Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
The Effects of Low Pressure Domiciliary Non-Invasive Ventilation on Clinical Outcomes in Patients with Severe COPD Regardless Having Hypercapnia
Authors Theunisse C, Ponssen HH, de Graaf NTC, Scholten-Bakker M, Willemsen SP, Cheung D
Received 9 November 2020
Accepted for publication 1 February 2021
Published 26 March 2021 Volume 2021:16 Pages 817—824
DOI https://doi.org/10.2147/COPD.S289099
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Christiaan Theunisse,1,2 Huibert H Ponssen,2 Netty T C de Graaf,1 Maaike Scholten-Bakker,1 Sten P Willemsen,3 David Cheung1
1Department of Pulmonology, Albert Schweitzer Hospital, Dordrecht, the Netherlands; 2Department of Intensive Care, Albert Schweitzer Hospital, Dordrecht, the Netherlands; 3Department of Biostatics, Erasmus University Medical Centre, Rotterdam, the Netherlands
Correspondence: David Cheung
Department of Pulmonology, Albert Schweitzer Hospital, Albert Schweitzerplaats 25, Dordrecht, AT, NL-3318, the Netherlands
Tel +31-78-6523328
Fax +31-78-6523354
Email [email protected]
Background: The effectiveness of non-invasive home ventilation in patients with severe chronic obstructive pulmonary disease (COPD) is lacking. Non-invasive home ventilation might be more effective when high ventilator settings are used. However, high ventilator settings might reduce patient adherence. We have developed a multidisciplinary approach (ventilation practitioners, 24 hours support of respiratory nurses, physicians) to non-invasive ventilation aimed at optimizing patient adherence using low ventilator settings in severe COPD patients with high disease burden irrespectively having hypercapnia.
Methods: We included in a proof of concept, prospective interventional study, 48 GOLD stage III–IV COPD patients with a high disease burden (≥ 2 exacerbations in a year, and Medical Research Council dyspnea scores ≥ 3). Outcome measures included hospital admissions, capillary pCO2, Medical Research Council dyspnea scores (MRC), Clinical COPD Questionnaire scores (CCQ) and Hospital Anxiety and Depression Scale (HADS).
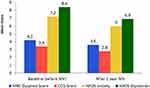
Results: After 1 year 32 patients could be evaluated. Hospital admissions decreased by 1.0 admission (mean difference ± SD: 1.0 ± 1.48; p = 0.001). In-hospital days decreased by 10.0 days (10.0 ± 15.48; p = 0.001). Capillary pCO2 decreased by 0.33 kPa (0.33 ± 0.81: p = 0.03). The MRC dyspnea score decreased by 0.66 (0.66 ± 1.35; p = 0.02). The CCQ score decreased by 0.59 (0.59 ± 1.39; p = 0.03). The HADS anxiety score decreased by 1.64 (1.64 ± 3.12; p = 0.01). The HADS depression score decreased by 1.64 (1.64 ± 3.91; p = 0.04).
Conclusion: A proof of concept multidisciplinary approach, using low pressure domiciliary non-invasive ventilation, aimed at optimizing patient adherence in severe COPD patients regardless having hypercapnia, reduced hospital admissions and improved symptoms and quality of life measures. This may imply that severe COPD patients with high disease burden, irrespective being hypercapnic, are candidates to be treated with low pressure domiciliary non-invasive ventilation.
Keywords: COPD, non-invasive ventilation, domiciliary, hospitalization, compliance
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive chronic lung disease, characterized by irreversible airflow obstruction and recurrent exacerbations.1 Treatment for COPD is based on pharmacological treatment, pulmonary rehabilitation, and sometimes long-term oxygen therapy. Exacerbations are a main origin of increased morbidity, mortality, and poor health status, and put a substantial burden on the health care system.2 Approximately 15% of COPD patients have exacerbations requiring hospital admission per year.3,4 Reducing exacerbation frequency is therefore an important therapeutic target.
Non-invasive ventilation (NIV) is a method of supplying ventilatory support via a mask and is successful in improving survival among patients with acute or acute-on-chronic hypercapnic respiratory failure in hospital.5,6 Evidence for domiciliary usage of NIV in non-acute COPD patients is more restricted despite a number of systematic reviews.7–10 Using this NIV strategy, a randomized controlled trial by Köhnlein et al demonstrated a survival benefit in stable hypercapnic COPD patients after 1 year of NIV.11 Therefore, domiciliary NIV is current a standard treatment for COPD patients with chronic hypercapnic respiratory failure and a considerable indication for home mechanical ventilation in Europe.12
Nevertheless, it remains unidentified what the mechanism is how chronic NIV can lead to these improved outcomes. It has been postulated that reduction in hypercapnia is considered for the beneficial effects of NIV and causing improved survival of patients on chronic NIV. However, without a change in hypercapnia, a survival benefit has also been shown and studies showing a reduction in hypercapnia could not demonstrate an improvement in survival.13–15 Therefore, chronic NIV might induce these positive results through several other mechanisms.
Domiciliary NIV can be a difficult to tolerate treatment for many patients, and they might feel uncomfortable.16 The most important parts determining the submission of the patient to NIV include patient’s discomfort, mask leak and fit, the presence of side effects (conjunctivitis, nasal congestion, and sleep interruption), and symptoms (morning headache, hypersomnolence, and dyspnea).17 Using high inspiratory positive airway pressure (IPAP) is suggested as the most familiar reason for treatment discordance.18 This might reduce patient adherence to non-invasive ventilation. Therefore, we developed a multidisciplinary approach to non-invasive ventilation aimed at optimizing patient acceptance and adherence. Low pressures are used in order to ventilate patients as comfortable as possible. We used this approach in severe COPD patients irrespectively having hypercapnia and compared hospital admissions and quality of life measures before, versus 1 year after starting non-invasive home ventilation. The aim of the present study was to investigate, in a proof of concept, whether low pressure domiciliary non-invasive ventilation aimed at optimizing patient acceptance and adherence can improve quality of life and reduce hospitalization in severe COPD patients with high disease burden irrespectively being hypercapnic.
Methods
Subjects
Patients were recruited from an outpatient population from the Department of Pulmonology in the Albert Schweitzer Hospital Dordrecht in the Netherlands between January 2014 till January 2015. Patients have an indication for chronic NIV when they have COPD diagnosed according to the GOLD criteria stage III–IV (FEV1 < 50%, FEV1/FVC ratio <70%),19 combined with a high disease burden ≥2 exacerbations in a year treated with corticosteroids or antibiotics,19 and high dyspnea symptoms using the Medical Research Council’s (MRC) dyspnea scale (≥3),19,20 and in a stable clinical condition. Patients were excluded if they had abnormalities of the lung or thorax other than COPD. Additional exclusion criteria were previously initiated NIV, obstructive sleep apnea, severe heart failure (New York Heart Association stage IV), and malignant comorbidities. Patients with acute respiratory failure or COPD exacerbation within the last 6 weeks prior to enrolment were not included in the study.19 The study protocol was approved by the Ethics Committee of the Albert Schweitzer Hospital, and the study was performed conforming to good clinical practice and the ethical standards as constituted by the Declaration of Helsinki. From all subjects, a written informed consent was obtained prior to the study.
Design and Measurements
The design was a proof of concept, prospective interventional study. Measurements (demographics, comorbidities, body mass index (BMI), medication, lung function, blood gasses, and ventilator settings) were carried out at baseline before NIV initiation, and during follow-up after 12 months of NIV treatment. Health-Related Quality of Life (HRQoL) measures were assessed at each visit. Dyspnea was assessed using the Medical Research Council’s (MRC) 5 points dyspnea scale20 and the COPD control status was measured with the 7 points Clinical COPD Questionnaire (CCQ).21 The Hospital Anxiety and Depression Scale (HADS) (≥8 point per domain) was conducted for screening of symptoms of anxiety and depression.22
Lung Function
Lung function parameters (Masterlab-Compact ® Labor, Jaeger, Hochberg, Germany) were carried out in accordance with international guidelines.24 Capillary blood gas measurements were taken from the arterialized earlobe AVL Omni ® (Roche Diagnostics GmbH, Graz, Austria). Partial pressure of carbon dioxide (pCO2), and bicarbonate concentration HCO3− were assessed. Pneumotachographic measurements were assessed (RSS 100 Research PneumoSeries, Hans Rudolph, Inc., Shawnee, KS, USA) using a flow sensor connected between the ventilatory mask and the exhalation port (Silentflow 2 ®, Weinmann, Hamburg, Germany) during NIV. Minute ventilation (MV) was defined as the product of tidal volume and respiratory rate (RR).
Non-Invasive Ventilation
According to a standardized protocol, the patients were admitted by the same registered nurse on NIV. NIV was provided through a pressure cycled ventilator, administering Bi-level inspiratory and expiratory positive airway pressure (BiPAP), with a backup respiratory rate (BiPAP, 9 VPAPTM S®, Resmed, San Diego, CA, USA). The efficacy of NIV during the night was monitored by the transcutaneous pCO2, and O2 saturation (TOSCA® 500, Linde Medical Sensors AG, Basel, Switzerland). Supplementary oxygen was administered through the ventilator. Patients were instructed using a commercially available nasal-mouth masks (Silentflow 2). Patients were instructed in non-invasive ventilation during a one-day visit in our clinic. Previously usage of home NIV was not allowed. Patients started on ST-mode, Bi-level positive airway pressure (BiPAP), with low pressures. Starting inspiratory positive airway pressure (IPAP) at 10 cm H2O and end expiratory positive airway pressure (EPAP) at 4 cm H2O. Pressure levels were gradually increased (maximum: EPAP 6 cm H2O – IPAP 16 cm H2O). Patients were advised to sleep with the BiPAP device; the assist mode (ST) was combined with a back-up respiratory rate mode (13 breaths per minute) in case of apnea. At home patients were attended by nurses specialized in pulmonary diseases. In case of pulmonary problems, patients were allowed to make phone calls 7 x 24 hour to the hospital. During the interview, accurate information was achieved regarding the side effects of NIV therapy and ventilator usage characteristics. The patients can also apply to the hospital at any time when needed. The compliance of NIV usage was achieved using the readout of the SD card from the ventilator’s software.
Subjective Preference
After one year NIV treatment, patients were given a questionnaire in Dutch that assessed their sleep quality and comfort with NIV. The two questions were as follows. 1) How is your sleep quality during treatment with NIV. The response is four points: worse, no difference, slightly better, much better. 2) How is your experience with NIV. The response is four points: negative, neutral, positive, very positive.23
Statistical Analyses
Statistical analysis was carried out using the IBM SPSS version 23 software (IBM Corporation, Armonk, NY, USA). Repeated measures analysis of variance was used to analyse the data. Effects that were significant on analysis of variance were analysed with Student´s t-tests. Differences within the groups were analysed with two-tailed, paired t-tests, and differences between the groups were analysed with unpaired t-tests. The summary statistics were expressed as means ± SD. Spearman tests were carried out to analyse the correlation between variables. A p-value of <0.05 was considered statistically significant. A significant improvement of sleep quality or patients' preference of NIV was defined if NIV was preferred by ≥50% of all treated patients. The minimal clinically relevant preference was estimated to be 60%.23
Results
In the study 48 patients were included, 32 patients were evaluated after 1 year (Table 1).
- One patient underwent lung transplantation (confirmation that the lung transplant was donated voluntarily with written informed consent and that this was conducted in accordance with the Declaration of Istanbul).
- Nine patients died within 1 year (4 patients died due to COPD exacerbations, 3 patients died due to pneumonia, 1 patient died due to pneumothorax and post-operative complications, 1patient died of unknown cause).
- Six patients did not endure the ventilation after 6 weeks because of not being able to sleep with NIV, progressive dementia and mask problems.
- Thirteen patients were normocapnic (capillary pCO2 ≤6.0 kPa).
- The duration of COPD was 6.9 years (mean ± SD: 6.9 ± 3.2 years).
 |
Table 1 Demographic and Clinical Characteristics of Patients at Baseline |
Effects of NIV on Hospital Admissions, Capillary pCO2, and Health-Related Quality of Life (HRQoL) Measures
Hospital admissions decreased by 1.0 admission (mean difference ± SD: 1.0 ± 1.48; p = 0.001).
In-hospital days decreased by 10.0 days (10.0 ± 15.48; p = 0.001) (Table 2). Capillary pCO2 decreased by 0.33 kPa (0.33 ± 0.81: p = 0.03). The MRC dyspnea score decreased by 0.66 (0.66 ± 1.35; p = 0.02). The CCQ score decreased by 0.59 (0.59 ± 1.39; p = 0.03). The HADS anxiety score decreased by 1.64 (1.64 ± 3.12; p = 0.01). The HADS depression score decreased by 1.64 (1.64 ± 3.91; p = 0.04) (Table 2) (Figure 1). Analyzing the group of 42 patients including the 10 patients who was transplanted or died during 1 year follow-up (mean follow up was 6.10 ± 2.68 months) and taking the interval of follow up into account, hospital admissions decreased significantly by 0.98 admission (0.98 ± 1.33; p = 0.001).
 |
Table 2 Hospital Admissions, Symptoms Scores and pCO2 Before and After 1 Year NIV |
Non-Invasive Ventilation
After 12 months, mean inspiratory positive airway pressure (IPAP) was 14.9 ± 2.8 cm H2O, expiratory positive airway pressure (EPAP) was 5.2 ± 1.5 cm H2O. After 12 months the mean patient’s compliance of using the ventilator was 6.5 ± 2.0 hours per night and at least 80% of the patients used NIV for more than 5 hours per night. The compliance to the ventilator did not differ between normocapnic patients and hypercapnic patients (p = 0.50) (Table 3).
 |
Table 3 NIV Usage, Type, and Setting |
Differences in Response Between Normocapnic and Hypercapnic Group
There was before the NIV was started no statistically differences between the normocapnic and hypercapnic group concerning age, BMI, FEV1, FVC, MRC, CCQ, HADS anxiety and HADS depression score, COPD history, hospital admission and in-hospital days (p > 0.10) (Table 1).
There was no statistical differences between the normocapnic group in comparison with the hypercapnic group in the change in hospital admissions (0.85 ± 1.14 and 1.11 ± 1.70 decrease respectively, p = 0.64), and in-hospital days (8.31 ± 12.35 and 11.16 ± 17.53 decrease respectively, p = 0.62). Furthermore, there was no significant differences between the groups in change in symptoms scores. MRC dyspnea score (0.82 ± 1.54 and 0.56 ± 1.25 decrease respectively, p = 0.63), CCQ score (0.91 ± 0.92 and 0.38 ± 1.61 decrease respectively, p = 0.33), HADS anxiety score (1.09 ± 2.39 and 2.0 ± 3.54 decrease respectively, p = 0.46), and HADS depression score (2.18 ± 2.79 and 1.29 ± 4.54 decrease respectively, p = 0.57).
Correlations Between Change in Capillary pCO2 and Change in Hospital Admission and Symptoms Scores
There was no significant correlation between delta capillary pCO2 (before and after 1 year of treatment) and the decrease in hospital admissions (correlation coefficient 0.07, p = 0.70). Furthermore, there was no significant correlation between delta capillary pC02 and the decrease in-hospital days (correlation coefficient 0.03 p = 0.88). Nor was there a significant correlation between delta capillary pCO2 and change in MRC dyspnea score (correlation coefficient 0.08, p = 0.70), CCQ score (correlation coefficient 0.04, p = 0.86), HADS anxiety score (correlation coefficient 0.15, p = 0.44), and HADS depression score (correlation coefficient 0.20, p = 0.30).
Sleep Quality
Within the study population of 32 patients, 27 patients (84%) have an improvement of their sleep quality NIV treatment. Only 5 patients (16%) did not have improvement in sleep quality after NIV treatment (Figure 2).
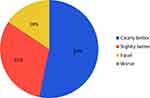 |
Figure 2 Sleep quality after one year of NIV (n=32). 31% of the patients showed some improvement in sleep quality, 53% did show a very clear improvement. |
Patient Preference
Within the study population of 32 patients, 28 patients (88%) were experienced positively about NIV treatment. Only 1 patient (3%) was negative about NIV treatment (Figure 3).
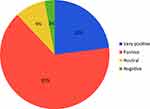 |
Figure 3 NIV experience after one year (n=32). 65% experienced NIV as positive, 23% as very positive. |
Discussion
The present study showed that multidisciplinary approach using low pressure domiciliary non-invasive ventilation aimed at optimizing patient acceptance and adherence in severe COPD patients (GOLD III and IV),19 regardless having hypercapnia, reduced the numbers of hospital admissions, in-hospital admission days, symptoms and quality of life measures in 1-year follow-up period. This suggests that NIV was improving symptoms and frequency of hospital admissions in a subgroup of patients irrespective of having hypercapnia and emphasizes the need to investigate further than pCO2 when considering to treat COPD patients with chronic NIV.
Patients with severe COPD (GOLD III and IV) are often also characterized by a very high disease burden.25 These patients experience severe dyspnea, fear and loss of quality of life.25 Patients stay often in hospitals during exacerbations of their disease.26 This is to our knowledge, the first study in the effects of NIV in patients with severe COPD irrespective of having hypercapnia. This study confirmed the results from Raveling et al27 that NIV might have their beneficial effects irrespective of the ability to improve pCO2 and highlighted the need to look further than hypercapnia. Furthermore, our findings are compatible with recent studies in which long-term NIV are successful in reducing COPD exacerbations and COPD-related hospital readmissions in a group of COPD patients with frequent exacerbations.28,29 Our study emphasizes the study by Murphy et al that low pressure non-invasive ventilation can improve symptoms and reduction in hospital admissions.30 Like others, in our study group, there was no correlation between pCO2 decline and reduction in hospital admissions or symptoms score.30 In addition, this study emphasized that long-term NIV therapy is feasible at home in severe COPD patients with improving sleep quality and patients preference.31
This study had a number of restrictions. First, not having a placebo-controlled design for this study is a potential draw back. Although the usage of a placebo group with sham device was considered since this procedure has been carried out previously in patients with obstructive sleep apnea using continuously positive airway pressure. However, the usage of a device through a face mask delivering zero pressure support could result in an increase in dynamic dead space, which could deteriorate respiratory failure in severe COPD patients. Furthermore, there was some doubt about the validity of blinding using sham device because both patients and investigators were able to classify the sham intervention, so limiting the rationale of this procedure. Second, not every patient experienced improvement by the treatment. Six from 48 patients did not endure the NIV therapy. However, this percentage is lower than the percentage failure in earlier studies.32 The design of the study with a multidisciplinary approach might explain this difference. By instruction of the patients in non-invasive ventilation during a one-day visit in our clinic and by visiting the patients at home 24/7 might elucidate these positive results. Furthermore, the results of this study confirmed that outpatient control of long-term non-invasive ventilation is feasible in severe patients with COPD.30 Third, the average IPAP was 14.9 cm H2O was relatively low in the present study. In recent studies, some investigators underline the importance of high-intensity non-invasive ventilation using high inspiratory pressure. On the other hand, by using low intensity NIV we demonstrated that low pressure NIV still can improve the quality of life measures and decrease the numbers of hospital admissions. Fourth, the usage of capillary blood sampling as a substitute for arterial blood sampling might introduce a bias. Lower complication rates, lower grade of invasiveness, higher grade of patient comfort and physician independence are arguments for the usage of capillary blood sampling. Capillary pCO2 was significantly decreased by NIV in this study and since capillary pCO2, and HCO3− were accurate measurements in comparison with arterial sampling methods,33 capillary blood sampling can be useful in our study approach. Finally, since we could not retrieve the precise causes of death for all patients in our study, we could not identify between COPD and other causes of death. All these restrictions might have biased our results. However, since there was still a significant reduction in hospital admissions in the whole group with the 10 patients who was transplanted or died during the year of follow-up, we are convinced that these restrictions have not biased our results.
An important finding of this study is that in stable COPD patients domiciliary low pressure NIV reduces the number of hospital admissions, symptoms and improves quality of life measures. Reducing symptoms, and prevention of exacerbations are thought to be essential in restricting COPD progression. A possible mechanism lowering the number of exacerbations is by improving the ventilation–perfusion (V/Q) matching. Airflow redistribution into the lungs may change on NIV, thereby improving ventilation-perfusion matching.34 De Backer et al demonstrated that NIV-treated patients who showed improvement in their blood gases, mass flow was redistributed toward areas with better perfusion.34 The improvement in ventilation-perfusion matching was positively correlated with the anxiety domain of the Severe Respiratory Insufficiency Questionnaire and the 6-minute walking test.35 Another mechanism of action could be mobilization of sputum. Positive end-expiratory pressure can improve mobilizing mucus,36 reducing the risk of infections, which are thought to be an important origin of COPD exacerbations. Finally, a possible mechanism for the beneficial effects of NIV in COPD could be explained in changes in lung function, airway obstruction and/or hyperinflation. NIV is capable to decrease hyperinflation.36 Diaz et al have demonstrated a decrease in residual volume and increase in intrinsic positive end-expiratory pressure with only short periods of NIV.36 Indeed, in nonhypercapnic patient with COPD and hyperinflation, NIV can improve both hyperinflation and exercise capacity.37
Conclusion
A proof of concept multidisciplinary approach using low pressure domiciliary non-invasive ventilation aimed at optimizing patient acceptance and adherence in severe COPD patients regardless having hypercapnia reduced hospital admissions, symptoms and improved a better quality of life. This may imply that severe COPD patients (GOLD III and IV), with high disease burden irrespective being hypercapnic, might be candidates to be treated by domiciliary low pressure NIV.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365.
2. Seemungal T, Donaldson G, Paul E, Bestall J, Jeffries D, Wedzicha J. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422.
3. Britton M. The burden of COPD in the U.K.: results from the Confronting COPD survey. Respir Med. 2003;97(Suppl):
4. Roberts CM, Lowe D, Bucknall CE, Ryland I, Kelly Y, Pearson MG. Clinical audit indicators of outcome following admission to hospital with acute exacerbation of chronic obstructive pulmonary disease. Thorax. 2002;57(2):137–141.
5. National Institute for Clinical Excellence (NICE). Chronic obstructive pulmonary disease: national clinical guideline for management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax. 2004;59(Suppl 1):1–232.
6. Ram FS, Picot J, Lightowler J, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2004;(1):CD004104.
7. Struik FM, Lacasse Y, Goldstein RS, Kerstjens HA, Wijkstra PJ. Nocturnal noninvasive positive pressure ventilation in stable COPD: a systematic review and individual patient data meta-analysis. Respir Med. 2014;108(2):329–337.
8. Struik FM, Lacasse Y, Goldstein R, Kerstjens HM, Wijkstra PJ. Nocturnal non-invasive positive pressure ventilation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;6:CD002878.
9. Shi JX, Xu J, Sun WK, Su X, Zhang Y, Shi Y. Effect of noninvasive, positive pressure ventilation on patients with severe, stable chronic obstructive pulmonary disease: a meta-analysis. Chin Med J (Engl). 2013;126(1):140–146.
10. Chandra K, Blackhouse G, McCurdy B, et al. Cost-effectiveness of interventions for chronic obstructive pulmonary disease (COPD) using an Ontario policy model. Ont Health Technol Assess Ser. 2012;12(12):1–61.
11. Köhnlein T, Windisch W, Köhler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014;2(9):698–705.
12. Crimi C, Noto A, Princi P, et al. Domiciliary non-invasive ventilation in COPD: an international survey of indications and practices. COPD. 2016;13(4):483–490.
13. Windisch W, Storre JH, Köhnlein T. Nocturnal non-invasive positive pressure ventilation for COPD. Expert Rev Respir Med. 2015;9(3):295–308.
14. McEvoy RD, Pierce RJ, Hillman D, et al. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax. 2009;64(7):561–566.
15. Struik FM, Sprooten RT, Kerstjens HA, et al. Nocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure: a randomised, controlled, parallel-group study. Thorax. 2014;69(9):826–834.
16. Wild MR, Engleman HM, Douglas NJ, Espie CA. Can psychological factors help us to determine adherence to CPAP? A prospective study. Eur Respir J. 2004;24(3):461–465.
17. Díaz-Lobato S, Alises SM, Rodríguez EP. Current status of noninvasive ventilation in stable COPD patients. Int J Chron Obstruct Pulmon Dis. 2006;1(2):129–135.
18. Aloia MS, Stanchina M, Arnedt JT, Malhotra A, Millman RP. Treatment adherence and outcomes in flexible vs standard continuous positive airway pressure therapy. Chest. 2005;127(6):2085–2093.
19. Rabe KF, Hurd S, Anzueto A, et al. Zielinski J: global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555.
20. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586.
21. van der Molen T, Willemse BW, Schokker S, Ten Hacken NH, Postma DS, Juniper EF. Development, validity and responsiveness of the clinical COPD questionnaire. Health Qual Life Outcomes. 2003;1:13.
22. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77.
23. Cheung D, Klink HCJ, Aalbers R. for the OZON study group. Improved lung function and symptom control with formoterol on demand in asthma. Eur Respir J. 2006;27:504–510.
24. Wanger J, Clausen JL, Coates A, et al. Viegi G: standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–522.
25. Johansson H, Berterö C, Berg K, Jonasson -L-L. To live a life with COPD – the consequences of symptom burden. Int J Chron Obstruct Pulmon Dis. 2019;14:905–909.
26. Le LAK, Johannessen A, Hardie JA, et al. Prevalence and prognostic ability of the GOLD 2017 classification compared to the GOLD 2011 classification in a Norwegian COPD cohort. Int J Chron Obstruct Pulmon Dis. 2019;23(14):1639–1655.
27. Raveling T, Bladder G, Vonk JM, et al. Improvement in hypercapnia does not predict survival in COPD patients on chronic noninvasive ventilation. Int J Chron Obstruct Pulmon Dis. 2018;13:3625–3634.
28. Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax. 2010;65(4):303–308.
29. Ankjaergaard KL, Maibom SL, Wilcke JT. Long-term non-invasive ventilation reduces readmissions in COPD patients with two or more episodes of acute hypercapnic respiratory failure. Eur Clin Respir J. 2016;3:28303.
30. Murphy PB, Hart N. Home non-invasive ventilation for COPD: how, who and when? Arch Bronconeumol. 2018;54:149–154.
31. Wilson ME, Dobler CC, Morrow AS, et al. Association of home noninvasive positive pressure ventilation with clinical outcomes in chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2020;323:455–465.
32. Bräunlich J, Dellweg D, Bastian A, et al. Nasal high-flow versus noninvasive ventilation in patients with chronic hypercapnic COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:
33. Kongstad HK, Rosendal CAH, Rasmussen BS, Weinreich UM. Agreement between arterial and non-arterialised fingertip capillary blood gas and acid-base values. Eur Clin Respir J. 2019;6(1):1644892. doi:10.1080/20018525.2019.1644892
34. De Backer L, Vos W, Dieriks B, et al. The effects of long-term noninvasive ventilation in hypercapnic COPD patients: a randomized controlled pilot study. Int J Chron Obstruct Pulmon Dis. 2011;6:615–624.
35. Hajian B, De Backer J, Sneyers C, et al. Pathophysiological mechanism of long-term noninvasive ventilation in stable hypercapnic patients with COPD using functional respiratory imaging. Int J Chron Obstruct Pulmon Dis. 2017;12:2197–2205.
36. Diaz O, Begin P, Torrealba B, et al. Effects of noninvasive ventilation on lung hyperinflation in stable hypercapnic COPD. Eur Respir J. 2002;20:1490–1498.
37. Koopman M, Spruit MA, Franssen FME, et al. Effects of non-invasive ventilation combined with oxygen supplementation on exercise performance in COPD patients with static lung hyperinflation and exercise-induced oxygen desaturation: a single blind, randomized cross-over trial. J Clin Med. 2019;8(11):2012.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.