Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
The Effectiveness and Safety of Dupilumab for the Treatment of Recalcitrant Chronic Actinic Dermatitis: A Case Series
Authors Chen J, Yu N, Wu W, Ou S, Chen Q, Zhu H
Received 24 May 2023
Accepted for publication 29 July 2023
Published 29 August 2023 Volume 2023:16 Pages 2357—2363
DOI https://doi.org/10.2147/CCID.S422683
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Jiaoquan Chen,* Nanji Yu,* Weihong Wu, Shanshan Ou, Quan Chen, Huilan Zhu
Department of Dermatology, Guangzhou Institute of Dermatology, Guangzhou, 510095, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huilan Zhu, Department of Dermatology Guangzhou Institute of Dermatology, 56 Hengfu Road, Guangzhou, 510095, People’s Republic of China, Email [email protected]
Background: Although dupilumab is an effective treatment approach for chronic actinic dermatitis (CAD) in some cases, its effectiveness and safety in CAD have not been sufficiently assessed.
Purpose: Evaluation of the effectiveness and safety of dupilumab in patients with recalcitrant CAD was performed.
Methods: We retrospectively reviewed the medical records of CAD patients treated with dupilumab. Data regarding demographics were collected, and disease severity scores were assessed using the following: Clinical Severity Score of CAD (CSS-CAD), Atopic Dermatitis Control Tool (ADCT), Dermatology Life Quality Index (DLQI), and Numeric Rating Scale (NRS)-itch scores.
Results: After 12 weeks of treatment, there was a significant decrease in disease severity scores of 16 CAD patients. Only one patient achieved a good response and most of the patients (62.5%, 10/16) had no significant symptom improvement after 4 weeks of treatment. However, after 12 weeks of treatment, 43.75% (7/16) of the patients reached excellent response (> 75% improvement of CSS-CAD), 31.25% (5/16) good response (50%– 75% improvement of CSS-CAD), 6.25% (1/16) partial response (25%– 50% improvement of CSS-CAD), and only 18.75% (3/16) no response (< 25% improvement of CSS-CAD). One patient complained of injection site reaction at the first injection.
Conclusion: This study supports dupilumab as an effective and safe treatment option for patients with recalcitrant CAD. Patients may require at least 4 weeks of treatment before the partial response is noted.
Keywords: chronic actinic dermatitis, dupilumab, photodermatitis, atopic dermatitis
Introduction
Chronic actinic dermatitis (CAD) is a persistent and recurrent eczematous dermatitis that predominantly affects the photo-exposed areas, along with photosensitivity. Patients with CAD, are extremely sensitive to ultraviolet (UV) included UVA, UVB, or visible radiation, and even brief sunlight exposure triggers eczema,1 thus needing to avoid outdoor activities. In recalcitrant cases, conventional treatments based on photoprotection, topical corticosteroids, and immunosuppressive agents have been tried, and in most cases, their effectiveness has been disappointing.2,3 Dupilumab is an IgG human monoclonal antibody that binds to the IL-4 receptor and has a well-established role in atopic dermatitis (AD), which is not responding to conventional immunosuppressive therapy. Dupilumab has proved to be safe and effective in several real-life studies both on clinical and psychological aspects of AD.4–7 CAD is an immune-mediated photodermatosis and has been reported to have a similar Th2-mediated inflammatory pathogenesis to AD. According to previous reports,8–13 dupilumab prescribed for CAD has shown promising results in the disease. However, the evidence supporting its efficacy in patients with CAD comes from small case series and case reports. Therefore, we aimed to conduct an analysis of our experience with dupilumab to gain a better understanding of the efficacy and safety of this approach in patients with recalcitrant CAD.
Methods
After obtaining institutional ethics committee approved by the Ethical Committee of Guangzhou Institute of Dermatology (No: gzsp202267), the study was analyzed using electronic medical records of patients from the dates of January 1, 2021, to December 1, 2022. Patients who suffered from CAD satisfied the following criteria:14 eczematoid skin lesion on sun-exposed areas lasting more than 3 months; abnormal phototesting of reduced minimal erythema dose (MED) in UVB, UVA, or visible wavelength. The patients’ age, gender, duration of CAD, personal atopic history, phototest results, and previous treatment method were collected and analyzed. All patients with recalcitrant CAD had previously received at least one conventional systemic agent; however, disease control was inadequate. After discussion with the patients and obtaining informed consent, patients were started treated a loading dose of dupilumab (600 mg) at the first week, followed by a maintenance dose (300mg) every 2 weeks. During the treatment, patients were allowed to use antihistamines, hydroxychloroquine, cyclosporin A, thalidomide, topical corticosteroids (low to mid-potency: hydrocortisone acetate cream or triamcinolone acetonide acetate cream), and topical calcineurin inhibitors.
The clinical response to dupilumab was assessed in terms of Clinical Severity Score of CAD15 (CSS-CAD, 0–240), Atopic Dermatitis control tool (ADCT, 0–24), Dermatology Life Quality Index (DLQI, 0–30), and Numeric Rating Scale (NRS)-itch (0–10) scores by two dermatologists at baseline to those after 4 weeks, 8 weeks, 12 weeks, and 16 weeks of treatment. The clinical response of CAD was evaluated on 4 weeks, 8 weeks, 12 weeks, and 16 weeks based on the decrease of the CSS-CAD score, including excellent (>75% improvement), good (50%–75% improvement), partial (25%–50% improvement), and no response (<25% improvement). Adverse events during the treatment period were also noted. Continuous variables were given as mean ± standard error. Baseline characteristics were analyzed by t-test, and analysis of variance (ANOVA) using SPSS version 26.0, with a significance level of <0.05.
Results
Baseline demographics and clinical characteristics are summarized in Table 1. We included 16 patients (Male: Female 12:4) with mean age of 53.56 ± 9.84 years (range 34–72 years) who were treated off-label with dupilumab. These patients suffered a mean CAD disease course of 3.75 ± 2.86 years. Four (25%) patients exhibited a personal atopic history of allergies, including AD (3/16) and allergic rhinitis (1/16). Phototest of CAD patients revealed that 11 cases were sensitive to both UVA and UVB. In contrast, among the remaining five patients, one was only sensitive to UVA and the other four cases only had UVB sensitivity. All patients had received prior topical and systemic treatments for CAD, with seven (43.75%) patients having been treated with hydroxychloroquine, ten (62.5%) patients with antihistamine, four (25%) patients with systemic glucocorticoids, six (37.5%) patients with traditional Chinese medicine (TCM), four (25%) patients with cyclosporin A, two (12.5%) patients with thalidomide, one (6.25%) patient with methotrexate, and one (6.25%) patient with tripterygium wilfordii. All patients had been treated with low to high-potency topical corticosteroids and (or) topical calcineurin inhibitors (TCI). Among those receiving dupilumab, 16 patients continued to use topical or systemic medications at some point during their treatment duration. Out of the 16 patients receiving a concomitant therapy, 12 (75%) used antihistamine, seven (43.75%) used hydroxychloroquine, one (6.25%) received cyclosporin A, one (6.25%) received thalidomide. All other systemic treatments were gradually tapered and finally withdrawn after 4–16 weeks of dupilumab treatment.
 |
Table 1 Clinical Data of 16 CAD Cases |
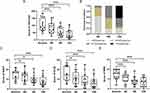
Sixteen patients completed the 12–16 weeks of treatment of dupilumab without interruption at our hospital. Figures 1 shows one of our patients before and 16 weeks after treatment with dupilumab. At baseline, the CSS-CAD score of the patients was 104.5 ± 42.05. Following the intervention, the CSS-CAD score revealed a progressive and significant reduction from baseline over the 12 weeks (40.75 ± 29.45, P< 0.001) of follow-up (Figure 2A). The CSS-CAD score analysis demonstrated that 31.25% (5/16) of the cases reached partial response, one patient obtained a good response, and 62.5% (10/16) received no response at week 4, 37.5% (6/16) of the cases reached partial response, 43.75% (7/16) obtained good response, and 18.75% (3/16) received no response at week 8, and 43.75% (7/16) of the patients reached excellent response, 31.25% good response (5/16), 6.25% partial response (1/16), and 18.75% (3/16) no response at week 12 (Figure 2B). Among the seven patients who completed the 16 weeks of treatment, one changed from no response to partial response, and two improved from good response to excellent response.
 |
Figure 1 Significant improvement with the use of dupilumab before (A) after administration for 4 weeks (B), 8 weeks (C), 12 weeks (D), and 16 weeks (E) in the same patient. |
Compared to baseline levels, the clinical indexes, ADCT, DLQI, and NRS were demonstrated rapid improvements in their self-reported following the administration of dupilumab at week 4, week 8, and week 12 (Figure 2C-E). At baseline, the ADCT score was 14.81 ± 4.9, while the DLQI score was 15.63 ± 7.38, and the NRS score was 7.19 ± 2.35 in this patient cohort, with these scores declining rapidly at week 12 to 8.06 ± 4.94, 7.19 ± 5.54, and 3.38 ± 2.26, respectively. Furthermore, at the week 16 visit, the patients demonstrated further improvements in the above clinical indexes. Overall, an adverse effect was reported by one of the patients, with the injection site reaction at the first injection and resolved within 3 hours after oral antihistamine.
Discussion
CAD is a chronic photosensitive disorder with erythematous, lichenified, papules, and plaques on sun-exposed areas. This retrospective study showed that CAD patients tend to afflict elder males disproportionately.16 The exact pathomechanism of CAD is not fully elucidated, so its therapeutic management remains very difficult and not standardized. However, some cases may have unsatisfactory control with conventional treatments, or their use may be precluded by adverse events, often leading to difficulties in treatment and recurrence or aggravation of the condition.3,17 This study showed that dupilumab is a relatively safe and effective treatment for recalcitrant CAD patients and results in significant improvement in the signs and symptoms and quality of life. After 12 weeks of treatment with dupilumab, the patients showed a significant decrease in their CSS-CAD scores compared to baseline, and 75% of the patients achieved a good-to-excellent response at week 12. There were also significant improvements in secondary outcome measures, such as ADCT, DLQI, and NRS scores compared to baseline. In the literature review, six studies8–13 includes 12 cases were treated with dupilumab after failing conventional treatments. All 12 patients experienced a partial-to-complete response after the treatment of dupilumab. These findings are in agreement with our study. Previous studies have reported that patients experienced significant improvement in their signs and symptoms within 4 weeks of initiating dupilumab treatment.8,12 However, in this retrospective investigation, noteworthy improvement of CSS-CAD, DLQI, and NRS was observed as early as week 4, while ADCT exhibited slightly delayed response. Additionally, at week 4, only a solitary patient achieved a good response; but with continuous treatment, the number of patients showing good-to-excellent responses significantly increased by week 8. These results suggest that patients with CAD may receive significantly improved symptoms after 4 weeks of dupilumab treatment.
Dupilumab is an IL-4 receptor alpha antagonist that blocks Th2 proinflammatory cytokines IL-4 and IL-13. Although previous reports8–13 and this study have found that dupilumab showed surprising efficacy in the treatment of CAD, it is challenging to speculate on the exact mechanism of dupilumab to improve the disease severity in patients with CAD. Ko et al found the role of Th1/Th2 misbalance in patients suffering severe CAD.15 Besides, UV irradiation may inhibit antigen presentation to Th1 cells but increase that to Th2 cells.18 It was reported that UV-induced CD3+, CD4+, and CD8+ cells increased in the more severe CSS-CAD subgroup and mediated their suppressive function by releasing immune regulatory cytokines such as IL-4 and IL-10.19 Therefore, it has been speculated that immune imbalance plays an important role in the pathogenesis of CAD. A retrospective study of CAD patients over a 5-year period found that 16 (21.6%) of CAD patients with an early age of onset had an atopic history.16 Furthermore, young AD patients suffering symptoms of CAD with photosensitive have also been reported before.20 In the current study, three patients had a history of AD and all received an excellent response after 12 weeks of treatment. The overlap and similar inflammatory nature of CAD and AD may provide a potential benefit for the direct efficacy of dupilumab on CAD. Interestingly, an excellent or good response was also seen in our patients (9/13, 69.23%) who did not have a history of AD. Besides, one changed from no response to partial response, and another improved from good response to excellent response after 16 weeks of treatment. These findings are in keeping with recently reviewed data demonstrating that dupilumab may be a viable treatment option for patients with refractory CAD regardless of a history of AD.8,9
In the literature review, adverse events occurred in 33.33% (4/12) of patients after treatment of dupilumab and presented by dupilumab facial redness and conjunctivitis.8–13 Only one of our patients experienced an injection site reaction at the first injection, and it was resolved within 3 hours after oral antihistamine.
In conclusion, based on the clinical improvement and absence of serious adverse events in our patients, these data support dupilumab as a valuable option for the treatment of resistant CAD. Our results also found that patients may require at least 4 weeks of treatment before a partial response is noted. Thus, clinical trials of dupilumab are indicated in CAD, given a sufficient number of patients available with this rare disorder. However, the mechanism of action of dupilumab in the treatment of CAD is unclear, and randomized controlled studies are required to draw any further conclusions.
Ethics Statement
The study protocol was approved by the Ethical Committee of Guangzhou Institute of Dermatology (No: gzsp202267). The patients in this manuscript have given written informed consent to publication of their case details.
Funding
The author(s) reported that there is no funding associated with the work featured in this article.
Disclosure
The authors declare no conflict of interest.
References
1. Tan KW, Haylett AK, Ling TC, et al. Comparison of demographic and photobiological features of chronic actinic dermatitis in patients with lighter vs darker skin types. JAMA Dermatol. 2017;153:427–435. doi:10.1001/jamadermatol.2016.5861
2. Agarwal P, Agarwal US, Meena RS, et al. A combination of oral azathioprine and methotrexate in difficult to treat dermatoses. Indian J Dermatol Ve. 2017;83:389–392.
3. Forsyth EL, Millard TP. Diagnosis and pharmacological treatment of chronic actinic dermatitis in the elderly: an update. Drug Aging. 2010;27(6):451–456. doi:10.2165/11315930-000000000-00000
4. Mastorino L, Viola R, Panzone M, et al. Dupilumab induces a rapid decrease of pruritus in adolescents: a pilot real-life study. Dermatol Ther. 2021;34(6):e15115. doi:10.1111/dth.15115
5. Mastorino L, Rosset F, Gelato F, et al. Chronic pruritus in atopic patients treated with dupilumab: real life response and related parameters in 354 patients. Pharmaceuticals-Base. 2022;15.
6. Mastorino L, Cantafio DV, Vecco C, et al. Impact of comorbidities in the response of atopic patients treated with dupilumab: a real-life study up to 36 weeks. J Eur Acad Dermatol. 2022;36(12):e1021–3. doi:10.1111/jdv.18427
7. Miniotti M, Ribero S, Mastorino L, et al. Long-term psychological outcome of patients with moderate-to-severe atopic dermatitis continuously treated with Dupilumab: data up to 3 years. Exp Dermatol. 2023;32(6):852–858. doi:10.1111/exd.14786
8. Ali K, Wu L, Lou H, et al. Clearance of chronic actinic dermatitis with dupilumab therapy in Chinese patients: a case series. Front Med-Lausanne. 2022;9:803692. doi:10.3389/fmed.2022.803692
9. McFeely O, Doyle C, Blasco MC, et al. Chronic actinic dermatitis successfully treated with methotrexate and dupilumab. Photodermatol Photo. 2022:57.
10. Chen JC, Lian CH. Chronic actinic dermatitis in an old adult significantly improved by dupilumab. Photodermatol Photo. 2022;38(2):176–177. doi:10.1111/phpp.12731
11. Chen J, Li H, Zhu H. Successful treatment of chronic actinic dermatitis with dupilumab: a case report and review of the literature. Clin Cosmet Inv Derm. 2021;14:1913–1917. doi:10.2147/CCID.S342401
12. Patel N, Konda S, Lim HW. Dupilumab for the treatment of chronic actinic dermatitis. Photodermatol Photo. 2020;36(5):398–400. doi:10.1111/phpp.12566
13. Verma L, Pratt M. A case report of therapeutically challenging chronic actinic dermatitis. Sage Open Med Case R. 2019;7:2050313X19845235.
14. Hawk JL. Chronic actinic dermatitis. Photodermatol Photo. 2004;20(6):312–314. doi:10.1111/j.1600-0781.2004.00129.x
15. Ko DY, Choi SH, Ha SM, et al. The clinical severity score of chronic actinic dermatitis correlates with in vivo photoallergic reactions and the immunologic parameters related to a shift towards Th2 immunity from the Th2/Th1 balanced status in patients with chronic actinic dermatitis. Photodermatol Photo. 2016;32(4):199–206. doi:10.1111/phpp.12244
16. Gu Q, Zhang Z, Yang J, et al. Chronic actinic dermatitis: a 5-year clinical analysis of 488 patients in China. Photodermatol Photo. 2022.
17. Safa G, Pieto-Le CC, Hervagault B. Recalcitrant chronic actinic dermatitis treated with low-dose thalidomide. J Am Acad Dermatol. 2005;52(5):E6. doi:10.1016/S0190-9622(03)01131-9
18. Ullrich SE. Does exposure to UV radiation induce a shift to a Th-2-like immune reaction? Photochem Photobiol. 1996;64(2):254–258. doi:10.1111/j.1751-1097.1996.tb02454.x
19. Kim JH, Kim HJ, Yoo DW, et al. A postulated model for photo immunopathogenesis of chronic actinic dermatitis around adaptive immunity, including Th17 cells, Tregs, TRMs, cytotoxic T cells, and/or common-gamma chain receptor+ cells. Photodermatol Photo. 2022.
20. Quatrano NA, Shvartsbeyn M, Meehan SA, et al. Chronic actinic dermatitis occurring in an adult with atopic dermatitis. Dermatol Online J. 2015;21.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.