CGGD treatment, a significant decrease in both index scores of patients was shown compared with pretreatment (all p < 0.01) (Additional File: Table S4 and Figure 3). The mean CNAG score of these patients decreased (positive change) by 8.7 points (TEI: 65.5% ± 18.1%) and the mean TCM syndrome scores decreased by 15.0 points (TEI: 70.9% ± 15.8%). In particular, some clinical symptoms nearly disappeared, such as dry throat and bitter taste in the mouth (TEI: 78.2% ± 28.2%), anorexia (TEI: 70.1% ± 41.6%), hypodynamia (TEI: 79.9% ± 30.0%) and belching (TEI: 77.1% ± 27.6%). The gastroscopy results showed that there was an improvement in the condition of the stomach after treatment with CGGD. An example is shown in Figure S2 and Additional File). All results proved that CGGD has an obvious therapeutic effect on CNAG with GHSC.
CGGD Ameliorated the Pharmacological Index of Patients
Six hormones related to gastrointestinal function (pharmacological index) were measured in healthy controls and patients before and after CGGD treatment (Table 1). For the patients, the pepsinogen level at baseline (127.07 ± 27.15 μg/L) was significantly elevated by 15.49% (p = 0.009) compared to the level at the 28-day point (146.75 ± 26.21 μg/L), close to that of the healthy controls (148.95 ± 32.01 μg/L). In addition, cholecystokinin and gastrin showed an increasing trend. In contrast, ghrelin, motilin, and secretin decreased after CGGD intervention, but without any significance. Specifically, after 28 days of CGGD treatment, secretin showed a significant decrease (199.44 ± 32.70 ng/L) (p = 0.005) compared to the healthy control (231.57 ± 47.36 ng/L).
Plasma Metabolic Patterns Differed Among Healthy Controls and Patients Before and After Treatment of CGGD
The typical total ion chromatograms (TIC) of UHPLC-Q-TOF/MS from plasma samples in positive and negative ion modes are shown in Figure S3. After data filtering, 6574 and 1672 feature ions were extracted from positive and negative ion modes, respectively. A PCA model was obtained to clearly visualize the overall differences in metabolite profiles among the three groups (Figure 4a). The QC samples were tightly clustered, indicating the stability of this method. A clear distinction among the three groups was also observed in the score plot, suggesting that there were different metabolic patterns among them. Specifically, a clear distinction between the patient and healthy control groups on the PCA score confirmed the perturbation of plasma metabolic profiles caused by CNAG with GHSC. After the intervention of CGGD, the patient group tended to shift towards the healthy control group, indicating that CGGD treatment could reverse the levels of the perturbation induced by the disease.
Metabolite Changes in CNAG Patients with Gallbladder-Heat Syndrome and Spleen-Cold Syndrome After CGGD Invention
OPLS-DA was conducted to further find the differential metabolites (Figure 4b and c). A 100-repeated permutation was completed to verify the reliability of the model. The OPLS-DA model presented with R2Y = 0.983, Q2 = 0.924 between patients and healthy controls, while it showed R2Y = 0.725, Q2 = 0.403 between patients before and after CGGD treatment. All p values of the permutations were lower than 0.01 (Figure 4d and e), indicating the model was statistically validated.
Subsequently, 81 metabolites were identified for patients before and after treatment with CGGD, including lipids, amino acids, steroids, and so on (Table S5). Among them, 15 metabolites were significantly altered in patients compared to healthy controls (Table 2), such as L-valine, L-leucine, indole, 5-hydroxyindole acetic acid, and phosphatidylcholine 18:1. After 28 consecutive days of CGGD treatment, the decrease in those metabolites was reversed (Figure 5).
 |
Table 2 Key Differential Metabolites Detected by UHPLC-Q-TOF/MS of Plasma Samples from CNAG Patients and Healthy Controls |
Effects of CGGD on CNAG with GHSC-Induced Metabolic Dysfunction and the Association Analysis of Metabolites and Clinical Parameters
Pathway analysis by Metaboanalyst 5.0 identified two top pathways (all p < 0.05) related to CGGD treatment: glycerophospholipid metabolism and branched-chain amino acids (BCAA) biosynthesis (Figure 6).
 |
Figure 6 Pathway enrichment analysis. (a) overview of pathways based on the KEGG pathway network (b) valine, leucine, and isoleucine biosynthesis (c) glycerophospholipid metabolism. |
Spearman correlation analysis was performed to explore the correlation between those metabolites and clinical parameters (Figure 7a). Specifically, ghrelin showed a positive correlation with indole. At the same time, valine, choline, and lysophosphatidylcholine showed a significant correlation with other metabolites (all p < 0.05, R2 > 0.7) (Figure 7b). This could be one of the underlying mechanisms of the therapeutic effects of CGGD.
Discussion
With the development of modern research in TCM, metabolomics is increasingly being applied to the study of syndrome differentiation in TCM, such as spleen-deficiency and qi-stagnation.10,18 However, there have been no reports on the mechanism research or related metabolomics studies regarding the pathogenesis of gallbladder heat and spleen cold syndrome, which is one of the most common clinical syndromes. Furthermore, Chaihu Guizhi Ganjiang Decoction, as a classic formula for treating digestive system disorders, also lacks any relevant metabolomics reports regarding its therapeutic effects on gastric diseases. Here, for the first time, we utilized metabolomics to study the mechanism of CGGD in treating CNAG with GHSC syndrome. Due to the varied properties of compounds, certain substances such as lipids and bile acids are more readily detected in positive mode, while others such as organic acids and phenylpropanoids are more easily detected in negative mode. To ensure comprehensive results, we conducted detections in both positive and negative modes.
TCM treatment evaluation is often based on a 28-day treatment cycle or a 56-day treatment cycle.19,20 Therefore, in this study, a 28-day treatment cycle was applied for CNAG with GHSC treatment, according to the clinical experience in our hospital. The quantitative rating scale (TEI) was utilized for the efficacy evaluation, which was commonly accepted in clinical studies.21 To our satisfaction, statistically significant differences were observed between the medication groups for the main outcome measures (both TCM syndrome and CNAG scores) at the 28-day point, suggesting real benefits from the CGGD treatment. Additionally, pharmacological parameters such as gastrointestinal hormones were first evaluated before and after CGGD treatment, with pepsinogen and secretin showing significant changes. As a promising biomarker for predicting gastric mucosal status, pepsinogen could decrease when the area of gastric fundic gland mucosa decreases.22 This was consistent with our results, confirming the protective effect of CGGD on the gastric mucosa.
A total of 107 compounds were characterized in CGGD, including flavonoids, triterpene saponins, phenols, and so on. Among these ingredients, many have been reported to have significant anti-inflammatory effects. Saikosaponin A (SSA), the main active component in Bupleuri Radix, effectively suppressed the expression of inducible nitric-oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in RAW264.7 cells stimulated with lipopolysaccharide (LPS), ultimately leading to the reduction of nitric oxide (NO) and prostaglandin E (PGE).23 In mice with LPS-induced acute lung injury, SSA also inhibited the expression of TNF-α and IL-1β.24 The inhibition of the NF-κB signaling pathway is believed to be the primary mechanism by which SSA exerts its anti-inflammatory effects.23,25 In addition to SSA, the entire class of saikosaponins is considered to possess pharmacological effects such as anti-inflammatory, immunomodulatory, and other activities.26 Baicalin, one of the active components of Scutellariae Radix, has been shown in previous studies to inhibit the activation of the TLR4/NF-κB p65 pathway and inflammation induced by LPS through the suppression of CD14 expression.27 Cai pointed out that baicalin can regulate macrophage polarization and promote inflammation recovery via the RhoA/ROCK pathway.28 According to Ma, baicalin inhibits the proinflammatory cytokines IL-1β, IL-6, MCP-1, and TNF-α, and suppresses the MAPK signaling pathway to alleviate the inflammatory response in diabetic nephropathy.29 Similarly, 8-shogaol present in Zingiberis Rhizoma reduces the levels of TNF-α, IL-1β, IL-6, and IFN-γ, and downregulates the mRNA and protein expression of iNOS and COX-2 in a mouse model of dextran sodium sulfate-induced colitis.30 Furthermore, glycyrrhizinic acid exhibits the same anti-inflammatory effects.31
The LC-MS-based metabolomics study suggested a metabolic account: CGGD showed a specific effect on BCAA biosynthesis and glycerophospholipid metabolism pathways. In detail, the metabolic changes in BCAAs are related to many diseases such as diabetes, heart failure, and cancer,32 as well as the emotional state. In a cross-sectional study of 3175 Iranian adults aged 18–55 years, Glareh found that the dietary intake of BCAAs was negatively correlated with the risk of depression and anxiety.33 In addition, valine was found at a lower level in patients with depression than in healthy people and could be reversed by Xiaoyaosan treatment.34 In addition, an increase in valine by supplementation improved the subhealth state.35 It was reported that the subhealth state mainly resulted in emotional problems such as depression and anxiety and often led to some symptoms of gastrointestinal disturbances and targeting emotion dysregulation might be a promising health promotion tactic among young adults with gastrointestinal symptoms and disorders.36,37 From this perspective, CGGD may improve the mental and emotional state of patients by changing the content of plasma branched-chain amino acids, thus playing an important role in the treatment.
Therefore, one possibility would be that the CGGD intervention changed the plasma BCAA level and then the brain BCAA level. It was reported that BCAAs are transported across the blood-brain barrier (BBB) by the large neutral amino acid transporter 1 (LAT1), which is responsible for the transport of many large neutral amino acids (LNAAs), including BCAAs and aromatic amino acids such as tryptophan and kynurenine.38,39 Competition for LAT1 transporters may cause tryptophan and kynurenine to accumulate in the brain, leading to decreased BACC levels. Tryptophan can be metabolized to serotonin (5-HT) and kynurenine, and the latter pathway accounts for approximately 90% of tryptophan metabolism.40 Kynurenine can be catalyzed by kynurenine-3-monooxygenase (KMO) to generate 3-hydroxy-kynurenine (3-HK), which is further metabolized to quinolinic acid (QUIN). Both 3-HK and QUIN are considered neurotoxic substances, that drive depressive behaviors in patients.41,42 Thus, two metabolic hypotheses were predicted for CGGD treatment: (1) the level of kynurenine transported across the BBB was decreased, and(2) the level of tryptophan in the brain converted into kynurenine was decreased. These factors potentially led to a decrease in neurotoxic substances such as 3-HK and QUIN in the brain, thus improving the mental and emotional state of patients. The improvement of patients’ mental and emotional state could inhibit the overactivated hypothalamic-pituitary-adrenal (HPA) axis and reduce the expression of corticotropin-releasing factor (CRF) and adrenocorticotropic hormone (ACTH), which are considered to be related to the excessive secretion of gastric acid, reduced gastric motility, and decreased gastric mucosal blood flow.36,43,44 To confirm this, we conducted separate tests to measure the levels of CRF and ACTH in the plasma before and after medication by ELISA (Additional File: The plasma content of CRF and ACTH). Consistent with our hypothesis, both CRF and ACTH exhibited a significant decrease after medication (Additional File and Figure S4). At the same time, our results showed that the levels of indole and 5-hydroxyindole acetic acid (5-HIAA) increased after taking CGGD, indicating that tryptophan was more inclined to metabolize to the indole pathway and 5-HT pathway, not kynurenine, after taking CGGD. More specifically, ghrelin was positively correlated with indole in our results. As confirmed in previous studies, ghrelin played an important role in accelerating gastric mucosal healing, alleviating inflammatory pain, and resisting anxiety (Figure 8).45,46
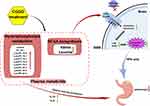 |
Figure 8 Possible therapeutic mechanism of CGGD in CNAG patients. |
Phosphatidylcholine (PC) is the most abundant phospholipid in eukaryotic membranes. Studies have shown that the main components of gastric mucosa are phosphatidylcholine and phosphatidylethanolamine, which form a hydrophobic phospholipid layer on the surface of gastric mucosa to provide a barrier against the harmful environment inside the stomach.47 A double-blind, randomized, placebo-controlled superiority study found that the use of a modified release phosphatidylcholine formula LT-02 in the treatment of ulcerative colitis was effective in reducing patients’ disease activity scores, promoting mucosal healing and improving clinical signs and symptoms, which was proven to be safe and effective for PC therapy.48 In our study, the PC level in the CGGD treatment group was significantly higher than that in the CNAG group, and the increased PC further drove the formation of a hydrophobic phospholipid layer in the gastrointestinal tract to better protect the gastric mucosa. Meanwhile, phosphatidylcholine was also an intermediate product of the mammalian CDP-choline cycle, a precursor of acetylcholine, which would result in the disturbance of the HPA axis. Additionally, phosphatidylcholine and phosphatidylethanolamine can further generate inositol triphosphate (IP3), diacylglycerol (DAG), and phosphatidic acid, the second messengers, thus playing an important role in cell proliferation.49,50 In our results, LPC 20:4 and LPE 20:4 also showed an increasing trend after CGGD invention. LPC 20:4 and LPE 20:4 exerted anti-inflammatory effects by regulating different inflammatory factors.51 The fitted parameters indicated that CGGD might enhance the protective effects of gastric mucosa, because it regulated the level of phospholipids, inhibited the release of pro-inflammatory mediators and upregulated the production of anti-inflammatory mediators (Figure 8).
There are several limitations in the current study. First, this was not a multicenter study. Moreover, a longer follow-up should be conducted to assess whether CGGD could sustain the benefits. Second, a metabolomics study was applied to explore the therapeutic mechanism of CGGD at the level of small molecules; experiments for verification are still missing for why BCAAs and glycerophospholipids accumulate after CGGD treatment, which calls for future investigations.
Conclusion
In this observational controlled before-after study, CGGD showed a significant therapeutic effect on CNAG with GHSC patients, and it could increase the pepsinogen level. The metabolomics study based on UHPLC-Q-TOF/MS found that the therapeutic effect was mainly achieved by increasing plasma BCAA and glycerophospholipid levels. Furthermore, these metabolites have an impact on the patients’ HPA axis, leading to decreased levels of CRF and ACTH. The potential link was also elucidated between the therapeutic markers and both the traditional TCM syndrome score and the serum pharmacological index. This study demonstrated that an LC-MS-based metabolomics study could be successfully applied for mechanism elucidation of TCM, also favoring the etiology and pathogenesis speculations of CNAG.
Abbreviations
ACTH, adrenocorticotropic hormone; BCAA, branched-chain amino acids; BBB, blood-brain barrier; CGGD, Chaihu Guizhi Ganjiang Decoction; CNAG, chronic non-atrophic gastritis; COX-2, cyclooxygenase-2; CRF, corticotropin-releasing factor; DAG, diacylglycerol; ESI, electrospray ion source; FC, fold change; HPA axis, hypothalamic-pituitary-adrenal axis; iNOS, inducible nitric-oxide synthase; IP3, inositol triphosphate; KMO, kynurenine-3-monooxygenase; LAT1, Large Neutral Amino Acid Transporter 1; LNAA, large neutral amino acids; LPS, lipopolysaccharide; m/z, mass-to-charge ratio; NO, nitric oxide; OPLS-DA, orthogonal partial least squares discriminant analysis; PC, phosphatidylcholine; PCA, principal component analysis; PGE, prostaglandin E; QC, quality control; QUIN, quinolinic acid; RSD, relative standard deviation; RT, retention time; SSA, Saikosaponin A; TCM, traditional Chinese medicine; TEI, therapeutic effect index; TIC, total ion chromatograms; UHPLC-Q-TOF/MS, ultra-high performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometer; VIP, variable importance projection; 3-HK, 3-hydroxy-kynurenine; 5-HIAA, 5-hydroxyindole acetic acid; 5-HT, serotonin.
Data Sharing Statement
The data generated in this study are available from the corresponding author upon request.
Ethics Approval and Informed Consent
The protocol of this study was approved by the Biomedical Research Ethics Committee of Shanghai Changzheng Hospital (Ethical Approval No. 2019SL033) and registered at the Chinese Clinical Trial Registry (ChiCTR2200066224). Written informed consent was signed by all the study participants.
Consent for Publication
All data sources and individual person’s datum submitted were accepted for publication.
Acknowledgments
The authors thank all the subjects who participated in this study. The clinical trial was supported by Guangdong Yifang Pharmaceutical Co., LTD., which offered the trial drugs.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This project was supported by the National Key R&D Program of China (2022YFC3501700), Science and Technology Commission of Shanghai Municipality (19401971700, 22S21901900), National Natural Science Foundation of China (82274059) and Deep Blue Project of Naval Medical University: long voyage talent program.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut. 2020;69(12):2113–2121. doi:10.1136/gutjnl-2020-320839
2. Fang JY, Du YQ, Liu WZ, et al. Chinese consensus on chronic gastritis (2017, Shanghai). J Dig Dis. 2018;19(4):182–203. doi:10.1111/1751-2980.12593
3. McCabe ME 4th, Dilly CK. New Causes for the Old Problem of Bile Reflux Gastritis. Clin Gastroenterol Hepatol. 2018;16(9):1389–1392. doi:10.1016/j.cgh.2018.02.034
4. Yang S, Zhang J, Yan Y, et al. Network pharmacology-based strategy to investigate the pharmacologic mechanisms of Atractylodes macrocephala Koidz. for the treatment of chronic gastritis. Front Pharmacol. 2020;10:1629. doi:10.3389/fphar.2019.01629
5. Qin F, Liu JY, Yuan JH. Chaihu-Shugan-San, an oriental herbal preparation, for the treatment of chronic gastritis: a meta-analysis of randomized controlled trials. J Ethnopharmacol. 2013;146(2):433–439. doi:10.1016/j.jep.2013.01.029
6. Liu GP, Yan JJ, Wang YQ, et al. Application of multilabel learning using the relevant feature for each label in chronic gastritis syndrome diagnosis. Evid Based Complement Alternat Med. 2012;2012:135387. doi:10.1155/2012/135387
7. Li YH, Tian FY, Wang CY, Li D, Li HQ. Retrospective analysis of 85 cases of Chaihu Guizhi Ganjiang decoction. J Basic Chin Med. 2022;28(06):944–947.
8. Khoo J, Rayner CK, Feinle-Bisset C, Jones KL, Horowitz M. Gastrointestinal hormonal dysfunction in gastroparesis and functional dyspepsia. Neurogastroenterol Motil. 2010;22(12):1270–1278. doi:10.1111/j.1365-2982.2010.01609.x
9. Ogiso K, Asakawa A, Amitani H, Inui A. Ghrelin: a gut hormonal basis of motility regulation and functional dyspepsia. J Gastroenterol Hepatol. 2011;26(3):67–72. doi:10.1111/j.1440-1746.2011.06630.x
10. Zhang J, Wang X, Shi X, et al. Combination of 15 lipid metabolites and motilin to diagnose spleen-deficiency FD. Chin Med. 2019;14:16. doi:10.1186/s13020-019-0238-9
11. Han Y, Sun H, Zhang A, Yan G, Wang XJ. Chinmedomics, a new strategy for evaluating the therapeutic efficacy of herbal medicines. Pharmacol Ther. 2020;216:107680. doi:10.1016/j.pharmthera.2020.107680
12. Ren JL, Dong H, Han Y, et al. Network pharmacology combined with metabolomics approach to investigate the protective role and detoxification mechanism of Yunnan Baiyao formulation. Phytomedicine. 2020;77:153266. doi:10.1016/j.phymed.2020.153266
13. Yang M, Jiang Z, Wen M, et al. Chemical Variation of Chenpi (Citrus Peels) and corresponding correlated bioactive compounds by LC-MS metabolomics and multibioassay analysis. Front Nutr. 2022;9:825381. doi:10.3389/fnut.2022.825381
14. Chinese Society of Gastroenterology, Cancer Collaboration Group of Chinese Society of Gastroenterology, Chinese Medical Association. Guidelines for diagnosis and treatment of chronic gastritis in China (2022, Shanghai). J Dig Dis. 2023;24(3):150–180. doi:10.1111/1751-2980.13193
15. The Gastroenterology of Chinese Association of Integrative Medicine. Consensus on diagnosis and treatment of chronic non-atrophic gastritis by integrated traditional Chinese and Western medicine (2017). Chin J Integr Tradit West Med Dig. 2018;26(01):1–8.
16. Zhen XY. Guidelines for Clinical Research on Chinese New Herbal Medicines. Beijing: Chinese Medical and Pharmaceutical Science; 2002.
17. Cui L, Li C, Shang Y, et al. Chaihu Guizhi Ganjiang decoction ameliorates pancreatic fibrosis via JNK/mTOR signaling pathway. Front Pharmacol. 2021;12:679557.
18. Zou M, Zhang YS, Feng JK, et al. Serum metabolomics analysis of biomarkers and metabolic pathways in patients with colorectal cancer associated with spleen-deficiency and qi-stagnation syndrome or damp-heat syndrome: a prospective cohort study. Front Oncol. 2023;13:1190706. doi:10.3389/fonc.2023.1190706
19. Chen HF, Gong Y, Huang Z, et al. Efficacy and safety of Chinese herbal medicine Qirui Weishu capsule in treating chronic non-atrophic gastritis: a multicentre, double-blind, randomized controlled clinical trial. J Ethnopharmacol. 2022;294:115341. doi:10.1016/j.jep.2022.115341
20. Ko MM, Jung J, Lee JE, et al. Metabolomic analysis of Gyejibongnyeong-Hwan for shoulder pain: a randomized, wait-list controlled pilot trial. Phytomedicine. 2022;104:154248. doi:10.1016/j.phymed.2022.154248
21. Lu ZH, Yang CL, Yang GG, et al. Efficacy of the combination of modern medicine and traditional Chinese medicine in pulmonary fibrosis arising as a sequelae in convalescent COVID-19 patients: a randomized multicenter trial. Infect Dis Poverty. 2021;10(1):31. doi:10.1186/s40249-021-00813-8
22. Miki K. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels – “ABC method”. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87(7):405–414. doi:10.2183/pjab.87.405
23. Lu CN, Yuan ZG, Zhang XL, et al. Saikosaponin a and its epimer saikosaponin d exhibit anti-inflammatory activity by suppressing activation of NF-κB signaling pathway. Int Immunopharmacol. 2012;14(1):121–126. doi:10.1016/j.intimp.2012.06.010
24. Du ZA, Sun MN, Hu ZS. Saikosaponin a Ameliorates LPS-induced acute lung injury in mice. Inflammation. 2018;41(1):193–198. doi:10.1007/s10753-017-0677-3
25. Liu M, Zhang G, Naqvi S, et al. Cytotoxicity of Saikosaponin A targets HEKa cell through apoptosis induction by ROS accumulation and inflammation suppression via NF-κB pathway. Int Immunopharmacol. 2020;86:106751. doi:10.1016/j.intimp.2020.106751
26. Li X, Li X, Huang N, Liu R, Sun R. A comprehensive review and perspectives on pharmacology and toxicology of saikosaponins. Phytomedicine. 2018;50:73–87. doi:10.1016/j.phymed.2018.09.174
27. Fu YJ, Xu B, Huang SW, et al. Baicalin prevents LPS-induced activation of TLR4/NF-κB p65 pathway and inflammation in mice via inhibiting the expression of CD14. Acta Pharmacol Sin. 2021;42(1):88–96. doi:10.1038/s41401-020-0411-9
28. Cai X, Shi Y, Dai Y, Wang F, Chen X, Li X. Baicalin clears inflammation by enhancing macrophage efferocytosis via inhibition of RhoA/ROCK signaling pathway and regulating macrophage polarization. Int Immunopharmacol. 2022;105:108532. doi:10.1016/j.intimp.2022.108532
29. Ma L, Wu F, Shao Q, Chen G, Xu L, Lu F. Baicalin alleviates oxidative stress and inflammation in diabetic nephropathy via Nrf2 and MAPK signaling pathway. Drug Des Devel Ther. 2021;15:3207–3221. doi:10.2147/DDDT.S319260
30. Kim HR, Noh EM, Kim SY. Anti-inflammatory effect and signaling mechanism of 8-shogaol and 10-shogaol in a dextran sodium sulfate-induced colitis mouse model. Heliyon. 2023;9:1.
31. Richard SA. Exploring the pivotal immunomodulatory and anti-inflammatory potentials of glycyrrhizic and glycyrrhetinic acids. Mediators Inflamm. 2021;2021:6699560. doi:10.1155/2021/6699560
32. Neinast M, Murashige D, Arany Z. Branched Chain Amino Acids. Annu Rev Physiol. 2019;81:139–164. doi:10.1146/annurev-physiol-020518-114455
33. Koochakpoor G, Salari-Moghaddam A, Keshteli AH, Afshar H, Esmaillzadeh A, Adibi P. Dietary intake of branched-chain amino acids in relation to depression, anxiety and psychological distress. Nutr J. 2021;20(1):11. doi:10.1186/s12937-021-00670-z
34. Liu X, Liu C, Tian J, et al. Plasma metabolomics of depressed patients and treatment with Xiaoyaosan based on mass spectrometry technique. J Ethnopharmacol. 2020;246:112219. doi:10.1016/j.jep.2019.112219
35. Dunstan RH, Sparkes DL, Roberts TK, Crompton MJ, Gottfries J, Dascombe BJ. Development of a complex amino acid supplement, Fatigue Reviva™, for oral ingestion: initial evaluations of product concept and impact on symptoms of sub-health in a group of males. Nutr J. 2013;12:115.
36. Nardone G, Compare D. The psyche and gastric functions. Dig Dis. 2014;32(3):206–212. doi:10.1159/000357851
37. Zvolensky M, Jardin C, Farris SG, et al. Gut interpretations: how difficulties in emotion regulation may help explain the relation of visceral sensitivity with depression and anxiety among young adults with gastrointestinal symptoms. Psychol Health Med. 2018;23(7):840–845. doi:10.1080/13548506.2018.1455984
38. Walker AK, Wing EE, Banks WA, Dantzer R. Leucine competes with kynurenine for blood-to-brain transport and prevents lipopolysaccharide-induced depression-like behavior in mice. Mol Psychiatry. 2019;24(10):1523–1532. doi:10.1038/s41380-018-0076-7
39. Novarino G, El-Fishawy P, Kayserili H, et al. Mutations in BCKD-kinase lead to a potentially treatable form of autism with epilepsy. Science. 2012;338(6105):394–397. doi:10.1126/science.1224631
40. Jenkins TA, Nguyen JC, Polglaze KE, Bertrand PP. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. 2016;8(1):56. doi:10.3390/nu8010056
41. Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13(7):465–477. doi:10.1038/nrn3257
42. Kruse JL, Cho JH, Olmstead R, et al. Kynurenine metabolism and inflammation-induced depressed mood: a human experimental study. Psychoneuroendocrinology. 2019;109:104371. doi:10.1016/j.psyneuen.2019.104371
43. Keller J, Gomez R, Williams G, et al. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry. 2017;22(4):527–536. doi:10.1038/mp.2016.120
44. Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. 2019;24(1):18–33. doi:10.1038/s41380-018-0017-5
45. Jaworek J, Konturek SJ. Hormonal protection in acute pancreatitis by ghrelin, leptin and melatonin. World J Gastroenterol. 2014;20(45):16902–16912. doi:10.3748/wjg.v20.i45.16902
46. Bali A, Jaggi AS. An integrative review on role and mechanisms of ghrelin in stress, anxiety and depression. Curr Drug Targets. 2016;17(5):495–507. doi:10.2174/1389450116666150518095650
47. Schmitz MG, Renooij W. Phospholipids from rat, human, and canine gastric mucosa. Composition and metabolism of molecular classes of phosphatidylcholine. Gastroenterology. 1990;99(5):1292–1296. doi:10.1016/0016-5085(90)91152-V
48. Karner M, Kocjan A, Stein J, et al. First multicenter study of modified release phosphatidylcholine ”LT-02” in ulcerative colitis: a randomized, placebo-controlled trial in mesalazine-refractory courses. Am J Gastroenterol. 2014;109(7):1041–1051. doi:10.1038/ajg.2014.104
49. Ridgway ND. The role of phosphatidylcholine and choline metabolites to cell proliferation and survival. Crit Rev Biochem Mol Biol. 2013;48(1):20–38. doi:10.3109/10409238.2012.735643
50. Gibellini F, Smith TK. The Kennedy pathway--De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life. 2010;62(6):414–428. doi:10.1002/iub.337
51. Grzelczyk A, Gendaszewska-Darmach E. Novel bioactive glycerol-based lysophospholipids: new data -- new insight into their function. Biochimie. 2013;95(4):667–679. doi:10.1016/j.biochi.2012.10.009
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.