Back to Journals » Therapeutics and Clinical Risk Management » Volume 18
The Effect of Trigeminal Ganglion Block on Trigeminocardiac Reflex in Elderly Patients with Trigeminal Neuralgia Undergoing Percutaneous Balloon Compression: A Randomized Controlled Study
Authors Zhang H, Liu M, Guo W, He J, Li J
Received 19 May 2022
Accepted for publication 18 November 2022
Published 9 December 2022 Volume 2022:18 Pages 1091—1098
DOI https://doi.org/10.2147/TCRM.S373370
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Deyun Wang
Huanhuan Zhang,1 Meinv Liu,1 Wenchang Guo,2 Jinhua He,1 Jianli Li1
1Department of Anesthesiology, Hebei General Hospital, Shijiazhuang City, Hebei Provence, People’s Republic of China; 2Department of Neurosurgery, Hebei General Hospital, Shijiazhuang City, Hebei Provence, People’s Republic of China
Correspondence: Jianli Li, Department of Anesthesiology, Hebei General Hospital, 348 Heping Road West, Shijiazhuang, Hebei Province, 050051, People’s Republic of China, Tel +86 311 8598 8447, Email [email protected]
Background: Trigeminal neuralgia (TN) is a facial neuropathic pain, which is defined as unilateral brief shock-like paroxysmal pain. Percutaneous balloon compression (PBC) was widely used to treat TN under general anesthesia. However, trigeminocardiac reflex (TCR) as a brainstem reflex can induce bradycardia or even cardiac arrest during PBC, which may result in catastrophic consequences in elderly patients. The aim of the present study was to investigate the effect of trigeminal ganglion block on TCR in elderly patients with TN during PBC.
Materials and Methods: Eighty-two elderly patients undergoing PBC were recruited to this randomized controlled study. The participants were randomly allocated to the control group (C group, n=41) and study group (S group, n=41). After anesthesia induction, 2% lidocaine 0.5 mL or an equal volume of normal saline was injected into Meckel’s cave. HR and MAP were recorded at seven time-points, and the TCR incidence was compared.
Results: HR decreased in the C group at the time of foramen ovale puncture (T5) and at the time of ganglion compression (T6) compared with that at the moment of needle puncture (T4) (P< 0.05), but almost no change in the S group. HR was lower in the C group compared with the S group at T5 and T6 (P< 0.05). MAP increased significantly at T5 and T6 compared with that at T4 in the C group (P< 0.05), but almost no increase in the S group. Compared with the C group, MAP was lower at T5 and T6 in the S group (P< 0.05). There were no significant differences in HR and MAP between the two groups at T1, T2, T3, T4 and T7. The incidence of bradycardia was higher in the C group than that in the S group (P< 0.05).
Conclusion: Trigeminal ganglion block was an effective approach to prevent TCR in elderly patients during PBC.
Keywords: trigeminal neuralgia, trigeminocardiac reflex, trigeminal ganglion block, elderly patients
Introduction
Trigeminal neuralgia (TN) is a severe unilateral brief facial pain, and the incidence is higher in elderly patients.1,2 TN can seriously affect the patient’s quality of life and may lead to suicide in severe cases.3,4 It is well known that sodium channel blockers, such as carbamazepine and oxcarbazepine, are advocated as the first-line treatment of TN.5 Surgical procedures should be considered when the medical treatment is ineffective or the adverse events become unacceptable.6 There are several percutaneous surgical options for TN, including glycerol blockade, balloon compression and radiofrequency thermocoagulation.7 Percutaneous balloon compression (PBC), firstly established by Mullan and Lichtor, was an effective and safe method to provide immediate therapeutic relief for patients with TN.8–10 It has been widely used in elderly patients due to the advantages of minimally invasive, short operation time and less complications. However, trigeminocardiac reflex (TCR) is common during PBC, which manifests as bradycardia, arrhythmia, asystole and increase in blood pressure.11 TCR often suddenly occurs especially at the moment of the needle punctures foramen ovale and the balloon compresses trigeminal ganglion, which can lead to cardiac arrest and death. Therefore, it is necessary to take effective measures to prevent TCR.
Numerous studies demonstrated that the sympathetic as well as parasympathetic nervous systems were involved in the TCR, which was triggered by stimulating the branches of trigeminal nerve.12 Nevertheless, the detailed pathophysiology mechanisms of TCR are still poorly understood.13 A recent study reported that pretreatment with atropine was effective to prevent the occurrence of TCR during PBC.14 However, the administration of anticholinergic agents sometimes can lead to cardiac arrhythmias or fail to terminate TCR.15,16 Currently, the use of anticholinergic drugs for minimizing TCR-related bradycardia remains disputable.17 Fortunately, nerve blocks were recommended as a simple and effective strategy to prevent TCR in some relevant studies.18,19 Otherwise, there was almost no randomized controlled research on this issue in elderly patients. This randomized controlled study aimed to investigate the effect of trigeminal ganglion block on TCR in elderly patients during PBC.
Materials and Methods
Patients and Setting
The study was approved by the ethics committee of the Hebei General Hospital (ethics approval no.2022–08) and registered in the Chinese Clinical Trial Registry (registration number ChiCTR2200057522) on March 14, 2022. The study was carried out in accordance with the ethical standards of the Declaration of Helsinki. Eighty-two patients with TN undergoing PBC were recruited to this study from March 1, 2022 to April 25, 2022, and all participants signed the informed consent. The surgeries were performed under general anesthesia in Digital Subtraction Angiography (DSA) complex operating room.
Inclusion criteria include classical trigeminal neuralgia, age ≥65, ASA physical status I to III, normal cardiopulmonary function. Exclusion criteria include secondary trigeminal neuralgia, bradycardia (HR <50 bpm), arrhythmia, drug allergy and overseas.
Anesthesia Management
Induction of anesthesia was achieved with etomidate 0.2 mg/kg, sufentanil 0.2 µg/kg and cisatracurium besilate 0.2 mg/kg in both groups. After endotracheal tube insertion, mechanical ventilation was performed with tidal volume of 6–8 mL/kg and ventilation frequency of 12–14 times/min. Then, anesthesia was maintained with propofol (2–6 mg/kg/h), remifentanil (0.1–0.15 µg/kg/min) and combined with 1% sevoflurane. Hemodynamic indexes were monitored continuously, and bispectral index (BIS) was kept between 40 and 60 during anesthesia. The endotracheal tube was removed at the conclusion of anesthesia. Then, the patients were transferred to the post-anesthesia care unit and returned to the wards when they were fully awake and Aldrete score was ≥9.
Surgery Procedure
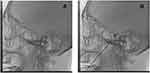
PBC and trigeminal ganglion block were performed by the same surgeon with Härtel’s technique.20 After anesthesia induction, the patients were positioned supine with the head in the natural median position. The same point on the skin was selected for both trigeminal ganglion block and operation. The needle entry point was selected at 2.5 cm outside the corner of the mouth on the side of the disease. Both trigeminal ganglion block and operation were performed under radiation. After anesthesia induction, a 22-gauge needle (0.7×90 mm) was inserted into the foramen ovale and the location of the needle tip was confirmed under radiation. When the needle reached its proper location, 2% lidocaine 0.5 mL or an equal volume of normal saline was injected into Meckel’s cave (Figure 1A). Then, the operation was performed after 3 minutes, the foramen ovale was punctured with a 14-gauge needle (2.1×150 mm) under the monitoring of the radiation. When the needle tip reaching the correct position, the needle core was withdrawn, and the 4# Fogarty balloon catheter was placed into the Meckel’s cave. Then, the balloon was inflated to compress the trigeminal ganglion for 1 to 2 min (Figure 1B). In the end, the emptied balloon and the puncture needle were withdrawn together when the operation finished.
Hemodynamic Management
In order to maintain hemodynamic stability during surgery, we used some vasoactive drugs as follows: (1) ephedrine 6 mg was given intravenously when the MAP decreased more than 20% of the baseline values; (2) nicardipine 0.2 mg was given intravenously when the MAP increased more than 20% of the baseline values; (3) esmolol 20 mg was injected intravenously if HR increased >100 bpm; (4) atropine 0.5 mg was administered intravenously if HR decreased <50 bpm. We injected isoproterenol intravenously when severe bradycardia occurred.
Randomization and Measurements
After inclusion in the study, patients were randomly divided into two groups at a ratio of 1:1 according to Microsoft Excel random number generator. HR and MAP were collected during the entire surgical procedure.
The data were obtained at seven time-points: 3 min after the patient entered the operating room (T1), 1 min after tracheal intubation (T2), during the trigeminal ganglion block (T3), at the moment of needle puncture (T4), at the time of foramen ovale puncture (T5), at the time of ganglion compression (T6) and at the moment of balloon release (T7).
Sample Size and Statistical Analysis
The sample size of our study was according to the data from a pilot study of patients undergoing PBC, which was calculated using G*power 3.1. A sample size of 41 patients in each group could detect a significance difference with an α level of 0.5 and a power of 80%. Considering for a dropout of 10%, we finally recruited 45 patients per group in this study.
IBM SPSS Statistics version 26.0 was used for statistical analysis. The Shapiro–Wilk test was used to determine whether variables were normally distributed. For variables normally distributed, continuous data were presented as mean ± standard deviation (SD). Student’s t-test was used in the comparisons of age, height and weight. Non-normal distribution data were presented as median (interquartile range, IQR) and were analyzed using the Wilcoxon test among the two groups. The values of HR and MAP at seven time-points among the two groups were assessed using one-way ANOVA. Categorical variables were expressed as numbers and percentages. The sex, laterality, ASA grade, and health status in two groups were compared using chi-square tests. The incidence of bradycardia between the two groups was analyzed using Fisher’s exact test. Two-sided P-value <0.05 was considered statistically significant difference.
Results
We recruited 90 elderly patients who were diagnosed with TN and underwent PBC between March 1, 2022 and April 25, 2022, 8 were excluded and 82 were included (Figure 2). The demographic characteristics of 82 participants are presented in Table 1. There were no differences in age (P=0.26), height (P=0.20), weight (P=0.26), sex (P=0.38), trigeminal neuralgia laterality (P=0.66), and ASA grade (P=0.39) between the two groups.
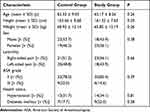 |
Table 1 Patients’ Characteristics of the Study |
 |
Figure 2 The study flow chart. |
HR and MAP of the two groups at the seven time-points in the study are presented in Figure 3. In the C group, HR was lower at T5 (56.07 ± 9.69 bpm) and T6 (55.80 ± 9.45 bpm) compared with that at T4 (67.22 ± 8.48 bpm; P<0.05). Whereas in the S group, there was no significant change in HR at T5 (65.73 ± 6.70 bpm) and T6 (65.76 ± 6.76 bpm) compared with T4 (65.80 ± 6.75 bpm; P>0.05). In addition, the changes of HR were not obvious at T3 (control: 66.18 ± 8.32 bpm; study: 64.39 ± 6.43 bpm) compared with T2 (control: 65.61 ± 8.10 bpm; study: 65.46 ± 7.33 bpm; P>0.05) in both groups. MAP was higher at T5 (98.00 ± 10.98 mmHg) and T6 (110.71 ± 6.60 mmHg) compared with T4 (83.88 ± 9.82 mmHg; P<0.05) in the C group, but there was no significant change at T5 (84.00 ± 11.05 mmHg) and T6 (83.39 ± 11.03 mmHg) compared with T4 (83.51 ± 9.66 mmHg; P>0.05) in the S group. Moreover, there was no significant difference in MAP at T3 (control: 83.76 ± 9.45 mmHg; study: 83.48 ± 9.03 mmHg) compared with T2 (control: 84.12 ± 12.63 mmHg; study: 84.88 ± 9.09 mmHg; P>0.05) in two groups.
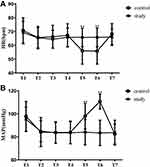 |
Figure 3 Changes in HR and MAP at seven time-points. (A) HR. (B) MAP. #P<0.05, study group compared with the control group. *P<0.05, T5 and T6 compared with T4. |
We further compared HR and MAP between the two groups at seven time-points. In the S group, HR was significantly higher compared with the C group at T5 (study, control: 65.73 ± 6.70, 56.07 ± 9.69 bpm) and T6 (study, control: 65.76 ± 6.76, 55.80 ± 9.45 bpm) (P< 0.05). Compared with the C group, MAP was significantly lower after nerve block with lidocaine at T5 (study, control: 84.00 ± 11.05, 98.00 ± 10.98 mmHg) and T6 (study, control: 83.39 ± 11.03, 110.71 ± 6.60 mmHg) (P< 0.05). There were no significant differences in HR and MAP between the two groups at T1, T2, T3, T4 and T7.
Seven of the 41 patients in the C group at T5 and T6 showed dramatic hemodynamic disturbances (Table 2). Puncturing the foramen ovale and compressing the ganglion respectively resulted in moderate bradycardia (HR <50 beats/min) in 4 patients (9.8%) and 4 patients (9.8%), and severe bradycardia (HR <40 beats/min) in 3 (7.3%) and 3 (7.3%). And 14 patients developed bradycardia (HR <50 beats/min) were administered with 0.5 mg atropine intravenously at T5 and T6. Moreover, we observed MAP significantly increased at T5 and T6 in most patients of the C group. MAP increased more than 20% from baseline during foramen ovale puncture and trigeminal ganglion compression in 19 patients and 23 patients, who were given nicardipine 0.2 mg intravenously.
 |
Table 2 The Incidence of Bradycardia in the Control Group and Study Group |
As shown in Figure 3, none of the patients developed bradycardia or an increase in MAP in the study group. Our study demonstrated that patients receiving trigeminal ganglion block showed stable hemodynamics.
Discussion
In our study, we observed that trigeminal ganglion block was an effective method to prevent the decrease in HR and the increase in MAP during the PBC, which was essential for elderly patients. And in the present study, the total incidence of TCR was 34.2% at the foramen ovale puncture and the ganglion compression.
PBC was recommended as the first-choice percutaneous procedure for the treatment of TN, and the efficacy and safety had been demonstrated.6,7,21 However, PBC-induced TCR has been reported in numerous studies,22 which is characterized by significant hemodynamic changes that may be fatal to elderly patients. The TCR is known to occur suddenly without any signal at the moment of puncturing the foramen ovale and compressing the trigeminal ganglion during PBC. In case of catastrophic consequences, it is crucial to take active measures to prevent the occurrence of TCR for anesthesiologists, especially in the elderly patients or patients with cardiovascular diseases.
Although PBC-induced TCR has been widely reported,14,15,23 the pathophysiology of the TCR has not been well elucidated. Some studies suggested that the stimulation of trigeminal nerve could activate both the sympathetic and the parasympathetic nerve systems, which might result in dramatic changes in hemodynamics.12,23 Severe hemodynamic disturbances are extremely dangerous for elderly patients, and it is a challenge for anesthesiologists to maintain hemodynamic stability in elderly patients during PBC. As a study reported, atropine was effective on reducing the incidence of bradycardia during PBC.14 However, some investigations showed that the TCR could not be fully prevented by atropine in patients.12,23 Recently, Chigurupati et al firstly reported that lidocaine was effective to prevent TCR by blunting the afferent arc in a patient undergoing microvascular decompression.24 Besides, another study from Tibano et al stated the effectiveness of trigeminal block on preventing TCR during PBC.25 However, data about the effect of trigeminal ganglion block on preventing TCR were lacking, especially for elderly patients undergoing PBC.
Local anesthetics were advocated as a strategy to prevent TCR by some researchers,24,25 which interrupted the propagation of nerve impulses by binding and inhibiting sodium channels.26 In our study, we performed trigeminal ganglion by injecting 2% lidocaine 0.5 mL into Meckel’s cave, and there was almost no change in HR and MAP at the moment of puncturing the foramen ovale and compressing the trigeminal ganglion. As the studies reported, smaller size of the needle and slighter stimuli intensity could decrease the incidence of TCR.27,28 A case report showed that a larger needle and more firm pressure might provoke TCR in a patient underwent extraction of a residual tooth root.27 Another case report stated that prolonged tension to nerves or their innervated structures could increase the risk of TCR.28 In our study, the magnitudes of the decrease in HR and the increase in MAP were slight during the trigeminal ganglion block with a 22-gauge needle in both groups, which might be due to the smaller needle size.
The total incidence of the TCR was 34.2% in the control group during PBC, which was inconsistent with other studies. A study showed that the incidence of the TCR was 10% during the treatment of trigeminal nerve.29 Another study reported that the prevalence of the TCR was 14.5% during neurointerventional procedures.30 Based on the above studies, the different incidence of TCR might be attributed to the type of the surgery, depth of anesthesia and participants’ factors.29,30 Although the exact incidence of the TCR remains uncertain, it is a common and severe complication that must be prevented especially in elderly patients.
Nonetheless, there are several limitations in this study. First, the sample size of the study was relatively small, so larger sample size is needed to validate the effect of trigeminal ganglion block on preventing TCR in elderly patients during PBC. Second, the observation time of this study was short, and the postoperative complications were not collected.
Conclusions
As a conclusion, trigeminal ganglion block with 2% lidocaine is effective to maintain hemodynamic stability in elderly patients during PBC. Thus, our study might provide a good solution for decreasing the incidence of TCR during PBC.
Data Sharing Statement
All the individual identifiable participant data generated during the present study will be available upon reasonable request from Hebei General Hospital. Email: [email protected]
Ethical Approval
The study has been approved by the ethics committee of the Hebei General Hospital (ethics approval no.2022–08) and registered in the Chinese Clinical Trial Registry (registration number ChiCTR2200057522).
Acknowledgments
The authors would like to thank Junfang Rong, MD, Tao Qian, MD and Xiujie Chang for their support of this study. The study was supported by the Key Research and Development Program of Hebei Province (19277714D).
Author Contributions
All authors made significant contributions to the design, acquisition or analysis of data; took part in writing the article or revising it critically for important content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cruccu G, Di Stefano G, Truini A. Trigeminal neuralgia. N Engl J Med. 2020;383(8):754–762. doi:10.1056/NEJMra1914484
2. Domages C, Brenet E, Labrousse M, et al. Efficacy and complications of microvascular decompression in patients over 70 years with trigeminal neuralgia. Acta Neurol Belg. 2022;122(3):615–623.
3. Freeman R, Edwards R, Baron R, et al. AAPT diagnostic criteria for peripheral neuropathic pain: focal and segmental disorders. J Pain. 2019;20(4):369–393.
4. Zakrzewska JM, Wu J, Mon-Williams M, et al. Evaluating the impact of trigeminal neuralgia. Pain. 2017;158(6):1166–1174.
5. Bendtsen L, Zakrzewska JM, Abbott J, et al. European Academy of Neurology guideline on trigeminal neuralgia. Eur J Neurol. 2019;26(6):831–849.
6. Noorani I, Lodge A, Vajramani G, et al. The effectiveness of percutaneous balloon compression, thermocoagulation, and glycerol rhizolysis for trigeminal neuralgia in multiple sclerosis. Neurosurgery. 2019;85(4):E684–E692.
7. Grewal SS, Kerezoudis P, Garcia O, et al. Results of percutaneous balloon compression in trigeminal pain syndromes. World Neurosurg. 2018;114:e892–e899.
8. Mullan S, Lichtor T. Percutaneous microcompression of the trigeminal ganglion for trigeminal neuralgia. J Neurosurg. 1983;59(6):1007–1012.
9. Jain A, Ibrahim B, Ali A, et al. Percutaneous balloon compression technique using intraoperative contrasted DynaCT for the treatment of refractory trigeminal neuralgia: initial experience. Neurosurg Rev. 2022;45(2):1393–1399.
10. Texakalidis P, Xenos D, Karras CL, et al. Percutaneous surgical approaches in multiple sclerosis-related trigeminal neuralgia: a systematic review and meta-analysis. World Neurosurg. 2021;146:342–350. doi:10.1016/j.wneu.2020.11.006
11. Meuwly C, Chowdhury T, Sandu N, et al. Definition and diagnosis of the trigeminocardiac reflex: a grounded theory approach for an update. Front Neurol. 2017;8:533. doi:10.3389/fneur.2017.00533
12. Meuwly C, Golanov E, Chowdhury T, et al. Trigeminal cardiac reflex: new thinking model about the definition based on a literature review. Medicine. 2015;94(5):e484. doi:10.1097/MD.0000000000000484
13. Leon-Ariza DS, Leon-Ariza JS, Gualdron MA, et al. Territorial and extraterritorial trigeminocardiac reflex: a review for the neurosurgeon and a type IV reflex vignette. Cureus. 2020;12(11):e11646.
14. Wang CM, Guan ZY, Zhao P, et al. The effect of atropine on trigeminocardiac reflex-induced hemodynamic changes during therapeutic compression of the trigeminal ganglion. J Neurosurg Anesthesiol. 2022;34(1):e40–e45.
15. Qin Q, Wang Y. Recurrent trigeminocardiac reflex in percutaneous balloon compression for trigeminal neuralgia: a case report. Medicine. 2020;99(44):e22467.
16. Schaller B, Cornelius JF, Prabhakar H, et al.; Trigemino-Cardiac Reflex Examination Group (TCREG). The trigemino-cardiac reflex: an update of the current knowledge. J Neurosurg Anesthesiol. 2009;21(3):187–195.
17. Borghei-Razavi H, Das P, Maurtua M, et al. Unusual appearance of trigemino-cardiac reflex during cerebellopontine angle surgery. World Neurosurg. 2018;112:298–299.
18. Schaller B, Probst R, Strebel S, et al. Trigeminocardiac reflex during surgery in the cerebellopontine angle. J Neurosurg. 1999;90(2):215–220.
19. Yoshida A, Seki T, Aratani Y, et al. Prevention of trigeminocardiac reflex-induced severe bradycardia during cerebral aneurysm clipping surgery by topical anesthesia of the dura surface and atropine administration: a case report. JA Clin Rep. 2022;8(1):2.
20. Härtel’s. Die Leitungsanästhesie und Injections-Behandlung des Ganglion Gasseri und der Trigeminusstämme [Conduction Anesthesia and Injection Treatment of the Ganglion Gasseri and the Trigeminal Strains]. Berlin: Springer; 1913.
21. Wang H, Chen C, Chen D, et al. Clinical analysis of the treatment of primary trigeminal neuralgia by percutaneous balloon compression. Front Surg. 2022;9:843982.
22. Meuwly C, Chowdhury T, Gelpi R, et al. The clinical surrogate definition of the trigeminocardiac reflex: development of an optimized model according to a PRISMA-compliant systematic review. Medicine. 2017;96(49):e9033.
23. Chen CY, Luo CF, Hsu YC, et al. Comparison of the effects of atropine and labetalol on trigeminocardiac reflex-induced hemodynamic alterations during percutaneous microballoon compression of the trigeminal ganglion. Acta Anaesthesiol Taiwan. 2012;50(4):153–158.
24. Chigurupati K, Vemuri NN, Velivela SR, et al. Topical lidocaine to suppress trigemino-cardiac reflex. Br J Anaesth. 2013;110(1):145.
25. Tibano AT, de Siqueira SR, da Nóbrega JC, et al. Cardiovascular response during trigeminal ganglion compression for trigeminal neuralgia according to the use of local anesthetics. Acta Neurochir. 2010;152(8):1347–1351.
26. Maniker RB, Damiano J, Ivie RMJ, et al. Perioperative breast analgesia: a systematic review of the evidence for perioperative analgesic medications. Curr Pain Headache Rep. 2022;26(4):299–321.
27. Sato K, Miyamae Y, Kan M, et al. Accelerated idioventricular rhythm following intraoral local anesthetic injection during general anesthesia. Anesth Prog. 2021;68(4):230–234.
28. Champion A, Masi J. Profound trigeminocardiac reflex from lingual nerve stimulation: a case report. J Dent Anesth Pain Med. 2022;22(1):61–65.
29. Leon-Ariza DS, Leon-Ariza JS, Nangiana J, et al. Evidences in neurological surgery and a cutting edge classification of the trigeminocardiac reflex: a systematic review. World Neurosurg. 2018;117:4–10.
30. Chowdhury T, Rizk AA, Azazi EA, et al. Brain and heart crosstalk during neurointerventional procedures: the role of the trigeminocardiac reflex: an updated systematic review. J Neurosurg Anesthesiol. 2022;34(3):282–287.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.