Back to Journals » Drug Design, Development and Therapy » Volume 18
The Effect of Platelet Activity, ABCB1 Genetic Polymorphism, and Renal Function on the Development of Ticagrelor-Related Dyspnea in Patients with Acute Coronary Syndrome
Authors Tamakauskas V, Žaliūnas R, Lesauskaitė V , Kupstytė-Krištaponė N, Čiapienė I, Šakalytė G, Plisienė J, Skipskis V, Tatarūnas V
Received 7 September 2023
Accepted for publication 21 December 2023
Published 23 January 2024 Volume 2024:18 Pages 109—119
DOI https://doi.org/10.2147/DDDT.S435477
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Vytenis Tamakauskas,1,2 Remigijus Žaliūnas,2 Vaiva Lesauskaitė,1 Nora Kupstytė-Krištaponė,1,2 Ieva Čiapienė,1 Gintarė Šakalytė,1,2 Jurgita Plisienė,2 Vilius Skipskis,1 Vacis Tatarūnas1
1Institute of Cardiology, Medical Academy, Lithuania University of Health Sciences, Kaunas, LT-50009, Lithuania; 2Department of Cardiology, Faculty of Medicine, Medical Academy, Lithuania University of Health Sciences, Kaunas, LT-50009, Lithuania
Correspondence: Vytenis Tamakauskas, Institute of Cardiology, Medical Academy, Lithuania University of Health Sciences, Sukilėlių g. 15, Kaunas, LT-50009, Lithuania, Tel/Fax +370 675 77 393, Email [email protected]
Purpose: The aim of this study was to determine the effect of ABCB1 genetic polymorphism and renal function on the occurrence of ticagrelor-related dyspnea.
Patients and Methods: A total of 299 patients with acute with type 1, 2, or 3 myocardial infarction (with and without ST-segment elevation), who underwent coronary angiography and PTCA with stent implantation and were treated with antiplatelet drugs (ticagrelor and aspirin), were enrolled in this prospective study. For all enrolled patient’s platelet aggregation (induction with high-sensitivity adenosine diphosphate, ADP HS) testing was performed using a MULTIPLATE® analyzer. Venous blood was also collected for genotyping.
Results: Patients experiencing ticagrelor-related dyspnea had lower ADP HS value (ADP HS ≤ 19.5 U; OR = 2.254; P = 0.009), higher creatinine concentration (> 90 μmol/l; OR = 3.414; P = 0.019), and lower GFR value (< 60 mL/min/1.73 m2; OR = 2.211; P = 0.035). ABCB1 T allele was associated with ticagrelor-related dyspnea (OR = 2.550; P = 0.04).
Conclusion: Ticagrelor-related dyspnea was found to be related to low platelet aggregation, increased plasma creatinine concentration, decreased GFR, and ABCB1 T allele. Carriers of the ABCB1 T allele had a higher plasma creatinine concentration that could be associated with an inhibitory effect of ticagrelor on P-glycoprotein function.
Keywords: ticagrelor, ticagrelor pharmacogenomics, ticagrelor related dyspnea, ABCB1 gene polymorphisms, platelet aggregation
Introduction
Ticagrelor in combination with aspirin is a first-line therapy in patients with acute coronary syndrome (ACS) who underwent percutaneous transluminal coronary angioplasty (PTCA) with stent implantation.1–4 The most recent guidelines by the European Society of Cardiology (ESC) and the American Heart Association (AHA) recommend this dual antiplatelet therapy as a first-line therapy for patients with ACS irrespective of initial treatment strategy.1,2,4 New data suggest that Ticagrelor in combination with aspirin provide more favorable outcomes not only for ACS patients who underwent PTCA with stent implantation, but also for secondary stroke prevention in patients with vascular risk factors.5
Ticagrelor, a cyclopentyltriazolo-pyrimidine (CPTP), is a direct-acting, reversible P2Y12 receptor antagonist. The drug inhibits the binding of endogenous adenosine diphosphate (ADP) to platelet membrane-bound P2Y12 receptors and blocks platelet aggregation. On average, maximum plasma concentration of ticagrelor is reached within 1.3–2 h, and the mean elimination half-life is 7–12 h.6,7 Studies involving animal and healthy volunteers have shown that urinary and fecal excretion of ticagrelor accounts for 26.5% and 57.8%, respectively, on the average.8,9 In the liver, ticagrelor is extensively metabolized into at least 10 metabolites that are detected in plasma, feces, and urine. Ticagrelor and its active metabolite M8 (AR-C124910XX) have been detected as major circulating components in plasma and feces, while M5 (AR-C133913XX) and M4 have been found to be major ticagrelor metabolites in urine. Administration of ticagrelor at therapeutic doses (90 mg of ticagrelor b.i.d.) results in clinically insignificant concentrations of other drug metabolites (M1, M2, and M7), which can be identified only at maximum tolerated dose or higher (900 and 1200 mg of ticagrelor).8,9
Treatment with ticagrelor faces numerous clinical challenges that make safe and continuous drug use difficult during the whole treatment period. Treatment with ticagrelor is most commonly discontinued due to ticagrelor-related adverse events by substituting it with less effective and genetic polymorphism-dependent dual therapy with clopidogrel and aspirin.10,11 Premature ticagrelor discontinuation increases the risk of stent thrombosis and recurrent ACS, leading to longer hospital stay and worse patient’s clinical outcomes.12 Dyspnea and bleeding are adverse events associated with ticagrelor usage. Different studies report that dyspnea of various intensities manifests in up to 38.6% of patients treated with ticagrelor.12,13 Premature discontinuation of ticagrelor usage occurs in up to 6.5% of patients experiencing dyspnea of moderate and more severe intensity.12,14 Such premature ticagrelor discontinuation increases the risk of stent thrombosis and recurrent ACS, leading to longer hospital stay and worse patient’s clinical outcomes.12
Up to date, literature data on the impact of genetic polymorphisms on ticagrelor antiplatelet effects and occurrence of adverse events are contradictory. Some researchers claim that the polymorphisms of CYP2C19, ABCB1, CYP3A4, and other genes do not have any impact on the pharmacodynamic effects of ticagrelor.15–17 Studies on healthy volunteers have shown that ABCB1 genetic polymorphism is not related to ticagrelor efficacy.7 Meanwhile, other studies have reported a possible impact of the CYP2C19 genetic polymorphism on the metabolism of ticagrelor or its active metabolite M8.18 The most recent data on experiments with the human umbilical vein endothelial and hepatocellular carcinoma HepG2 cell lines provide evidence that ticagrelor can have an effect on the expression of CYP4F2 and CYP4A11 gene.19
It has been reported that ticagrelor usage could lead to an increase in the levels of creatinine, but it is not known how this could affect the occurrence of ticagrelor-related adverse events.20 Recent literature suggests that the ABCB1 genetic polymorphism is associated with renal function and has an effect on the properties of certain drugs.21,22 In our previous study, involving 277 patients with ACS, we determined platelet aggregation values that could predict the development of ticagrelor-related dyspnea,23 however we did not analyze the impact of renal function in patients with dyspnea induced by ticagrelor therapy. The aim of this study was to determine the effect of ABCB1 genetic polymorphism and renal function on the occurrence of ticagrelor-related dyspnea. In this study, we used data from patients from our previous study,23 by complementing them with data from additional 22 patients.
Materials and Methods
Patients and Investigations
A total of 967 patients according to the “Fourth Universal Definition of Myocardial Infarction”24 with acute myocardial infarction type 1, 2, or 3 (with and without ST-segment elevation) were treated at the Cardiovascular Centre of the Šiauliai Republican Hospital from January 2020 to September 2021. During this period, 299 patients were eligible to be enrolled in the prospective study based on the inclusion criteria.23 Figure 1 depicts the study flow chart.
 |
Figure 1 Flow chart of the study. |
The inclusion and exclusion criteria of the study are shown in Table 1.
 |
Table 1 Inclusion and Exclusion Criteria of the Study |
During the treatment period, patients underwent routine examinations based on the ESC guidelines for the management and treatment of ACS.1,3,4 All patients received a loading dose of ticagrelor (180 mg) regardless of initial treatment, followed by a maintenance dose of 90 mg twice daily for 12 months. All patients received a loading dose of aspirin (300 mg) regardless of initial treatment, followed by a maintenance dose of 100 mg once daily. The severity of dyspnea was rated from 0 to 4 scores by using the modified Borg acute dyspnea scale, which was used in early clinical trials of ticagrelor.25 All enrolled patients 24–36 h after admission were divided into 2 groups considering the absence or presence of dyspnea, which was considered as ticagrelor related after the evaluation and exclusion of other possible causes for dyspnea occurrence (Table 1).23 Characteristics of the study population and the results of clinical investigations are shown in Table 2. GFR was calculated using the Modification of Diet in Renal Disease (MDRD) equation and was expressed in mL/min/1.73 m2.26
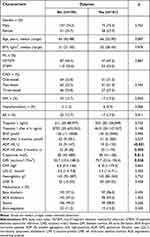 |
Table 2 Characteristics of the Study Population |
On the enrollment into the study and after 3 months, the flowing additional investigations were done:23
- Venous blood was drawn for genotyping that was carried out at the Laboratory of Molecular Cardiology, Institute of Cardiology, Medical Academy, Lithuanian University of Health Sciences. DNA was extracted from blood by a salting-out method. The determination of genetic variants was performed using TaqMan molecular markers (Thermo Fisher Scientific, USA), TaqMan Universal Master Mix (Thermo Fisher Scientific, USA), and PCR grade water. The QuantStudio 5 and 3 Real-Time PCR systems (Thermo Fisher Scientific, USA) were used. A total of 12 genetic variants – FGB C148T (rs1800787), CYP4F2 (rs3093135, rs1558139, rs2108622, and rs2074902) and CYP2C19 *2 and*17 (rs4244285 and rs12248560), CYP2C9 *15 (rs72558190), ABCB1 (rs1045642), COX-2 (rs689465), PAI-1 (rs5918), CYP1A2*1C (rs2069514) – were analyzed;
- Platelet aggregation (induction with high-sensitivity adenosine diphosphate, ADP HS) testing was performed by using a MULTIPLATE® analyzer and reagents for the determination of P2Y12 receptor activity.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics 22 software. Variants of the genes analyzed are given in percentage. Continuous data are expressed as medians. Categorical and nominal data were compared with the chi-square test (Pearson criterion); when the frequency in at least one cell of a contingency table was small (<5), the Fisher’s exact test was used. Two data proportions were compared with the z-test. To compare two independent samples, the Mann–Whitney U-test was used. Binary logistic regression analysis (BACKWARD STEPWISE (Wald) and ENTER approaches) was carried out to determine risk factors associated with the development of dyspnea. To identify the variables as significant, cutoff points of p<0.25 were used for the multivariate analysis. The variables from the univariate analysis were included in the multivariate analysis model. The level of significance was set at p < 0.05. An ADP HS value that predicts dyspnea was determined by applying the ROC curve analysis. This method was used to determine a platelet aggregation threshold that predicts the development of dyspnea by calculating and assessing the maximum values of the Youden index.
Results
Characteristics of the Study Population
In this study, there were more men than women (n = 220; 74.2%). MI with ST-segment elevation was more common than MI without ST-segment elevation among patients (n = 163; 54.9%). All the patients had hypertension and dyslipidemia. Conventional treatment with beta blockers, angiotensin-converting enzyme inhibitors, statins, and antiplatelet drugs was administered. Diabetes mellitus (CD) was diagnosed in 14.1% of patients, and they received metformin most frequently. Hypothyroidism was recorded in 3% of patients. Patients with atrial fibrillation (AF) (n = 42; 14.1%) were given a combination of ticagrelor and low-dose (110 mg × 2/d) dabigatran etexilate; aspirin for these patients was prescribed only during hospitalization (6 days on average). The enrolled patients most frequently had advanced coronary lesions, ie two- or three-vessel coronary artery disease (66.5%). The value of ≤19.5 U ADP HS was documented in 99 patients (33.1%).
During the study, 101 (33.8%) patients experienced ticagrelor-related dyspnea; 198 patients did not complain about dyspnea. Fifteen patients (5%) had insignificant bleeding from the digestive tract confirmed by esophagogastroduodenoscopy, but there was no need to discontinue treatment with antiplatelet drugs. Bleeding was not associated with ADP HS, dyspnea, or renal function.
Higher creatinine concentrations and lower glomerular filtration rates (GFR) were documented more frequently in patients experiencing dyspnea.
Associations Between Clinical and Genetic Variables and Ticagrelor-Related Dyspnea
Ticagrelor-related dyspnea was not associated with patients’ gender, age, body mass index (BMI), ACS type, extent of CAD, comorbidities, administered medications, and heart failure criteria (BNP, LVEF) (Table 2).
Dyspnea experienced by patients was associated with platelet aggregation (ADP HS) both during the first evaluation and after 3 months as well as with renal function (Table 2). Patients who experienced dyspnea had a lower ADP HS value both during the first evaluation (P < 0.001) and after 3 months (P = 0.005). In the group of patients with ticagrelor-related dyspnea, a greater creatinine concentration (P = 0.012) and a lower GFR were documented (P = 0.028).
An ADP HS cutoff value of ≤19.5 U was found to be associated with the development of dyspnea (n = 299; p < 0.001; AUC = 0.710; 95% CI: 0.646–0.774; sensitivity of 57.0%; specificity of 78.6%; Youden J index = 0.356) (Figure 2).
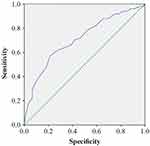 |
Figure 2 ROC curve analysis of ADP HS for the prediction of dyspnea. |
The ABCB1 (rs1045642) gene polymorphism was determined in 184 patients. Carriers of the T allele (heterozygous, ie CT, or homozygous, ie TT) had a higher creatinine concentration than those homozygous for the C allele (CC) (P = 0.007) (Table 3).
 |
Table 3 Associations Between Gene Polymorphisms and Renal Function |
Logistic Regression Analysis Model for the Evaluation of Clinical and Genetic Risk Factors for the Development of Dyspnea
Univariate analysis showed that patients with hypothyroidism were at a fourfold greater risk of dyspnea (OR = 4.084, P = 0.04); however, multivariate analysis did not confirm this association.
In multivariate logistic regression analysis, the following risk factors were found to be associated with a greater risk of dyspnea: an ADP HS value of ≤19.5 U (OR = 2.254; P = 0.009), GFR of <60 mL/min/1.73 m2 (OR = 2.211; P = 0.035), creatinine concentration of >90 mol/L (OR = 3.414; P = 0.019), and ABCB1 T allele (CT and TT) (OR = 2.550; P = 0.04) (Table 4).
 |
Table 4 Risk Factors for the Development of Dyspnea |
Other clinical and genetic factors did not have any impact on the development of ticagrelor-related dyspnea.
Discussion
The aim of this study was to evaluate the effect of the ABCB1 rs1045642 genetic polymorphism and renal function on the development of ticagrelor-related dyspnea. The results of our study show significant interactions between the development of ticagrelor-related dyspnea and the ABCB1 genetic polymorphism as well as renal function. Ticagrelor-related dyspnea was associated with lower platelet aggregation value, lower GFR, higher creatinine concentration, and ABCB1 T allele (CT and TT).
Up to date, research on the impact of the ABCB1 rs1045642 genetic polymorphism in patients using P2Y12 receptor inhibitors has not confirmed any significant relationship between the pharmacodynamic or pharmacokinetic effects of ticagrelor and polymorphisms of this or other genes.15–17 Literature data on the ABCB1 rs1045442 genetic polymorphism are mostly linked to a reduced activity of transmembrane P-glycoprotein (P-gp) and worse renal function, and this in turn can have an impact on the elimination of ticagrelor and its active metabolites and can increase drug antiplatelet activity as well as the frequency of ticagrelor-related adverse events.21,22,27
It is known that P-gp is encoded by the ABCB1 gene; therefore, the polymorphisms of this gene can influence P-gp expression and properties. The physiological role of P-gp is to protect cells from toxic substances and metabolites by controlling their uptake to a cell and elimination from it. P-gp is linked to resistance to multiple drugs for cancer treatment and immunosuppression after renal transplantation; therefore, it has been extensively studied recently. P-gp is abundant in the epithelial tissues of various organs (kidney, intestine, etc.) and endothelia of the blood–brain barrier. P-gp is also associated with the nephrotoxicity of particular drugs after renal transplantation.28–30 The uptake of substances into cells of renal and other tissues and at the blood–brain barrier is P-gp dependent; therefore, high P-gp levels can limit the uptake of sufficient amounts of a used drug into cells, thus preventing adequate therapeutic effectiveness. Meanwhile, reduced P-gp activity can contribute to diminished elimination of a drug and its metabolites from the cells of renal, neural, or other tissues and with increasing concentrations of a drug and its metabolites, various drug-related adverse events can occur.30
Up to 26.5% of ticagrelor and its active metabolite M5 (AR-C133913XX) is excreted via urine.8,9 It has been reported that serum levels of creatinine can increase during treatment with ticagrelor. This increase could probably be linked to ticagrelor-dependent changes in adenosine levels, but more extensive studies have not been performed.20,31–33 Moreover, evidence suggests that ticagrelor possesses the characteristics of P-gp inhibitor and, therefore, can inhibit P-gp activity.34–36 The results of this study showed that an increased creatinine concentration (>90 mol/L) and a lower GFR (<60 mL/min/1.73 m2) were associated with 3.41- and 2.21-fold, respectively, greater risk of ticagrelor-related dyspnea. An increased creatinine concentration and a reduced GFR might be related not only to ABCB1 genetic polymorphism-dependent inhibition of P-gp activity but also to the inhibitory effect of ticagrelor itself on P-gp. This hypothesis is partly confirmed by the results of other studies showing that the ABCB1 genetic polymorphism can affect P-gp activity,22,37 which, in turn, can have an impact on the activity of ticagrelor and its active metabolites and thus increase the occurrence of drug-related adverse events.
Yan et al reported that the ABCB1 CC genotype had an influence on early renal function recovery in kidney transplant patients treated with tacrolimus.38 Meanwhile, other authors explored associations between ABCB1 genetic polymorphism and hypertension-induced target organ damage and found that hypertensive patients with the ABCB1 TT genotype were at a higher risk of renal function injury than those with the CC genotype.21 Liu et al also concluded that the ABCB1 TT genotype was associated with a lower GFR as compared with the CC genotype and might therefore confer susceptibility to nephropathy.39 Wallentin et al investigated the effects of CYP2C19 and ABCB1 genetic polymorphisms on the outcomes of treatment with ticagrelor and did not find any impact of these genetic polymorphisms on bleeding or frequency of recurrent ACS.15 Meanwhile, the results of our study showed that the carriers of ABCB1 T allele (CT or TT) were at a 2.55-fold greater risk of the development of ticagrelor-related dyspnea. As mentioned earlier, patients with the ABCB1 T allele had a greater creatinine concentration and a lower GFR that could also be linked to the inhibitory effect of ticagrelor on P-gp function. Other authors also found that the T allele of the ABCB1 gene was related to worse renal function.21,22 In addition, research on animals suggested that the ABCB1 TT genotype could be associated with lower P-gp expression and thus indirectly with a reduced elimination of a drug or its metabolites from cells.21,27
Our previous study showed that patients with ticagrelor-related dyspnea had lower platelet aggregation values, possibly indicating higher plasma concentrations of ticagrelor and its active metabolites.23 In this study, involving a larger sample size, we also observed that ticagrelor-related dyspnea was associated with lower values of platelet aggregation. A platelet aggregation (ADP HS) value of ≤19.5 U increased the risk of dyspnea by 2.25 times.
In order to clarify the effect of renal function and ABCB1 genetic polymorphism on the development of ticagrelor-related dyspnea, larger scale studies employing genotyping and investigations of ticagrelor metabolites in urine are needed.
Conclusion
Ticagrelor-related dyspnea was found to be related to low platelet aggregation (ADP HS ≤ 19.5 U), increased plasma creatinine concentration, decreased GFR, and ABCB1 T allele. Carriers of the ABCB1 T allele had a higher plasma creatinine concentration that could be associated with an inhibitory effect of ticagrelor on P-gp function.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by Kaunas Regional Biomedical Research Ethics Committee (permission No. P1-BE-2-19/2019, dated 10 June 2019).
Data Sharing Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions and data protection policies.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Funding
This research received no external funding.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Collet JP. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. In: European Heart Journal. Vol. 42. Oxford University Press;2021:1289–1367. doi:10.1093/eurheartj/ehaa575
2. Lawton JS, Lawton JS, Tamis-Holland JE, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines.. J Am Coll Cardiol. 2022;79(2):e21–e129. doi:10.1016/j.jacc.2021.09.006
3. Ibanez B. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. In: European Heart Journal. Vol. 39. Oxford University Press;2018:119–177. doi:10.1093/eurheartj/ehx393
4. Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes.. Eur Heart J. 2023;44(38):3720–3826. doi:10.1093/eurheartj/ehad191
5. Bálint A, Tornyos D, El Alaoui El Abdallaoui O, Kupó P, Komócsi A. Network meta-analysis of ticagrelor for stroke prevention in patients at high risk for cardiovascular or cerebrovascular events. Stroke. 2021;52(9):2809–2816. doi:10.1161/STROKEAHA.120.032670
6. Nawarskas JJ, Clark SM. Ticagrelor: a novel reversible oral antiplatelet agent. Cardiol Rev. 2011;19(2):95–100. doi:10.1097/CRD.0b013e3182099d86
7. Teng R. Ticagrelor: pharmacokinetic, pharmacodynamic and pharmacogenetic profile: an update. In: Clinical Pharmacokinetics. Vol. 54. Springer International Publishing;2015:1125–1138. doi:10.1007/s40262-015-0290-2
8. Li Y, Landqvist C, Grimm SW. Disposition and metabolism of ticagrelor, a novel P2Y 12 receptor antagonist, in mice, rats, and marmosets. Drug Metab Dispos. 2011;39(9):1555–1567. doi:10.1124/dmd.111.039669
9. Teng R, Oliver S, Hayes MA, Butler K. Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos. 2010;38(9):1514–1521. doi:10.1124/dmd.110.032250
10. Wang X, Xi S, Liu J, et al. Switching between ticagrelor and clopidogrel in patients who underwent percutaneous coronary intervention: insight into contemporary practice in Chinese patients. Euro Heart J Suppl. 2016;18:F19–F26. doi:10.1093/eurheartj/suw034
11. Cakal S, Cakal B, Güven Z, et al. Switching ticagrelor to 600 mg or 300 mg clopidogrel loading bridge in patients with unstable angina. J Clin Med. 2021;10(11). doi:10.3390/jcm10112463
12. Arora S. Premature Ticagrelor Discontinuation in Secondary Prevention of Atherosclerotic CVD: JACC Review Topic of the Week. J Am Colle Cardiol. 2019;73(19):2454–2464. doi:10.1016/j.jacc.2019.03.470
13. Storey RF. Incidence of dyspnea and assessment of cardiac and pulmonary function in patients with stable coronary artery disease receiving ticagrelor, clopidogrel, or placebo in the ONSET/OFFSET study. J Am Coll Cardiol. 2010;56(3):185–193. doi:10.1016/j.jacc.2010.01.062
14. Wang Y. Ticagrelor versus Clopidogrel in CYP2C19 Loss-of-Function Carriers with Stroke or TIA. N Engl J Med. 2021;385(27):2520–2530. doi:10.1056/nejmoa2111749
15. Armstrong M. Articles Eff ect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–1328. doi:10.1016/S0140
16. Máchal J. CYP2C19 and CYP3A4 activity and ADP-induced platelet reactivity in prasugrel- or ticagrelor-treated STEMI patients: monocentric study in Prague-18 trial participants. Xenobiotica. 2020;50(8):929–938. doi:10.1080/00498254.2020.1731625
17. Dong P, Yang X, Bian S. Genetic polymorphism of CYP2C19 and inhibitory effects of ticagrelor and clopidogrel towards post-percutaneous coronary intervention (PCI) platelet aggregation in patients with acute coronary syndromes. Med Sci Monit. 2016;22:4929–4936. doi:10.12659/MSM.902120
18. Zhu Q. Pharmacokinetic and pharmacogenetic factors contributing to platelet function recovery after single dose of ticagrelor in healthy subjects. Front Pharmacol. 2019;10. doi:10.3389/fphar.2019.00209
19. Meskauskaite U, Andruskeviciute S, Ciapiene I, Giedraitiene A, Lesauskaite V, Tatarunas V. Pleiotropic effects of ticagrelor: influence on CYP4F2 gene and protein expression in HUVEC and HepG2, and Escherichia coli bacterial survival. Drug Des Devel Ther. 2022;16:2559–2568. doi:10.2147/DDDT.S357985
20. Wallentin L. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N Engl J Med. 2009;361(11):1045–1057. doi:10.1056/nejmoa0904327
21. Chen X, Zhou T, Yang D, Lu J. Association between ABCB1 gene polymorphism and renal function in patients with hypertension: a case-control study. Med Sci Monit. 2017;23:3854–3860. doi:10.12659/MSM.902954
22. Bochud M. Association of ABCB1genetic variants with renal function in Africans and in Caucasians. BMC Med Genomics. 2008;1(1). doi:10.1186/1755-8794-1-21
23. Tamakauskas V. Factors determining ticagrelor-induced dyspnea in patients with acute coronary syndrome. Appl Sci. 2022;12(19). doi:10.3390/app121910021
24. Thygesen K, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40(3):237–269. doi:10.1093/eurheartj/ehy462
25. Wittfeldt A. Ticagrelor enhances adenosine-induced coronary vasodilatory responses in humans. J Am Coll Cardiol. 2013;61(7):723–727. doi:10.1016/j.jacc.2012.11.032
26. Kallner A, Khatami Z. How does the MDRD Study equation compare with serum creatinine in routine healthcare? Anatomy of MDRD-eGFR. Scand J Clin Lab Invest. 2008;68(sup241):39–45. doi:10.1080/00365510802144789
27. Uhr M, Holsboer F, Mü MB, Penetration of endogenous steroid hormones corticosterone, cortisol, aldosterone and progesterone into the brain is enhanced in mice deficient for both mdr1a and mdr1b P-glycoproteins.
28. Foote CJ. MDR1 C3435T polymorphisms correlate with cyclosporine levels in de novo renal recipients. Transplant Proc. 2006;38(9):2847–2849. doi:10.1016/j.transproceed.2006.08.120
29. Hauser IA, Koziolek M, Hopfer U, Thévenod F. Therapeutic concentrations of cyclosporine A, but not FK506, increase P- glycoprotein expression in endothelial and renal tubule cells. Kidney Int. 1998;54(4):1139–1149. doi:10.1046/j.1523-1755.1998.00095.x
30. Hoffmeyer S. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo; 1999. Available from: www.pnas.org.
31. Alsharif KF. Ticagrelor potentiates adenosine-induced stimulation of neutrophil chemotaxis and phagocytosis. Vascul Pharmacol. 2015;71:201–207. doi:10.1016/j.vph.2015.02.006
32. Li X, Wang Q, Xue Y, Chen J, Lv Q. Ticagrelor compared with clopidogrel increased adenosine and cyclic adenosine monophosphate plasma concentration in acute coronary syndrome patients. Basic Clin Pharmacol Toxicol. 2017;120(6):610–614. doi:10.1111/bcpt.12752
33. van Vuren AJ, de Jong B, Bootsma HPR. Ticagrelor-induced renal failure leading to statin-induced rhabdomyolysis. Neth J Med. 2015;2015:4.
34. Official reprint from UpToDate®. Inhibitors and inducers of P-glycoprotein (P-gp) drug efflux pump (P-gp multidrug resistance transporter); 2022. Available from: https://www.uptodate.com/.
35. Horn JR, Hansten PD. Ticagrelor Interactions. 2018.
36. Wessler JD, Grip LT, Mendell J, Giugliano RP. The P-glycoprotein transport system and cardiovascular drugs. J Am Colle Cardiol. 2013;61(25):2495–2502. doi:10.1016/j.jacc.2013.02.058
37. Taubert D. Impact of P-glycoprotein on clopidogrel absorption. Clin Pharmacol Ther. 2006;80(5):486–501. doi:10.1016/j.clpt.2006.07.007
38. Yan L, Li Y, Tang JT, An YF, Wang LL, Shi YY. Donor ABCB1 3435 C>T genetic polymorphisms influence early renal function in kidney transplant recipients treated with tacrolimus. Pharmacogenomics. 2016;17(3):249–257. doi:10.2217/pgs.15.165
39. Liu M. A functional common polymorphism of the ABCB1 gene is associated with chronic kidney disease and hypertension in Chinese. Am J Hypertens. 2013;26(12):1428–1436. doi:10.1093/ajh/hpt126
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
