Back to Journals » Therapeutics and Clinical Risk Management » Volume 20
The Efficacy and Safety of Diazepam for Intraoperative Blood Pressure Stabilization in Hypertensive Patients Undergoing Vitrectomy Under Nerve Block Anesthesia: A Prospective, Single-Center, Double-Blind, Randomized, Controlled Trial
Authors Qian T , Gong Q, Shu Y, Shen H, Wu X, Wang W, Zhang Z, Cao H, Xu X
Received 11 October 2023
Accepted for publication 18 December 2023
Published 12 January 2024 Volume 2024:20 Pages 9—18
DOI https://doi.org/10.2147/TCRM.S441152
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Deyun Wang
Tianwei Qian,1– 5,* Qiaoyun Gong,1– 5,* Yiyang Shu,1– 5 Hangqi Shen,1– 5 Xia Wu,1– 5 Weijun Wang,1– 5 Zhihua Zhang,1– 5 Hui Cao,1– 5 Xun Xu1– 5
1Department of Ophthalmology, Shanghai General Hospital, Shanghai Jiao Tong University, Shanghai, People’s Republic of China; 2National Clinical Research Center for Eye Diseases, Shanghai, People’s Republic of China; 3Shanghai Key Laboratory of Ocular Fundus Diseases, Shanghai, People’s Republic of China; 4Shanghai Engineering Center for Visual Science and Photomedicine, Shanghai, People’s Republic of China; 5Shanghai Engineering Center for Precise Diagnosis and Treatment of Eye Disease, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hui Cao; Zhihua Zhang, Tel +86 (0) 13611878111 ; +86 (0) 13817901235, Fax +86 021-63240090, Email [email protected]; [email protected]
Purpose: To evaluate the effectiveness and safety of diazepam in maintaining stable intraoperative blood pressure (BP) in hypertensive patients undergoing vitrectomy under nerve block anesthesia.
Methods: A total of 180 hypertensive patients undergoing vitrectomy with nerve block anesthesia were randomized into two groups. The intervention group was given oral diazepam 60 min before operation, while the control group was given oral placebo 60 min before operation. The primary outcome is the effective rate of intraoperative BP control, defined as systolic blood pressure (SBP) during the operation maintained < 160 mmHg at all timepoints. The logistic regression model will be performed to analyze the compare risk factors for ineffective BP control.
Results: The effective rate of intraoperative SBP control in the diazepam group was significant higher than that in the placebo group from 15 min to 70 min of the surgery (P < 0.05). The proportion of patients with SBP ≥ 180 mmHg at any timepoint from operation to 1 h postoperation was higher in the placebo group (12.22%) than in the diazepam group (2.22%) (P = 0.0096). We observed that the change in SBP from baseline consistently remained higher in the placebo group than in the diazepam group. In the logistic regression analysis, age, years of diagnosed hypertension and SBP 1h before surgery were significant risk factors for ineffective BP control.
Conclusion: This study provides robust evidence supporting the effectiveness of oral diazepam as a pre-surgery intervention in maintaining stable blood pressure during vitrectomy in hypertensive patients.
Trial Registration: Chinese Clinical Trial Registry (ChiCTR), ChiCTR2100041772.
Keywords: nerve block anesthesia, vitrectomy, diazepam, blood pressure stabilization
Introduction
Vitrectomy is a surgical procedure performed on the eye to remove the vitreous humor, a gel-like substance that fills the space between the lens and the retina. This procedure is commonly employed to treat various conditions affecting the vitreous, including retinal detachment,1 macular hole,2 epiretinal membrane,3 diabetic retinopathy,4 and vitreous hemorrhage.5 To ensure the patient’s comfort throughout the procedure, they are typically placed under anesthesia. The surgeon then makes small incisions in the eye and introduces tiny instruments, such as a light source and a vitrectomy probe, to carefully extract the vitreous gel from the eye.
General anesthesia (GA) and local anesthesia (LA) are two commonly used techniques in surgical procedures. GA ensures that the patient is completely unaware and unresponsive during the procedure, effectively eliminating any pain or discomfort. This level of anesthesia allows for precise control over the patient’s airway and breathing, making it suitable for lengthy and invasive surgeries. However, it is important to note that GA affects the entire body, which can lead to potential side effects6 such as nausea, vomiting, sore throat, and postoperative drowsiness. Furthermore, GA generally requires a longer recovery time after the surgery is completed. It is worth noting that GA carries a higher risk of cardiovascular accidents,7 which necessitates careful monitoring and management during the procedure.
On the other hand, LA, while maintaining consciousness, provides localized numbness and pain relief, minimizing the risk of systemic complications in ophthalmic surgeries and promoting faster postoperative recovery. This allows patients to resume their normal daily activities more quickly. In the context of vitrectomy surgery, for instance, the postoperative position is often face-down,8 which contradicts the supine position without a pillow required for general anesthesia. Therefore, when feasible, considering both the overall economic benefits9 and postoperative recovery, prioritizing vitrectomy surgery with LA is recommended.10 It is important to acknowledge that patients who undergo vitrectomy under LA will still experience some level of pain and anxiety throughout the procedure.11 This can potentially lead to fluctuations in blood pressure (BP) and heart rate, thereby increasing the risk of cardiovascular accidents and retinal hemorrhage. As a result, maintaining stable intraoperative blood pressure during vitrectomy is crucial for ensuring patient safety.
In clinical practice, it has been observed that oral diazepam, a commonly used medication for anxiety, can effectively alleviate anxiety and reduce intraoperative blood pressure fluctuations in anxious patients before surgery. Diazepam is widely utilized as a sedative and analgesic medication during surgical procedures,12 owing to its pharmacological properties as a benzodiazepine. It possesses sedative, anxiolytic, muscle relaxant, and anticonvulsant qualities, making it a versatile option for managing anxiety and promoting relaxation in patients before surgery. Importantly, when used as a single dose, diazepam has been reported to have a favorable safety profile with no significant adverse reactions documented.12
To the best of the authors’ knowledge, no randomized controlled trials have been conducted to evaluate the effects of diazepam in vitrectomy. Hence, we conducted a prospective, single-center, double-blind, randomized, controlled trial to examine the efficacy and safety of preoperative oral diazepam on intraoperative BP stabilization in hypertensive patients undergoing vitrectomy under nerve block anesthesia.
Methods
Trial Design and Patients
This prospective, single-center, double-blind, randomized, controlled clinical trial (RCT) is currently being conducted at Shanghai General Hospital, affiliated with Shanghai Jiaotong University. The trial was conducted in accordance with the Declaration of Helsinki and the retrospective review of data was approved by the Institutional Review Board of Shanghai General Hospital, approval number 2020-119. This study aims to investigate the effects of oral diazepam on hypertensive patients undergoing vitreoretinal surgery. Participants were recruited prospectively and consecutively at Shanghai General Hospital from August 2021 to March 2023. Prior to participation, all human subjects were fully informed about their responsibilities and the nature of the procedures involved and provided written informed consent. The trial has been registered with the Chinese Clinical Trial Registry (No. ChiCTR2100041772).
The detailed protocol for the trial, including the inclusion and exclusion criteria, was previously published13 and the protocol followed the Consolidated Standards of Reporting Trials guidelines.14,15 Participants have the right to withdraw their consent and discontinue participation at any time. Following comprehensive ophthalmic and physical examinations, the patients will be randomized into two groups. The intervention group was given oral diazepam 2.5 mg 60 min before vitrectomy. The control group was given oral starch placebo 60 min before vitrectomy. The effects of diazepam last for several hours after administration, so side effects should be closely monitored postoperatively. When reporting this randomised controlled trials, we followed the Consolidated Standards of Reporting Trials (CONSORT) statement.16
Outcomes
During the study, continuous electrocardiogram (ECG) monitoring was conducted throughout the entire procedure. BP measurements will be taken every 5 minutes during the surgery and for 1 hour post-operation in the same ward using consistent equipment. The primary outcome is the effective rate of intraoperative BP control, defined as BP <160 mmHg at every time point during vitrectomy. Additionally, we collected other information to evaluate the effectiveness of diazepam. These include: (1) the proportion of patients with systolic blood pressure (SBP) ≥180 mmHg at any timepoint from operation to 1 h post-operation; (2) the change in mean SBP measured by patients during operation performed from baseline; (3) the change in mean heart rate measured during operation performed from baseline; and (4) the number of patients with intraoperative and postoperative adverse reactions during treatment and within 12 weeks. Moreover, the operation record of each enrolled patient and the duration of the operation will be collected for analysis. Additionally, we conducted a comparison of the clinical characteristics between the effective BP control group and the ineffective BP control group, performing logistic regression analysis to identify risk factors for ineffective BP control. BP monitoring in this study was performed by a single anesthetist, and all vitreoretinal surgeries were performed by the same experienced specialist surgeon team.
Statistical Analysis
Statistical analyses were performed using SPSS 22.0 (IBM Corporation, Chicago, IL) and Prism 8.01 (GraphPad Software, San Diego, CA). Quantitative variables (eg, systolic BP, age) are expressed as the mean ± standard deviation (SD). The Kolmogorov–Smirnov test was used to determine whether quantitative variables fit with normal distribution. The unpaired t-test was performed for normally distributed data, and the Wilcoxon signed-rank test was performed for abnormal distributed data. While the categorical variables (eg, gender) are described as numbers and frequencies, and the differences were assessed using Chi-square test. The logistic regression model was used to compare the outcomes. P < 0.05 (two-sided) was considered statistically significant.
Results
Clinical Characteristics
The baseline and preoperative clinical characteristics of all patients were summarized in Table 1. A total of 180 patients were enrolled in the trial. The patients were divided into two groups: 90 patients (47 males and 43 females) in the diazepam group and 90 patients (41 males and 49 females) in the placebo group. The groups were not significantly different with respect to age, sex, diagnosis, year of diagnosed hypertension or duration of surgery (P > 0.05 for all, Table 1). All enrolled patients undergo surgery between 9am and 11am, and there was no difference in operative time by the surgeon. There was no difference in SBP or diastolic blood pressure (DBP) at admission, before surgery, before nerve block or after nerve block between the two groups (P > 0.05 for all). Moreover, the heart rate at 1 h before surgery was not significantly different between the two groups (P = 0.44). Furthermore, there was no significant difference in all the mean values of SBP, DBP, heart rate, and SpO2 between the two groups at each measurement point.
 |
Table 1 Baseline and Preoperative Clinical Characteristics of All Patients |
Primary Outcome
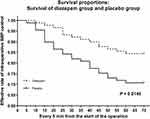
In both groups, the number of newly emerging patients with SBP≥160 mmHg at 0–50 min in the placebo group was greater than that in the diazepam group. And the effective BP control has a significant difference between the two groups from 15 min to 70 min (P < 0.05) (Table 2). The Kaplan-Meier survival plots were drawn and the Mantel-Cox test was used to investigate the overall effective BP control rate between diazepam and placebo group (Figure 1). Patients in the diazepam group had significantly higher overall effective rates of BP control during the surgery than patients in the placebo group (P = 0.0146).
 |
Table 2 Primary Outcome: Comparison of Effective Rate of Intraoperative SBP Control Between Diazepam and Placebo Group |
Secondary Outcomes
The proportion of patients with SBP ≥180 mmHg at any timepoint from operation to 1 h post operation was higher in the placebo group (12.22%) than in the diazepam group (2.22%) (P = 0.0096, Table 3).
 |
Table 3 Secondary Outcomes: Comparison of Diazepam and Placebo Group |
We observed that the change in SBP from baseline consistently remained higher in the placebo group than in the diazepam group. However, this difference was statistically significant at three time points: 10 minutes, 15 minutes, and 20 minutes (P = 0.0304, 0.0192, 0.0252, respectively). There were no significant differences in SBP between the two groups at other time intervals (P > 0.05, Table 3).
Safety
We collected data on adverse reactions within 12 weeks of surgery, including corneal edema, subconjunctival hemorrhage, high intraocular pressure, endophthalmitis, abnormal heart rate, and side effects related to the drug. Neither group experienced abnormal heart rates or adverse reactions to the drug (Table 3).
In the diazepam group, we observed 1 (1.11%) case of corneal edema, 5 (5.56%) cases of subconjunctival hemorrhage, and 6 (6.67%) cases of high IOP. In the control group, there were 2 (2.22%) cases of corneal edema, 4 (4.44%) cases of subconjunctival hemorrhage, 5 (5.5%) cases of high intraocular pressure, and 1 (1.11%) case of endophthalmitis. However, there was no statistically significant difference in the occurrence of adverse reactions between the two groups (P > 0.05).
Effective BP Control Group vs Ineffective BP Control Group
The clinical characteristics of the effective BP control group and ineffective BP control group are shown in Table 4. In the effective control group, there were 140 participants, with 67 males and 73 females. Among them, 77 individuals received diazepam, while 63 received a placebo. In the ineffective control group, there were 40 participants, including 21 males and 19 females. Of these, 13 individuals received diazepam, while 27 received a placebo. The effective control group had a lower average age, duration of hypertension, SBP and DBP compared to the ineffective control group (P < 0.05).
 |
Table 4 Comparison of Clinical Characteristics Between the Effective BP Control Group and Ineffective BP Control Group |
Risk Factors for Ineffective BP Control
The summary after logistic regression analysis for every feature was detailed in Table 5. In the logistic regression analysis, age (P = 0.028, odds ratio (OR) = 1.057), years of diagnosed hypertension (P < 0.001, OR = 5.882) and baseline SBP (P < 0.001, OR = 2.912) emerged as risk factors for ineffective BP control.
 |
Table 5 Logistic Regression Analysis of Risk Factors for Ineffective BP Control |
Discussion
The results of this prospective study revealed that preoperative oral diazepam in hypertensive patients has a notable impact on maintaining stable BP levels during surgery. Notably, the diazepam group consistently demonstrated a higher effective rate of intraoperative SBP control than the placebo group from the beginning of the surgical procedure. These findings underscore the importance of utilizing preoperative diazepam as a strategy to effectively regulate and stabilize BP during surgery in hypertensive patients.
The results of the secondary outcomes indicate a significant difference in blood pressure changes between the two groups at 10–20 min. This time period coincides with 70–80 min after oral diazepam, which is when diazepam reaches its effective concentration. This could be a possible explanation for this finding, further supporting the significant effect of diazepam in maintaining blood pressure. This finding suggests that when performing procedures that involve bleeding, it may be beneficial to avoid operating during this time window. By doing so, it is possible to potentially reduce the risk of bleeding to some extent. Further analysis is needed to understand the underlying factors contributing to the observed differences and their clinical significance.
Age, baseline BP, and years of diagnosed hypertension all influence the effectiveness of intraoperative blood pressure control and should be taken into comprehensive consideration. Therefore, a holistic approach to patient evaluation and treatment is crucial to optimize blood pressure control and overall patient outcomes.
In clinical applications, we found that oral diazepam before surgery can significantly relieve anxiety in patients.17 Anxiety can cause blood pressure fluctuations during surgery and increase the possibility of cardiovascular accidents. We hypothesized that preoperative oral diazepam was effective and safe in maintaining stable blood pressure during surgery. We hope our results can provide reliable evidence to guide patients with hypertension to control their blood pressure with diazepam before surgery.
Diazepam, also known as Valium,18 is a medication belonging to the benzodiazepine class. Benzodiazepines are a type of central nervous system depressant that can inhibit different parts of the central nervous system.19 They produce various clinical effects depending on the dose, ranging from mild sedation to hypnosis and even coma.20 Although the exact sites and mechanisms of action for benzodiazepines are not fully understood, it is believed that they enhance or facilitate the function of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) and its receptors.21 GABA primarily exerts inhibitory effects at the pre- and postsynaptic levels in different regions of the central nervous system. GABA receptors are functional supramolecular units also referred to as benzodiazepine-GABA receptor-chloride ion complex components. These receptor complexes are found in the neuronal cell membrane and regulate cell firing by functioning as gating mechanisms for chloride channels. When GABAA receptors are activated, chloride channels open, allowing chloride ions to flow across the neuronal cell membrane.22 This causes hyperpolarization of postsynaptic neurons, inhibiting their firing and resulting in a decrease in neuronal excitability and a reduction in the release of excitatory neurotransmitters. Benzodiazepines such as diazepam increase the frequency of chloride channel openings, possibly by enhancing the binding of GABA to its receptors or facilitating the interaction between GABA receptors and chloride ion channels. It has specific effects, including anxiolytic,23 sedative,24 and hypnotic effects,25 as well as amnesic, anticonvulsant,26 and skeletal muscle relaxant effects.20 In our study, the focus was on the anti-anxiety, sedative, and hypnotic effects of diazepam.
When diazepam tablets are taken orally, they are rapidly and completely absorbed, with a bioavailability of approximately 76%.27 Peak plasma concentrations are achieved within 0.5 to 2 hours,28 and steady-state blood concentrations are generally reached between 4 and 10 days.29 The half-life of diazepam ranges from 36.62–334.69 hours.30 Diazepam exhibits high plasma protein binding, with a rate of up to 99%. It is highly lipophilic, allowing it to easily cross the blood‒brain barrier. The majority of diazepam metabolism occurs in the liver, resulting in the formation of metabolites such as nordiazepam and oxazepam, which also possess varying degrees of pharmacological activity. The half-life of nordiazepam can range from 57.81–516.32 hours.30 Diazepam undergoes enterohepatic circulation, and long-term use of the medication can lead to accumulation. Metabolites can remain in the blood for several days or even weeks and are eliminated slowly after discontinuation. The primary route of excretion for diazepam and its metabolites is through the kidneys, either in the form of free or conjugated metabolites.
However, this study has some limitations. BP fluctuates throughout the day, and there may be rhythmic variations between morning and afternoon blood pressure readings. Not all surgeries occur at the same time of day. Moreover, patients are in a state of wakefulness during surgery, and environmental factors such as sounds in the operating room and interactions with doctors may affect their emotions, potentially influencing BP levels. Our study specifically targeted hypertensive patients and did not include individuals who do not have hypertension but experience extreme anxiety. To improve the generalizability of our research findings, it would be advantageous to incorporate this group in future studies. By including individuals without hypertension but with significant anxiety, we can gain a more comprehensive understanding of the effects of preoperative oral diazepam and its implications for maintaining stable blood pressure during surgery.
Conclusions
In summary, this study provides robust evidence supporting the effectiveness of oral diazepam as a pre-surgery intervention in maintaining stable blood pressure during vitrectomy in hypertensive patients. Our research findings offer valuable insights for enhancing preoperative care for hypertensive patients. Specifically, for patients with long-term hypertension, oral diazepam proves to be highly effective in reducing anxiety levels and enhancing overall surgical safety.
Abbreviations
RRD, Rhegmatogenous retinal detachment; ERM, epiretinal membrane; MH, Macular hole; BP, Blood pressure; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; CI, confidence interval; OR, odds ratio; M, Mean; SD, Standard deviation.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from corresponding authors on reasonable request.
Statement of Ethics
This study was conducted in accordance with the Declaration of Helsinki and the retrospective review of data was approved by the Institutional Review Board of Shanghai General Hospital, approval number 2020-119. Written informed consent was obtained from each participant before undergoing surgery.
Acknowledgments
This study was supported by members of the Department of Ophthalmology in Shanghai General Hospital.
Author Contributions
All authors made contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Special Project for Clinical Research in Health Industry of Shanghai Health Commission (No. 20214Y0045), the Clinical Research Innovation Plan of Shanghai General Hospital (No. CTCCR-2021C02) and the Three-year Action Plan for the Construction of Shanghai’s Public Health System (GWVI-11.2-YQ25).
Disclosure
The authors declare no competing interests.
References
1. Nagpal M, Chaudhary P, Wachasundar S, Eltayib A, Raihan A. Management of recurrent rhegmatogenous retinal detachment. Indian J Ophthalmol. 2018;66(12):1763–1771. doi:10.4103/ijo.IJO_1212_18
2. Stappler T, Montesel A, Konstantinidis L, Wolfensberger TJ, Eandi CM. Inverted Internal Limiting Membrane Flap Technique for Macular Hole Coexistent with Rhegmatogenous Retinal Detachment. Retina. 2022;42(8):1491–1497. doi:10.1097/IAE.0000000000003509
3. Chua PY, Sandinha MT, Steel DH. Idiopathic epiretinal membrane: progression and timing of surgery. Eye. 2022;36(3):495–503. doi:10.1038/s41433-021-01681-0
4. Berrocal MH, Acaba-Berrocal L. Early pars plana vitrectomy for proliferative diabetic retinopathy: update and review of current literature. Curr Opin Ophthalmol. 2021;32(3):203–208. doi:10.1097/ICU.0000000000000760
5. Wakabayashi T, Patel N, Bough M, et al. VITRECTOMY FOR VITREOUS HEMORRHAGE ASSOCIATED WITH RETINAL VEIN OCCLUSION: visual Outcomes, Prognostic Factors, and Sequelae. Retina. 2023;43(9):1506–1513. doi:10.1097/IAE.0000000000003839
6. Wilson RP. Complications associated with local and general ophthalmic anesthesia. Int Ophthalmol Clin. 1992;32(4):1–22. doi:10.1097/00004397-199223000-00001
7. Breen P, Park KW. General anesthesia versus regional anesthesia. Int Anesthesiol Clin. 2002;40(1):61–71. doi:10.1097/00004311-200201000-00006
8. Seno Y, Shimada Y, Mizuguchi T, Tanikawa A, Horiguchi M. Compliance with the Face-down Positioning after Vitrectomy and Gas Tamponade for Rhegmatogenous Retinal Detachments. Retina. 2015;35(7):1436–1440. doi:10.1097/IAE.0000000000000479
9. Kumar CM, Seet E, Eke T, Irwin MG, Joshi GP. Peri-operative considerations for sedation-analgesia during cataract surgery: a narrative review. Anaesthesia. 2019;74(12):1601–1610. doi:10.1111/anae.14845
10. Xiang Y, Ye W, Sun N, Jin X. Analgesic and Sedative Effects of Dezocine and Midazolam During Vitrectomy. Curr Eye Res. 2016;41(11):1460–1464. doi:10.3109/02713683.2015.1128551
11. Lai MM, Lai JC, Lee WH, et al. Comparison of retrobulbar and sub-Tenon’s capsule injection of local anesthetic in vitreoretinal surgery. Ophthalmology. 2005;112(4):574–579. doi:10.1016/j.ophtha.2004.10.043
12. Lin IP, Chang PC. Physiological reaction of anxious patients taking sedative medications before and after periodontal surgery. J Dent Sci. 2023;18(1):345–352. doi:10.1016/j.jds.2022.08.031
13. Qian T, Gong Q, Chen C, et al. Preoperative oral diazepam for intraoperative blood pressure stabilisation in hypertensive patients undergoing vitrectomy under retrobulbar nerve block anaesthesia: study protocol for a randomised controlled trial. Trials. 2022;23(1):723. doi:10.1186/s13063-022-06686-y
14. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. doi:10.1016/j.ijsu.2011.10.001
15. Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2011;9(8):672–677. doi:10.1016/j.ijsu.2011.09.004
16. Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18. doi:10.1186/1741-7015-8-18
17. Hock LE, Kennedy S, Wilson CW, et al. Oral anxiolytics prior to routine resident cataract surgery eliminate need for intravenous sedation at a Veterans Affairs Hospital. Am J Ophthalmol Case Rep. 2022;25:101379. doi:10.1016/j.ajoc.2022.101379
18. Murray JB. Diazepam (Valium): its dependency liability. J Psychol. 1990;124(6):655–674. doi:10.1080/00223980.1990.10543259
19. Wick JY. The history of benzodiazepines. Consult Pharm. 2013;28(9):538–548. doi:10.4140/TCP.n.2013.538
20. Olkkola KT, Ahonen J. Midazolam and other benzodiazepines. Handb Exp Pharmacol. 2008;182:335–360.
21. Vutskits L, Gascon E, Tassonyi E, Kiss JZ. Clinically relevant concentrations of propofol but not midazolam alter in vitro dendritic development of isolated gamma-aminobutyric acid-positive interneurons. Anesthesiology. 2005;102(5):970–976. doi:10.1097/00000542-200505000-00016
22. Sivilotti L, Nistri A. GABA receptor mechanisms in the central nervous system. Prog Neurobiol. 1991;36(1):35–92.
23. Baldwin DS. Clinical management of withdrawal from benzodiazepine anxiolytic and hypnotic medications. Addiction. 2022;117(5):1472–1482. doi:10.1111/add.15695
24. Chen SC, Rex DK. Review article: registered nurse-administered propofol sedation for endoscopy. Aliment Pharmacol Ther. 2004;19(2):147–155. doi:10.1111/j.0269-2813.2004.01833.x
25. Mandrioli R, Mercolini L, Raggi MA. Metabolism of benzodiazepine and non-benzodiazepine anxiolytic-hypnotic drugs: an analytical point of view. Curr Drug Metab. 2010;11(9):815–829. doi:10.2174/138920010794328887
26. Kienitz R, Kay L, Beuchat I, et al. Benzodiazepines in the Management of Seizures and Status Epilepticus: a Review of Routes of Delivery, Pharmacokinetics, Efficacy, and Tolerability. CNS Drugs. 2022;36(9):951–975.
27. Dhillon S, Oxley J, Richens A. Bioavailability of diazepam after intravenous, oral and rectal administration in adult epileptic patients. Br J Clin Pharmacol. 1982;13(3):427–432. doi:10.1111/j.1365-2125.1982.tb01397.x
28. Friedman H, Greenblatt DJ, Peters GR, et al. Pharmacokinetics and pharmacodynamics of oral diazepam: effect of dose, plasma concentration, and time. Clin Pharmacol Ther. 1992;52(2):139–150. doi:10.1038/clpt.1992.123
29. Divoll M, Greenblatt DJ, Ochs HR, Shader RI. Absolute bioavailability of oral and intramuscular diazepam: effects of age and sex. Anesth Analg. 1983;62(1):1–8. doi:10.1213/00000539-198301000-00001
30. Wang LL, Ren XX, He Y, et al. Study on the Pharmacokinetics of Diazepam and Its Metabolites in Blood of Chinese People. Eur J Drug Metab Pharmacokinet. 2020;45(4):477–485. doi:10.1007/s13318-020-00614-8
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.