Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
The Efficacy and Safety of Avanafil During a Treatment of Male Erectile Dysfunction: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Authors Warli SM, Steven S, Kadar DD, Prapiska FF, Siregar GP
Received 19 May 2023
Accepted for publication 29 June 2023
Published 18 July 2023 Volume 2023:19 Pages 629—644
DOI https://doi.org/10.2147/TCRM.S419408
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Syah Mirsya Warli,1,2 Steven Steven,3 Dhirajaya Dharma Kadar,1 Fauriski Febrian Prapiska,1 Ginanda Putra Siregar1
1Division of Urology, Department of Surgery, Faculty of Medicine, Universitas Sumatera Utara – Haji Adam Malik General Hospital, Medan, Indonesia; 2Department of Urology, Universitas Sumatera Utara Hospital, Universitas Sumatera Utara, Medan, Indonesia; 3Department of Urology, Faculty of Medicine, Universitas Indonesia - Haji Adam Malik General Hospital, Medan, Indonesia
Correspondence: Syah Mirsya Warli, Division of Urology, Department of Surgery, Faculty of Medicine, Universitas Sumatera Utara, Dr. Mansyur No. 5 Medan, North Sumatera, 20154, Indonesia, Tel +6261-8364930, Email [email protected]
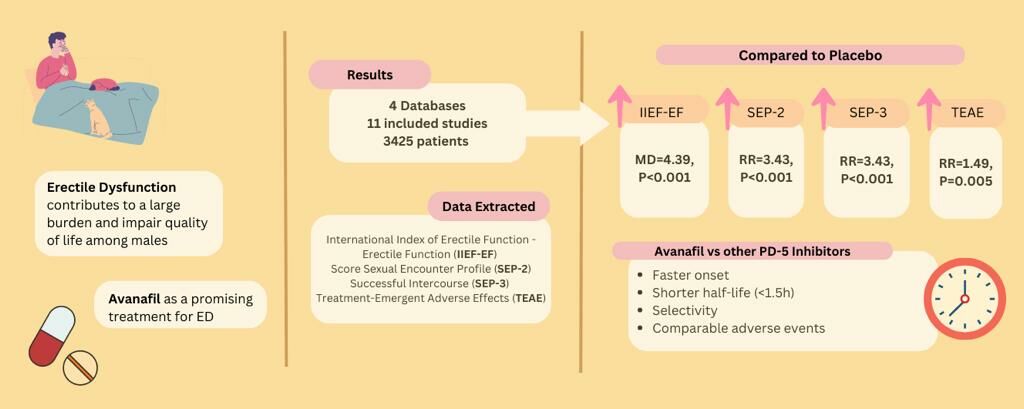
Purpose: Erectile dysfunction (ED) contributes to a large burden and impairs the quality of life among males. Avanafil appears to be a promising treatment for ED; however, its efficacy and safety profile remain unclear. This study aimed to evaluate the efficacy and safety of avanafil for the treatment of ED.
Patients and Methods: An extensive search of PubMed, ScienceDirect, Web of Science, and Embase databases with 11 publications was performed, with outcomes evaluated are International Index of Erectile Function – Erectile Function (IIEF-EF), Sexual Encounter Profile (SEP), and Treatment-Emergent Adverse Events (TEAE). Statistical parameter Mean Difference (MD) and Risk Ratio (RR) with 95% Confidence Interval (CI) were used to measure effect size.
Results: The pooled estimates demonstrated that changes in IIEF-EF function (MD=4.39, 95% CI [3.41, 5.37], p< 0.001), SEP-2 (RR=3.43, 95% CI [2.79, 4.22], p< 0.001), SEP-3 (RR=2.30, 95% CI [2.01, 2.62], p< 0.001), and TEAE (RR=1.49, 95% CI [1.12, 1.96], p=0.005) were significantly higher in the avanafil group than in the placebo group. Moreover, 200 mg avanafil was superior to that mg 100 mg-avanafil, indicated by the IIEF-EF score (MD=− 1.15, 95% CI [− 1.40, − 0.89], p< 0.001). In contrary, there were no significant differences in SEP-2 (RR=0.90, 95% CI [0.75, 1.08], p=0.26), SEP-3 (RR=0.92, 95% CI [0.81, 1.05], p=0.21) and TEAE (RR=1.00, 95% CI [0.87, 1.15], p=0.99) for both 100 mg and 200 mg doses.
Conclusion: This review highlights the potential use of this drug in ED treatment. Further large-scale Randomized Controlled Trials investigations involving various racial groups are required to confirm these findings.
Keywords: avanafil, erectile dysfunction, meta-analysis, systematic review, randomized controlled trial
Graphical Abstract:
Introduction
Erectile dysfunction (ED), the most prevalent sexual health issue among men, is defined as a recurrent inability to establish or maintain sufficient penile erection for satisfied-sexual performance.1 The global prevalence of ED ranges from 3% to 76.5%.2 Approximately 40% of males have suffered some form of erectile dysfunction by the time reaching their forties, and this number is predicted to grow by approximately 10% per decade.3 Recent research indicates that the frequency of ED among young men is as high as 30%.4 Cardiovascular disease, diabetes, hyperlipidemia, hypertension, smoking, obesity are well known risk factors for ED.1 Although ED is not a life-threatening condition, it constitutes a large burden due to its high prevalence and impact on quality of life, becoming a risk factor for the development of cardiovascular disease, dementia, and all-cause mortality.5
Since the discovery and introduction of sildenafil, a phosphodiesterase type-5 (PDE-5) inhibitor, has been considered as the first-line therapy for treating ED in a wide range of patients with diverse etiologies of sexual dysfunction.6 Currently, four drugs are approved by the Food and Drug Administration (FDA) for the treatment of ED: sildenafil, vardenafil, tadalafil, and the most recent addition, avanafil.7 However, owing to sporadic failures and unpleasant side effects, many patients are dissatisfied with initial PDE-5 inhibitors (sildenafil, vardenafil, and tadalafil). As a new PDE-5 inhibitor with strong PDE-5 inhibition, it is more promising than the others because of its selectivity and minimal adverse events.8
Avanafil has been identified to be a promising treatment for ED. Avanafil was approved for the treatment of ED in the US and Europe in 2012 and 2013.9 This drug also has the advantages of rapid onset of action (Tmax 35 min) and short half-life (< 1.5 h) compared to other PDE5 inhibitors.10 It works by mediating the breakdown of cyclic guanosine monophosphate (cGMP), inducing smooth muscle relaxation in the corpus cavernosum of the penis, increasing local blood flow, and leading to erection.11 However, little information is available on its efficacy and safety. The objectives of this systematic review and meta-analysis were to include more relevant randomized controlled trials (RCTs) and comprehensively analyze the efficacy and safety of this drug in the treatment of ED, which may resolve some of the current controversies regarding drug use. In addition, the current study compared different dosages to provide updated clinical evidence regarding avanafil treatment in ED.
Materials and Methods
Search Strategy
In this review, the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 guidelines were chosen as a guideline.12 We performed an electronic-based data search in PubMed, ScienceDirect, Web of Science, and Embase to identify all RCT studies on the treatment of ED with avanafil published up to October 24th, 2022. The following keywords were used are “avanafil”, or “stendra”, and/or “erectile dysfunction” and “impotence”. The search language was limited to English, and no restrictions on publication date were set for the search.
Selection of Studies and the Eligibility Criteria
After removing duplicates, the remaining articles were filtered by reviewing their titles and abstracts, and potentially relevant articles were screened. Finally, the selected articles with available full texts were retrieved and assessed according to the eligibility criteria. All of these processes were independently conducted by two reviewers.
The articles met the following criteria: (1) All patients were 18 years or older and were clinically diagnosed with any severity of ED; (2) Studies investigating ED with avanafil treatment; (3) The control groups in the studies were placebo, another PDE-5 inhibitor, or a different dose of avanafil; (4) The studies demonstrated at least one outcome of the efficacy and safety profile of avanafil treatment with a clear analysis; and (5) Randomized controlled trials (RCTs) were included in the review. The exclusion criteria were as follows: (1) Irrelevant titles or abstracts, (2) Irretrievable full-text, (3) Studies based on animal experiments, and (4) Studies that were non-RCTs.
Data Extraction and Quality Assessment
Data extraction was performed independently and the results were checked for certainty. The following relevant data were collected for each included study: first author’s name, year of publication, study location, study design, sample size, population age and characteristics, dose and duration of avanafil treatment, ED severity, and mean duration of ED in months. The following outcome data were extracted: changes in the International Index of Erectile Function – Erectile Function (IIEF-EF) score (before and after Avanafil treatment), changes in the Sexual Encounter Profile (SEP)-2, in which successful vaginal penetration occurred, and changes in SEP-3 (successful intercourse) as efficacy measurements of avanafil treatment. The IIEF-EF was introduced as a patient questionnaire to measure various aspects of erectile performance and to assess disease severity.13 Changes in IIEF-EF were used to assess the efficacy of avanafil. The IIEF has been widely accepted for its sensitivity and specificity; thus, it has been recommended as a primary endpoint for clinical trials of ED and for the diagnostic evaluation of ED severity.14 In addition, treatment-emergent adverse events (TEAE), including headache and flushing, and serious adverse events (SAE) were extracted as safety measures for avanafil treatment. In defining TEAE, the incidence of adverse events that are experienced during treatment, whether the events have been absent before the treatment or worsens relative to the pretreatment state.
Quality assessment of the studies included in this study was classified into “low risk of bias”, “some concerns”, or “high risk of bias” according to the Cochrane risk of bias tool for randomized controlled trials (RoB ver.2).12
Statistical Analysis
All analyses were performed using Review Manager version 5.4 (The Cochrane Collaboration, The Nordic Cochrane Center, Copenhagen, Denmark). The mean difference (MD) and risk ratio (RR) were used as the effect indexes for continuous and dichotomous data, respectively, while p-value and 95% confidence intervals (CI) were given for both data. A meta-analysis of each outcome was conducted only if two or more studies reported the same type of data. Heterogeneity between studies was determined using Cochran’s Q and I2 statistics. When there was statistical homogeneity between studies (p-value >0.1, I2 < 50%), a fixed-effects model was chosen for the meta-analysis. Otherwise, a random effects model was used. Potential publication bias was visually observed using Begg’s funnel plots. Statistical p-value <0.05 was set at p < 0.05.
Results
Study Characteristics
The initial database search yielded 1278 articles. A total of 33 duplicates were removed, and 10 articles were marked as ineligible by automation tools. After reviewing 1235 articles by title and abstract, 1147 were excluded. Fifteen reports were irretrievable because of inaccessible full text. Subsequently, 73 reports were assessed according to the eligibility criteria. The overall screening process of this systematic review and meta-analysis resulted in the inclusion of eleven RCTs studies,15–25 as demonstrated in the PRISMA flow diagram (Figure 1).
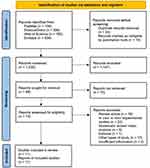 |
Figure 1 PRISMA flow chart of the study selection process.10 |
The characteristics of the 11 RCTs studies involving 3452 patients are summarized in Table 1. The dosage and treatment duration of avanafil varied among studies. All studies were conducted on three large continents: America (n=6), Asia (n=4), and Africa (n=1). Details of the study outcomes are presented in Table 2. The quality assessment of each study using the RoB tool is presented (Figure 2), which resulted in seven RCT studies to be classified as “low risk” of bias, three RCT studies to be classified as “some concerns”, and one RCT study to be classified as “high risk” of bias. The funnel plot provided a qualitative estimation of the publication bias of the studies, and no evidence of bias was found through visual inspection, as suggested by the symmetry of the funnel plot (Figure 3).
 |
Figure 2 Quality assessment of individual studies assessed with robvis: (A) risk of bias graph; (B) risk of bias summary.26 |
 |
Figure 3 Funnel plot of the studies represented in the meta-analysis. Abbreviations: MD, mean difference; SE, standard error. |
 |
Table 1 Characteristics of the Included Studies |
 |
Table 2 Outcome of the Individual Studies |
IIEF-EF Score
Six RCTs studies involving 1245 patients17,18,20,21,23,24 documented changes in IIEF-EF scores and were included in this meta-analysis. A pooled estimate showed that the changes in the IIEF-EF score of the avanafil group were significantly higher than those of the placebo group (MD=4.39, 95% CI [3.41, 5.37], p<0.001). Changes in IIEF-EF score were measured at the end of the treatment period and compared with the baseline score. Subsequently, subgroup analysis was performed according to the treatment dose. Similar results were obtained in the subgroup analysis, in which both 100 mg avanafil (MD=3.82, 95% CI [2.38, 5.25], p<0.001) and 200 mg avanafil (MD=4.96, 95% CI [3.47, 6.44], p<0.001) showed statistically significant differences compared with those in the placebo group (Figure 4A).
Successful Vaginal Penetration
Seven RCT studies involving 1379 patients17,18,20,21,23–25 documented changes in SEP-2 or successful vaginal penetration and were included in this meta-analysis. The pooled estimate demonstrated significant improvements in the SEP-2 scores of patients in the avanafil group compared to those in the placebo group (RR=3.43, 95% CI [2.79, 4.22], p<0.001). A subgroup analysis of patients treated with 100 mg and 200 mg avanafil in the ED was also performed. Similarly, both 100 mg avanafil (RR=3.25, 95% CI [2.42, 4.37], p<0.001) and 200 mg avanafil (RR=3.62, 95% CI [2.70, 4.84], p<0.001) showed statistically significant differences compared to the placebo group (Figure 4B).
Successful Intercourse
The SEP-3 or successful intercourse data included in the meta-analysis were obtained from seven RCT studies involving 1379 patients.17,18,20,21,23–25 The pooled estimate demonstrated that patients in the avanafil group had significantly greater improvements than those in the placebo group (RR=2.30, 95% CI [2.01, 2.62], p<0.001). Similarly, the results from subgroup analysis demonstrated statistically significant differences, both in the 100 mg avanafil (RR=2.20, 95% CI [1.82, 2.66], p<0.001) as well as in 200 mg-avanafil (RR=2.39, 95% CI [1.99, 2.88], p<0.001) groups compared to the placebo group (Figure 4C).
Treatment-Emergent Adverse Events (TEAE)
The TEAE data included in the meta-analysis were obtained from seven RCTs studies, involving 1429 patients treated with avanafil for ED.17,18,20,21,23–25 The pooled estimate revealed that the number of TEAE was significantly higher in the avanafil group than that in the placebo group (RR=1.49, 95% CI [1.24, 1.78], p<0.001) (Figure 5). Similarly, subgroup analysis suggested that the number of TEAE increased significantly in the 100 mg group compared to that in the placebo group (RR=1.49, 95% CI [1.12, 1.96], p=0.005), as well as in the 200 mg group (RR=1.50, 95% CI [1.15, 1.96], p=0.003). In addition, most of the selected studies reported more occurrences of TEAE than those in SAE, in which both headache and flushing were the two most common adverse events following avanafil treatment among many types of TEAE that occurred.
 |
Figure 5 Forest plot for comparison of TEAE in Avanafil (100mg and 200mg subgroup) and placebo group. |
Dose Comparison of Avanafil Treatment
This analysis investigated whether there were any differences in the efficacy and safety of ED treatment with 100 mg or 200 mg avanafil. Eight RCTs reported changes in the IIEF-EF score with two separate doses of avanafil,17–21,23–25 whereas seven RCTs reported changes in SEP-2, SEP-3, and TEAE.17,18,20,21,23–25 The pooled estimate demonstrated that 200 mg avanafil was superior to 100 mg avanafil in terms of IIEF-EF score (MD=−1.15, 95% CI [−1.40, −0.89], p<0.001). In contrary, there were no significant differences in SEP-2 (RR=0.90, 95% CI [0.75, 1.08], p=0.26), SEP-3 (RR=0.92, 95% CI [0.81, 1.05], p=0.21) and TEAE (RR=1.00, 95% CI [0.87, 1.15], p=0.99) between both 100 mg and 200 mg doses, in which these results suggested that both doses are similarly effective and relatively safe for patients with ED (Figure 6).
Sensitivity Analysis
A sensitivity analysis was performed by dividing the included studies into Asian and Caucasian groups. There were no significant differences in the overall pooled estimate of change in the IIEF-EF score, SEP-2, and SEP-3 in either the Asian or Caucasian study groups. However, omitting Caucasian groups in the TEAE analysis demonstrated no significant differences between the avanafil treatment and placebo groups (RR=1.92, 95% CI [1.00, 3.66], p=0.05). In addition, there were no significant differences in the efficacy of avanafil-100 mg and 200 mg in terms of IIEF-EF changes (MD=−0.93, 95% CI [−2.57, 0.72], p=0.27) among the Asian groups. In contrast, the Caucasian group showed that 200 mg avanafil was superior to 100 mg avanafil (MD=−1.16, 95% CI [−1.42, −0.89], p<0.001). Moreover, there were no significant differences between the 100 mg- and 200 mg- avanafil groups regarding to the changes in SEP-2, SEP-3, and TEAE between either Asian or Caucasian groups.
Discussion
This study demonstrated the evidence of RCTs studies on ED treatment. Overall, patients administered avanafil demonstrated significant improvements in the IIEF-EF domain score compared to those in the placebo group across all included RCTs. Moreover, treatment with avanafil also showed significant improvements in erectile function compared to the placebo group, as assessed by SEP-2 and SEP-3, which represent the ability of the penis to penetrate the vagina and measure how long erection is enough to have a successful intercourse.
Although the safety profile of avanafil was more likely to be associated with TEAE in the treatment groups than in the placebo groups, they were generally mild and well tolerated. The two most common adverse events following avanafil treatment were headaches and flushing. These effects have also been commonly reported for sildenafil, vardenafil, and also tadalafil treatments.28 Moreover, the cause of headaches induced by PDE-5 inhibitors is a nonvascular mechanism. In addition, people most likely complain about altered color vision because to the inhibition of PDE-6. Although none of the PDE-5 inhibitors are selective for the receptor PDE5, and avanafil is known to be the most selective PDE-5 inhibitor. A study comparing the selectivity of avanafil and sildenafil revealed that avanafil inhibited PDE-6 and PDE-1 to a lesser extent than sildenafil.8 Thus, avanafil is unlikely to affect retinal function at pharmacologically appropriate doses.
Among all included RCTs, studies showed that 100 and 200 mg doses of avanafil were similarly effective in improving SEP-2 and SEP-3 in patients with ED. These findings are in agreement with those of a previous study by Goldsteina et al and a meta-analysis by Li et al, which revealed no statistically significant differences in SEP-2 between the two doses.8,18 Likewise, there were also no differences in SEP-3 between the groups, which is in line with the findings of a previously published meta-analysis incorporating four RCTs.27 In contrast, the SEP-3 finding from Li et al discovered a higher proportion of successful intercourse among patients receiving 200 mg of avanafil than among those receiving a lower dose.8 However, different studies, in terms of population area, race, sample size, and age, may have contributed to this disparity. The treatment of patients with ED may be affected by a combination of factors.29 Consequently, as it is debatable whether high doses of avanafil have an advantage in improving SEP-3 in patients with ED, further objective evaluation of the impact of avanafil on improving SEP-3 at different doses is warranted. The results of this review also indicated that 200 mg of avanafil was superior to 100 mg in terms of improving the IIEF-EF domain score, consistent with the findings of previous studies that found that 200 mg of avanafil can significantly increase IIEF-EF in better scores than 100 mg doses.8,30 When compared to other PDE-5 inhibitors, Avanafil seems to have a quicker onset of effects, occurring within 15 minutes. This characteristic could be advantageous for individuals who cannot anticipate sexual activity more than 15–30 minutes in advance.8
Regarding the influence of race and ethnicity on efficacy, a review by Pyrigidis et al30 on the PDE-5 inhibitor effect generally showed no significant difference in the efficacy or side effects of sildenafil between African American and Caucasian men. Another review by Smith et al31 found no significant differences in the efficacy or side effects of tadalafil between Asians and Caucasians. Further studies on avanafil could explore whether there is a difference in its efficacy among different races.
The safety profile of 100 mg avanafil appeared to be comparable to that of 200 mg avanafil. Overall, the risks of TEAE were similar between the different dosage groups. Other adverse events, such as nasopharyngitis, dizziness, and back pain, were also reported, but their incidence was low; thus, they were not included in this study. It is encouraging to note that adverse events are generally mild and most patients can tolerate them. Moreover, major problems with SAE have rarely been reported across RCTs. A previous study reported that sublingual nitroglycerin had a lesser effect on blood pressure and heart rate after oral administration of avanafil for 60 minutes. Similar adverse events have been reported in other PDE-5 inhibitors and is comparable with avanafil.8 Adverse events associated with a clinically significant reduction in systolic blood pressure (≥30 mmHg) induced by avanafil were less common than sildenafil.32 Thus, patients who cannot tolerate TEAE due to sildenafil may benefit from avanafil.
As a systematic review and meta-analysis, this study has some limitations. First, not all RCTs included in this study were double-blind and placebo-controlled; thus, the risk of bias regarding allocation concealment in these studies cannot be eliminated. Additionally, some of the included studies had small sample sizes. Notably, data from unpublished studies were not included in this review, and these factors may have resulted in bias. Furthermore, as the primary population studies in this review were dominated by Caucasians and Asians, the clinical effects of avanafil in ED patients of other races remain unclear. This is further exacerbated by the lack of studies in Europe and Oceania. Additional RCTs with larger samples and diverse races are required to better understand the efficacy and safety of therapies for ED.
Conclusion
This review suggests that avanafil is an effective and well-tolerated therapy for erectile dysfunction among men. Considering its efficacy and safety, 200 mg of avanafil has the potential to be the chosen dose for the treatment of erectile dysfunction. This study may have substantial implications for clinical practice and ED research. However, additional large-scale RCTs involving a variety of racial groups and disease severities are necessary to corroborate these findings.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sooriyamoorthy T, Leslie SW. Erectile dysfunction; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562253/.
2. Kessler A, Sollie S, Challacombe B, Briggs K, Van Hemelrijck M. The global prevalence of erectile dysfunction: a review. BJU Int. 2019;124(4):587–599. PMID: 31267639. doi:10.1111/bju.14813
3. Ferrini MG, Gonzalez-Cadavid NF, Rajfer J. Aging related erectile dysfunction-potential mechanism to halt or delay its onset. Transl Androl Urol. 2017;6(1):20–27. doi:10.21037/tau.2016.11.18
4. Nguyen HMT, Gabrielson AT, Hellstrom WJG. Erectile dysfunction in young men-a review of the prevalence and risk factors. Sex Med Rev. 2017;5:508–520. doi:10.1016/j.sxmr.2017.05.004
5. Elterman DS, Bhattacharyya SK, Mafilios M, Woodward E, Nitschelm K, Burnett AL. The quality of life and economic burden of erectile dysfunction. Res Rep Urol. 2021;13:79–86. doi:10.2147/RRU.S283097
6. Andersson KE. PDE5 inhibitors - pharmacology and clinical applications 20 years after sildenafil discovery. Br J Pharmacol. 2018;175(13):2554–2565. doi:10.1111/bph.14205
7. Yafi FA, Jenkins L, Albersen M, et al. Erectile dysfunction. Nat Rev Dis Primers. 2016;2:16003. PMID: 27188339; PMCID: PMC5027992. doi:10.1038/nrdp.2016.3
8. Li J, Peng L, Cao D, He L, Li Y, Wei Q. Avanafil for the treatment of men with erectile dysfunction: a systematic review and meta-analysis of randomized controlled trials. Am J Mens Health. 2019;13(5):1557988319880764. doi:10.1177/1557988319880764
9. Jiann B-P. Evolution of phosphodiesterase type 5 inhibitors in treatment of erectile dysfunction in Taiwan. Urol Sci. 2016;27(2):66–70. ISSN 1879-5226. doi:10.1016/j.urols.2016.04.003
10. Burke RM, Evans JD. Avanafil for treatment of erectile dysfunction: review of its potential. Vasc Health Risk Manag. 2012;8:517–523. doi:10.2147/VHRM.S26712
11. Peak TC, Kammel K, Sangkum P, R.b.w T. Hellstrom W.J.G.update on the treatment of erectile dysfunction. In: Reference Module in Biomedical Sciences. Elsevier;2015. ISBN 9780128012383. doi:10.1016/B978-0-12-801238-3.06062-1
12. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372. doi:10.1136/BMJ.N71
13. Katz EG, Tan RB, Rittenberg D, Hellstrom WJ. Avanafil for erectile dysfunction in elderly and younger adults: differential pharmacology and clinical utility. Ther Clin Risk Manag. 2014;10:701–711. doi:10.2147/TCRM.S57610
14. Utomo E, Blok BF, Pastoor H, Bangma CH, Korfage IJ. The measurement properties of the five-item International Index of Erectile Function (IIEF-5): a Dutch validation study. Andrology. 2015;3(6):1154–1159. doi:10.1111/andr.12112
15. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366. doi:10.1136/BMJ.L4898
16. Belkoff LH, McCullough A, Goldstein I, et al. An open-label, long-term evaluation of the safety, efficacy and tolerability of avanafil in male patients with mild to severe erectile dysfunction. Int J Clin Pract. 2013;67(4):333–341. doi:10.1111/IJCP.12065
17. Elkamshoushi AM, Badae NM, Kabary MG, et al. Evaluation of daily avanafil efficacy in improving the endothelial function in Egyptian males with erectile dysfunction. Andrologia. 2021;53(1). doi:10.1111/AND.13833
18. Goldstein I, McCullough AR, Jones LA, et al. A randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of avanafil in subjects with erectile dysfunction. J Sex Med. 2012;9(4):1122–1133. doi:10.1111/J.1743-6109.2011.02629.X
19. Goldstein I, Jones LARA, Belkoff LH, et al. Avanafil for the treatment of erectile dysfunction: a multicenter, randomized, double-blind study in men with diabetes mellitus. Mayo Clin Proc. 2012;87(9):843–852. doi:10.1016/j.mayocp.2012.06.016
20. Hellstrom WJG, Freier MT, Serefoglu EC, et al. Onset of action and time to efficacy of avanafil, a novel, rapid-onset PDE5 inhibitor in men with mild to severe erectile dysfunction: data from Phase 2 and Phase 3 clinical trials. J Sex Med. 2012;9(4, S):E600. doi:10.1016/j.juro.2012.02.2002
21. Hellstrom WJG, Kaminetsky J, Belkoff LH, et al. Efficacy of avanafil 15 minutes after dosing in men with erectile dysfunction: a randomized, double-blind, placebo controlled study. J Urol. 2015;194(2):485–492. doi:10.1016/J.JURO.2014.12.101
22. Jiang H, Lin H, Li F, et al. Efficacy and safety of avanafil in Chinese subjects with erectile dysfunction: a multi-center, randomized, double-blinded, placebo-controlled Phase III clinical trial. Sex Med. 2021;9(3):100337. doi:10.1016/j.esxm.2021.100337
23. Kumar M, Pathade AD, Gupta SV, et al. Efficacy and safety of avanafil as compared with sildenafil in the treatment of erectile dysfunction: a randomized, double blind, multicenter clinical trial. Int J Urol. 2022;29(4):351–359. doi:10.1111/iju.14785
24. Mulhall JP, Burnett AL, Wang R, et al. A phase 3, placebo controlled study of the safety and efficacy of avanafil for the treatment of erectile dysfunction after nerve sparing radical prostatectomy. J Urol. 2013;189(6):2229–2236. doi:10.1016/J.JURO.2012.11.177
25. Park HJ, Kim SW, Kim JJ, et al. A randomized, placebo-controlled, double-blind, multi-center therapeutic confirmatory study to evaluate the safety and efficacy of avanafil in Korean patients with erectile dysfunction. J Korean Med Sci. 2017;32(6):1016–1023. doi:10.3346/JKMS.2017.32.6.1016
26. Swearingen D, Nehra A, Morelos S, Peterson CA. Hemodynamic effect of avanafil and glyceryl trinitrate coadministration. Drugs Context. 2013;2013. doi:10.7573/dic.212248
27. Zhao C, Kim SW, Yang DY, et al. Efficacy and safety of avanafil for treating erectile dysfunction: results of a multicentre, randomized, double-blind, placebo-controlled trial. BJU Int. 2012;110(11):1801–1806. doi:10.1111/J.1464-410X.2012.11095.X
28. Kedia GT, Uckert S, Assadi-Pour F, Kuczyk MA, Albrecht K. Avanafil for the treatment of erectile dysfunction: initial data and clinical key properties. Ther Adv Urol. 2013;5(1):35–41. doi:10.1177/1756287212466282
29. Wang X, Yang X, Cai Y, Wang S, Weng W. High prevalence of erectile dysfunction in diabetic men with depressive symptoms: a meta-analysis. J Sex Med. 2018;15(7):935–941. doi:10.1016/j.jsxm.2018.05.007
30. Pyrgidis N, Mykoniatis I, Haidich A-B, et al. The effect of phosphodiesterase-type 5 inhibitors on erectile function: an overview of systematic reviews. Front Pharmacol. 2021;12. doi:10.3389/fphar.2021.735708
31. Smith WB, McCaslin IR, Gokce A, Mandava SH, Trost L, Hellstrom WJ. PDE5 inhibitors: considerations for preference and long-term adherence. Int J Clin Pract. 2013;67(8):768–780. doi:10.1111/ijcp.12074
32. McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2020. doi:10.1002/jrsm.1411
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


