Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
The Efficacy and Safety of Oral and Topical Spironolactone in Androgenetic Alopecia Treatment: A Systematic Review
Authors Wang C , Du Y, Bi L, Lin X, Zhao M, Fan W
Received 24 November 2022
Accepted for publication 28 February 2023
Published 9 March 2023 Volume 2023:16 Pages 603—612
DOI https://doi.org/10.2147/CCID.S398950
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Chaofan Wang, Yimei Du, Lingbo Bi, Xuewen Lin, Min Zhao, Weixin Fan
Department of Dermatology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, People’s Republic of China
Correspondence: Weixin Fan, Department of Dermatology, The First Affiliated Hospital of Nanjing Medical University, No. 300 Guangzhou Road, Gulou District, Nanjing, Jiangsu Province, People’s Republic of China, Tel +86 13327805737, Email [email protected]
Introduction: Androgenetic alopecia (AGA) has negative impacts on both men and women in terms of appearance and mental stress. Spironolactone is a synthetic aldosterone receptor antagonist known to stimulate hair growth and has been widely used by dermatologists to treat AGA.
Objective: To conduct a systematic review evaluating the efficacy and safety of topical and oral spironolactone in AGA treatment.
Methods: We searched PubMed, Embase, the Cochrane Library, and the Web of Science until October 23rd, 2022, for human studies evaluating the efficacy of spironolactone for the treatment of AGA, regardless of doses and routes.
Results: We retrieved 784 papers and ultimately 7 articles matched our inclusion criteria and comprised 618 AGA patients (65 men, 553 women), 414 of them received spironolactone treatment. Oral spironolactone doses ranged from 25mg to 200mg daily, with the vast majority between 80mg and 110 mg. Dosage forms for topical spironolactone use include gels of 1% and solutions of 5% twice daily. Both oral and topical spironolactone have been shown efficacy for alopecia recovery, but topical use has significantly fewer side effects and is suitable for any gender. It showed better efficacy in combination with other therapies such as oral or topical minoxidil compared with monotherapy.
Conclusion: Spironolactone is an effective and safe treatment of androgenic alopecia which can enhance the efficacy when combined with other conventional treatments such as minoxidil. Topical spironolactone is safer than oral administration and is suitable for both male and female patients, and is expected to become a common drug for those who do not have a good response to minoxidil. Furthermore, more high-quality clinical randomized controlled studies should be performed.
Keywords: androgenetic alopecia, spironolactone, dosage, combine therapy, efficacy, adverse effects
Introduction
Androgenetic alopecia (AGA), also known as male pattern baldness (MPB) or female pattern hair loss (FPHL), is the most common type of progressive hair loss condition. According to statistics, nearly 80% of men and half of women can be affected by AGA during their lifetime,1 and the anxiety of thinning hair may bring a significant negative impact on a patient’s physical and mental health.
AGA is characterized by the miniaturization of hair follicles in bald areas, which may be driven by the conversion of testosterone to dihydrotestosterone (DHT) by 5-α reductase or genetic alterations in the androgen receptor.2 Currently, only minoxidil and finasteride have been approved by Food and Drug Administration (FDA) as pharmacological treatments for AGA.3 Finasteride reduces the conversion of testosterone to DHT by inhibiting the activity of 5α-reductase. Minoxidil is a potassium channel opener, which can stimulate the proliferation of hair follicle epithelial cells and promote hair growth. However, considering the chronic duration of AGA treatment and possible side effects of monotherapy, many other alternative drugs such as dutasteride, prostaglandin analogs, hormonal therapy, and ketoconazole have also been used in treating AGA.4 Treatment methods include oral, topical, percutaneous injection and other ways. In recent years, in addition to traditional oral and external drugs, transdermal administrations including spraying, massaging and microneedling are widely used in skin diseases because of their small side effects, painlessness, and self-administration.5 In AGA, after applying the drug onto the scalp transdermally, it penetrates the stratum corneum, reaches the epidermis and dermis to act on hair follicles, avoiding fluctuations in blood concentration and first-pass hepatic biotransformation caused by systemic medication.5
Spironolactone is a synthetic aldosterone receptor antagonist, it reduces total testosterone levels and blocks the androgenetic receptors in target tissues, which is now often used by dermatologists to diminish the effects of testosterone on skin and hair such as FPHL, acne, and hirsutism, especially in female patients.6 Therefore, we conducted a systematic review to assess the efficacy and safety of spironolactone in the clinical treatment of AGA, and applications of different dosage forms and doses were all included. As far as we know, this is the first systematic review to report both oral and topical spironolactone applications in AGA therapy.
Materials and Methods
Objective and Registration
The objective of this systematic review is to evaluate the efficacy and safety of spironolactone at different doses and dosage forms in the application of AGA treatment. This present systematic review is registered in PROSPERO, an international prospective register of systematic reviews, with the reference code CRD42022373534.
Literature Search
We searched PubMed, Embase, the Cochrane Library, and the Web of Science for relevant studies published up to October 23rd, 2022. The following retrieval terms were combined with Boolean operators (AND, OR, NOT): (Alopecia OR Androgenic Alopecia OR Baldness OR Hair Loss OR Male Pattern Alopecia OR Male Pattern Baldness OR Female Pattern Baldness OR Androgenetic Alopecia OR Pattern Baldness OR Alopecia, Androgenetic OR Pseudopelade) AND (Spironolactone OR Spirolactone OR Veroshpiron OR Verospirone OR Verospiron OR Aquareduct OR Aldactone). Additionally, reference lists of original articles and review articles were searched to avoid missing any other relevant studies.
Inclusion and Exclusion Criteria
Inclusion criteria: (1) Study population: enrolled at least 20 patients, both men and women at any age diagnosed as AGA; (2) Intervention: patients receive spironolactone in any concentration or dosage form, monotherapy or combined with other medications; (3) Comparison: a placebo or any other treatment options for AGA; (4) Outcomes: total hair density, vellus hair density, hair diameter, adverse events, or any other outcomes reported in the included studies; (5) Study type: we included any type of clinical studies such as randomized controlled trials (RCTs), non-randomized comparative study, retrospective study, prospective study and observational study; (6) The publication was in English.
Exclusion criteria: (1) Review, meta-analysis, case report, guideline, animal experiment, and conference abstract; (2) Insufficient posted result or unavailable full text; (3) The study contents or outcome measures were not consistent.
Study Selection
Three review authors screened the abstracts of studies from the initial searches independently and further obtained full-text copies of all relevant and potentially relevant studies that met the specific inclusion criteria. Three authors next independently assessed the full text and decide the eligibility of included studies. All disagreements between these reviewers were discussed, and if no consensus was reached, we consulted the other professional researcher in our team. All irrelevant studies will be excluded and their reasons for exclusion will be reported in the selecting flow diagram following PRISMA statements.7
Risk of Bias
The quality of the included studies was independently assessed by three reviewers using the Cochrane Collaboration’s Risk of Bias Assessment tool8 via the software Review Manager 5.4.1. for RCTs, the Newcastle-Ottawa Scale (NOS)9 for remaining studies. Three authors discussed together to resolve any disagreement on the assessment and to reach a consensus.
Data Extraction
Data were independently collected by three investigators. Disagreements on collected data were settled by consensus or by consulting the other professional researcher. For each included study, “levels of evidence” were assessed according to the “Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence”, and the following data were recorded: first author, year of publication, study design, the number of patients, specific treatment regimen, drug comparison, length of treatment, adverse effects and outcomes.
Results
Study Selection and Characteristics
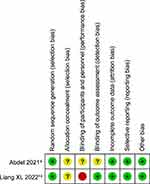
We identified 784 articles through a database search. After removing duplicates and irrelative records, we screened the titles and abstracts of remaining studies and full text if necessary. Ultimately 7 records4−10−15 were enrolled for final analysis (Figure 1). A total of 618 patients (65 men, 553 women) with androgenetic alopecia were included across two RCTs, one non-randomized comparative study, one prospective study, two retrospective studies and one single-arm study, and 414 of them received spironolactone treatment. The complete risk of bias assessment is shown in Figure 2 and Table 1. Table 2 summarizes the characteristics of 7 included studies, whose outcomes can be found in Table 3.
 |
Table 1 Risk of Bias of Non-RCTs Assessed by Newcastle-Ottawa Scale |
 |
Table 2 Characteristics of Included Studies |
 |
Table 3 Outcomes of Included Studies |
 |
Figure 1 Literature Selecting Flow Chart. Notes: Flow chart depicting PRISMA search for studies using spironolactone for the treatment of AGA. |
Gender Difference
All five studies11–15 of oral spironolactone as one of the interventions included only female patients. Two studies4,10 treated with topical spironolactone gels or solutions included a total of 65 men and 115 women, and there was no significant difference in gender between the groups reported.
Efficacy of Intervention
Oral Spironolactone
Five included studies11–15 reported the use of oral spironolactone in 295 female patients. In four of the five records,11–14 with a total of 195 participants, 81% (158/195) of them reported improvement in hair growth by photographic assessment or diverse scores after receiving spironolactone at doses ranging between 25mg and 200mg daily, with the vast majority from 80mg to 110 mg, alone or in combination with other therapies. A prospective study14 concluded that spironolactone 200 mg/day was equally effective in either restoring hair count or preventing progressive hair loss compared with cyproterone acetate at a dose of either 50 mg/day or 100 mg/day for 10 days every menstrual cycle. In a single-arm study,15 100 female patients utilized a once-daily capsule containing spironolactone 25 mg and minoxidil 0.25 mg and followed prospectively for 12 months, as a result, the mean value of the scores representing the severity of hair shedding and hair density decreased gradually at 3, 6, 9, 12 months after the intervention. Most participants received the treatment for up to one year or more, yet the minimum duration of oral spironolactone use in these included studies was 24 weeks. Burns11 and Sinclair15 compared the efficacy of spironolactone duration at 12 and 6 months, and both reported a greater improvement in the former than the latter.
Topical Spironolactone
Two recent trials4,10 using topical spironolactone as AGA treatment were attractive.
The RCT with 60 patients (Male 39, Female 21)4 reported the use of spironolactone gel 1%, and clinical improvement assessed by scalp photography was shown in 80% (16/20) of patients receiving topical spironolactone gel 1% alone for 12 months, while 90% (18/20) with minoxidil gel 5% alone and 100% (20/20) with combination gel of minoxidil and spironolactone. The anagen hair increased, telogen and vellus hairs decreased and the terminal hair/vellus hair (T/V) ratio increased compared with baseline significantly both after 12 months of use of spironolactone gel or mixed gel.
In one non-randomized comparative study,10 spironolactone solution 5% and a combined solution of minoxidil 5% and spironolactone 5% played roles for topical intervention to 40 AGA patients respectively compared with minoxidil solution 5% only. Patients only need to gently massage the scalp after applying 1 mL of the solution twice daily without other treatment. This study had the shortest treatment period, lasting only 12 weeks. Hair shaft diversity and vellus hair decreased while upright regrowing hair increased, and follicular unit with only one emerging hair decreased in patients with mixed solutions, but not in mono-spironolactone users. The quartile grading scale also increased after the intervention.
Combined Therapy
Six studies4,10–13,15 reported spironolactone in combination with other therapies including topical or oral minoxidil, low-level laser light device, iron supplementation and other unspecified agents as AGA treatment for a total of 273 patients. 94.5% (258) of them were deemed effective after undergoing combination therapy. Three studies compared the combination with minoxidil or spironolactone alone, and as a result, spironolactone-containing combination therapy showed more hair improvement than monotherapy, manifested in but not limited to increased hair density and shaft diameter, decreased follicular unit with only one emerging hair, better physician evaluation and patients’ satisfaction.
Adverse Effects
Four studies4,11,13,15 reported the occurrence of adverse effects and were involved in the analysis of safety. The most frequent side effect in patients using topical spironolactone or minoxidil gel was contact dermatitis, mainly presented as pruritus, burning and scaling in 20% of the patients, but no one discontinued treatment. However, the separate side effects are not mentioned specifically, so we cannot be sure whether this adverse effect was caused by minoxidil or spironolactone. But it stated that patients using only spironolactone gel showed minimal and tolerable side effects. No history of headache, dizziness, facial hypertrichosis, gynecomastia, breast tenderness and menstrual irregularity. No side effects on libido and sexual performance have been found. For oral spironolactone, side effects were reported from a total of 216 patients including dizziness/headache 14 (6.5%), menstrual disorder 17 (7.9%), rash 5 (2.3%), nausea 2 (0.9%), increased urination 2 (0.9%), breast tenderness 1 (0.5%), hyperkalemia 2 (0.9%), facial hypertrichosis 9 (4.1%), trichomadesis aggravating 4 (1.9%), scalp pruritus 8 (3.7%), increased scurf 6 (2.7%), edema of the limbs 1 (0.5%), palpitation 3 (1.4%), postural hypotension 2 (0.9%) and other 7 (3.2%). Of these patients, only 7 (3.2%) discontinued treatment due to adverse effects. Furthermore, those with a mean dose of 80–110mg orally daily experienced significantly more side effects than 25mg daily. According to the statistical results, the common side effects in those taking oral spironolactone averaging around 100mg or more per day were menstrual disorder and headache. While in Sinclair’s 201815 study, neither was found in 100 women treated with 25 mg of spironolactone daily.
Discussion
Spironolactone is a potassium-sparing diuretic and structural antagonist of aldosterone whose conventional indications include ascites and peripheral edema associated with portal hypertension and hyperaldosteronism, nephrotic syndrome, primary hyperaldosteronism and hypertension.16 Its antiandrogenic effects were first discovered in treating congestive heart failure, due to some endocrinological adverse effects such as gynecomastia in males and menstrual abnormalities in females after medication.17 Spironolactone can competitively block androgen receptors as well as inhibit ovarian androgen production. It is also a weak inhibitor of androgen synthesis.18 As AGA is generally thought to be caused by excessive production of DHT or alteration of androgen receptors, spironolactone is now becoming the most commonly used, off-label anti-androgen for the treatment of female AGA with 100 to 200 mg as the usual oral daily dose.19 In the seven studies we analyzed, there is no denying that spironolactone was effective in promoting hair regrowth, whether oral or topical. We just displayed five clinical trials using an oral route and two a topical route in this systematic review, yet no study directly comparing the effects of topical and oral spironolactone was found after a detailed literature search. Based on the results of Burns’s11 and Sinclair’s15 research, the efficacy of spironolactone at a 12-month treatment was significantly better than that of 6 months. At present, we have not found clinical trials that directly compare the efficacy of different dose gradients of spironolactone in AGA. In the studies included herein, most doses of oral spironolactone ranged from 80 to 200 mg, and small doses such as 25mg of spironolactone were used with minoxidil only. Notably, significant improvement was expected to show after 12 months of oral use at 80mg to 200mg daily. Six included studies used spironolactone plus minoxidil as one of the interventions, and three of them compared this effect with minoxidil alone, consistently confirming that this combination can potentiate the effect of minoxidil and increase efficacy. Additionally, many studies and cases also reported better efficacy of this combined therapy for AGA. A 53-year-old woman with clinical evidence and histological evidence of AGA was successfully treated with spironolactone 200 mg daily and 5% minoxidil solution with an additive effect on hair regrowth.20 In one case report with six adolescent girls diagnosed with AGA, the combination of oral minoxidil and spironolactone led to objective improvement in 5 of 6 patients.21
No serious adverse effects have been published in the analyzing literature, the most common side effects of oral spironolactone reported as menstrual disorder (7.9%), dizziness or headache (6.5%), facial hypertrichosis (4.1%), rash (2.3%) and hyperkalemia (0.9%), most of them are endurable and self-resolving, few patients (only 3.2%) discontinued spironolactone because of side effects. Two records of topical spironolactone just reported mild contact dermatitis in a small number of patients and no participants terminated treatment for it. Actually, men suffer from AGA in population and performance much higher than women. However, the vast majority of these studies recruited female participants, this may be related to the fact that side effects such as gynecomastia, decreased libido and relative impotence caused by hyperestrogenemia are more pronounced after using oral spironolactone in men,22 which is not mentioned in studies of topical spironolactone. Therefore, it is reasonable to believe that topical spironolactone is a therapy propelled by its minimal side effect profile and ability to be prescribed to almost all patients regardless of age or gender, especially those who are contraindicated or non-responders for minoxidil and other lines of treatment.
There are also several ongoing trials whose objective is to validate the effects of spironolactone on AGA, but whose results have not been published yet, not detailed or incomplete. For example, Ramos and his group are recruiting 60 women with AGA to compare the effect of oral minoxidil and spironolactone versus topical minoxidil for female pattern hair loss in a blind-eye RCT.23 Afsaneh is planning to conduct a randomized comparative study to compare the effect of minoxidil and spironolactone with minoxidil and finasteride in 60 women with AGA.24 Moreover, Pimpakarn has published a protocol of a randomized, double-blind, placebo-controlled RCT to compare the efficacy and safety of oral spironolactone combined with 3% topical minoxidil for the treatment of AGA in 48 premenopausal women.25 A study assessed the efficacy of topical spironolactone (1%, once daily) in 26 AGA patients (16 male/10 female) by dermoscopy, significant increases in total terminal, terminal hair, total hair count after treatment with topical 1% spironolactone were seen, leading to the conclusion that spironolactone is an effective and harmless treatment in both females and males with AGA.26 The specific results of these trials are expected to further confirm the effect of spironolactone on AGA in the near future.
It should also be added that we failed to conduct a meta-analysis due to different reported parameters for outcome of selected studies, which attracted our attention. Thus, we suggest that an objective and practical assessing criteria need to be established to evaluate the severity of AGA for global clinical dermatologists.
Conclusion
Based on the analysis of the above studies, we support the role of spironolactone in treating AGA, and with better results in hair regrowth than monotherapy when combined with other treatments such as minoxidil. Topical spironolactone tends to be a low-risk adjunctive or alternative therapy for both male and female patients. Moreover, it must be stated that traditional therapies as topical minoxidil and oral finasteride still remain dominant and invaluable, but for those who with a negative or unsatisfactory response to them, topical and oral spironolactone may serve as an effective choice. Additionally, more high-quality RCTs and large-scale prospective studies are necessary in order to make comprehensive analyses and better delineate the efficacy and safety of spironolactone for AGA.
Abbreviations
AGA, androgenetic alopecia; MPB, male pattern baldness; FPHL, female pattern hair loss; DHT, dihydrotestosterone; FDA, Food and Drug Administration; RCT, randomized controlled trials; NOS, Newcastle-Ottawa Scale; OCEBM, Oxford Centre for Evidence-Based Medicine; T/V, terminal hair/vellus hair.
Data Sharing Statement
All data analyzed in this systematic review can be searched in publicly available databases including PubMed, Embase, the Cochrane Library, and the Web of Science.
Ethics Approval
This is a review article and ethics approval should have been obtained by the original author.
Acknowledgments
We are thankful to Nanjing Medical University Library for providing a literature search platform and all the reviewers who made contribution to this article.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81972954).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nestor MS, Ablon G, Gade A, Han H, Fischer DL. Treatment options for androgenetic alopecia: efficacy, side effects, compliance, financial considerations, and ethics. J Cosmet Dermatol. 2021;20(12):3759–3781. doi:10.1111/jocd.14537
2. Gupta AK, Mays RR, Dotzert MS, Versteeg SG, Shear NH, Piguet V. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32(12):2112–2125. doi:10.1111/jdv.15081
3. Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77(1):136–141.e135. doi:10.1016/j.jaad.2017.02.054
4. Abdel-Raouf H, Aly UF, Medhat W, Ahmed SS, Abdel-Aziz RTA. A novel topical combination of minoxidil and spironolactone for androgenetic alopecia: clinical, histopathological, and physicochemical study. Dermatol Ther. 2021;34(1):e14678. doi:10.1111/dth.14678
5. Gowda BHJ, Ahmed MG, Sahebkar A, Riadi Y, Shukla R, Kesharwani P. Stimuli-responsive microneedles as a transdermal drug delivery system: a demand-supply strategy. Biomacromolecules. 2022;23(4):1519–1544. doi:10.1021/acs.biomac.1c01691
6. Ioannides D, Lazaridou E. Female pattern hair loss. Curr Probl Dermatol. 2015;47:45–54.
7. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
8. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi:10.1136/bmj.d5928
9. Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10. doi:10.1111/jebm.12141
10. Ammar AM, Elshahid AR, Abdel-Dayem HA, Mohamed AA, Elsaie ML. Dermoscopic evaluation of the efficacy of combination of topical spironolactone 5% and minoxidil 5% solutions in the treatment of androgenetic alopecia: a cross sectional-comparative study. J Cosmet Dermatol. 2022;2022:1.
11. Burns LJ, De Souza B, Flynn E, Hagigeorges D, Senna MM. Spironolactone for treatment of female pattern hair loss. J Am Acad Dermatol. 2020;83(1):276–278. doi:10.1016/j.jaad.2020.03.087
12. Famenini S, Slaught C, Duan L, Goh C. Demographics of women with female pattern hair loss and the effectiveness of spironolactone therapy. J Am Acad Dermatol. 2015;73(4):705–706. doi:10.1016/j.jaad.2015.06.063
13. Liang X, Chang Y, Wu H, et al. Efficacy and safety of 5% minoxidil alone, minoxidil plus oral spironolactone, and minoxidil plus microneedling on female pattern hair loss: a prospective, single-center, parallel-group, evaluator blinded, randomized trial. Front Med. 2022;9:1. doi:10.3389/fmed.2022.905140
14. Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152(3):466–473. doi:10.1111/j.1365-2133.2005.06218.x
15. Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57(1):104–109. doi:10.1111/ijd.13838
16. Carone L, Oxberry SG, Twycross R, Charlesworth S, Mihalyo M, Wilcock A. Spironolactone. J Pain Symptom Manage. 2017;53(2):288–292. doi:10.1016/j.jpainsymman.2016.12.320
17. Searle TN, Al-Niaimi F, Ali FR. Spironolactone in dermatology: uses in acne and beyond. Clin Exp Dermatol. 2020;45(8):986–993. doi:10.1111/ced.14340
18. Sinclair R, Patel M, Dawson TL
19. Fabbrocini G, Cantelli M, Masarà A, Annunziata MC, Marasca C, Cacciapuoti S. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4(4):203–211. doi:10.1016/j.ijwd.2018.05.001
20. Hoedemaker C, van Egmond S, Sinclair R. Treatment of female pattern hair loss with a combination of spironolactone and minoxidil. Australas J Dermatol. 2007;48(1):43–45. doi:10.1111/j.1440-0960.2007.00332.x
21. Olamiju B, Craiglow BG. Combination oral minoxidil and spironolactone for the treatment of androgenetic alopecia in adolescent girls. J Am Acad Dermatol. 2021;84(6):1689–1691. doi:10.1016/j.jaad.2020.10.097
22. Aguilar Medina DA, Cazarín J, Magaña M. Spironolactone in dermatology. Dermatol Ther. 2022;35(5):e15321. doi:10.1111/dth.15321
23. c26hx RBR. Oral minoxidil and spironolactone versus topical minoxidil for female pattern hair loss; 2017. Available from: https://trialsearchwhoint/Trial2aspx?TrialID=RBR-6c26hx.
24. Irct20211210053343N. Comparison of the effect of minoxidil and spironolactone with minoxidil and finasteride in the treatment of Androgenic Alopecia; 2021. Available from: https://trialsearchwhoint/Trial2aspx?TrialID=IRCT20211210053343N1.
25. Tctr. Efficacy and safety of oral spironolactone combined with 3% topical minoxidil for the treatment of female pattern hair loss in premenopausal women: a randomized, double- blind, placebo-controlled study; 2022. Available from: https://trialsearchwhoint/Trial2aspx?TrialID=TCTR20220613005.
26. Soliman AM, Hussein SM, El-Alim SHA, et al. Assessment of the efficacy of topical antiandrogen; spironolactone in patients with androgenetic alopecia by dermoscopy. J Pak Assoc Dermatol. 2022;32(3):493–501.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.