Back to Journals » Vascular Health and Risk Management » Volume 18
The Effect of Low Preoperative Ejection Fraction on Mortality After Cardiac Surgery in Indonesia
Authors Kurniawaty J , Setianto BY, Supomo, Widyastuti Y , Boom CE
Received 1 December 2021
Accepted for publication 10 March 2022
Published 24 March 2022 Volume 2022:18 Pages 131—137
DOI https://doi.org/10.2147/VHRM.S350671
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Pietro Scicchitano
Juni Kurniawaty,1 Budi Yuli Setianto,1 Supomo,1 Yunita Widyastuti,1 Cindy E Boom2
1Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia; 2Harapan Kita National Heart Center, Jakarta, Indonesia
Correspondence: Juni Kurniawaty, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia, Tel +62 274 514495, Email [email protected]
Background: Among cardiac surgery patients, low preoperative left ventricular ejection fraction (LVEF) is common and has been associated with poor outcomes. The objective of this study was to assess the association between LVEF and postoperative mortality in patients undergoing open-heart surgery in several hospitals in Indonesia.
Methods: We conducted a multicenter study with the retrospective design using data from patients undergoing open-heart surgery in 4 institutions in Indonesia. Data regarding LVEF and other potential risk factors were extracted from medical records and compiled in one datasheet. Statistical analyses were performed to assess if low LVEF was associated with postoperative mortality and identify other potential risk factors.
Results: A total of 4789 patients underwent cardiac surgery in participating centers during the study period. Of these, 189 subjects (3.9%) had poor preoperative LVEF. Poor LVEF was associated with postoperative mortality (adjusted OR 2.761, 95% CI 1.763– 4.323, p < 0.001). Based on types of surgery, LVEF had a significant association with mortality only in CABG patients, while there was no such association in valve surgery and inconclusive in congenital surgery patients. Other significant independent predictors of in-hospital mortality included age more than 65 years old, non-elective surgery, the complexity of procedures, history of cardiac surgery, organ failure, CARE score ≥ 3, NYHA class ≥ III, and poor right ventricular function.
Conclusion: Patients with low preoperative LVEF undergoing open-heart surgery had a higher risk of postoperative mortality. Cardiac surgery can be performed with acceptable mortality rates. Accurate selection of patients, risk/benefit evaluation, and planning of surgical and anesthesiological management are mandatory to improve outcomes.
Keywords: open cardiac surgery, left ventricular ejection fraction, mortality, retrospective study
Introduction
Left Ventricular Ejection Fraction (LVEF) indicates the efficiency of the ventricle and is regarded as an optimal marker of LV function. LVEF has been considered as among the strongest predictors of clinical outcomes after cardiac surgery.1,2 A low EF has been suggested as a critical predictor of poor outcome and included in all currently available scoring systems. Indeed, low EF has been associated with postoperative complications, such as Low Cardiac Output Syndrome (LCOS), need for inotropic support, acute renal failure, respiratory failure, pneumonia, stroke, sepsis, deep sternal wound infection, and bleeding.3–7
Left ventricular ejection fraction can be measured with different modalities. Cardiac magnetic resonance (CMR) has been considered the gold standard for LVEF calculation.8 However, most of the hospitals in Indonesia lack this technology. On the other hand, transthoracic echocardiography (TTE) is one of the most common procedures performed for LVEF measurement as part of the pre-operative evaluation. TTE is more affordable and provides immediate results. However, there is a concern of TTE-derived LVEF accuracy, and it may differ from LVEF measured from other modalities.9,10
Identifying patients who are at risk of poor outcomes are critical in the decision-making process. Several perioperative variables have been suggested as potential predictors of mortality, for example, acute renal failure, diabetes mellitus, chronic obstructive pulmonary disease. Scoring systems based on these risk factors have been applied in clinical practice to identify patients with high mortality risk. Cardiac surgery procedures have experienced significant advancement that leads to better surgical outcomes and lower postoperative mortality rates.11,12 The improvement in cardiac surgery may render available scoring systems obsolete and previously established predictors of postoperative mortality not applicable. Improvement in anesthesia, surgical techniques, and perioperative management resulted in better safety and outcome among cardiac surgery patients. Cardiac surgery outcomes improved in the past few years, and the current scoring systems are prone to overestimate poor outcomes.12 Khaled et al13 reported that CABG patients with low LVEF had acceptable mortality of 5.4%. Another study by Elassy et al14 also found that LVEF < 35% was not a significant predictor of mortality. These suggested improvements in cardiac surgery patients; thus, the role of LVEF in predicting postoperative mortality should be re-evaluated.
We performed a multicenter study to assess the association between low LVEF and postoperative mortality among open-heart surgery patients. We also identify potential risk factors of mortality in these patients.
Methods
We retrospectively reviewed medical records of adult patients who underwent cardiac surgery procedures at participating institutions; Sardjito general hospital, Yogyakarta; Karyadi general hospital, Semarang; Abdul Wahab Sjahranie general hospital, Samarinda; and Harapan Kita national heart center, Jakarta. We did not seek written consent for this retrospective observational study since all patients’ data were anonymized and de-identified before analysis.
Open cardiac surgery patients between 2016–2020 at the aforementioned hospitals were identified and had their medical records reviewed. Patients who had an aortic dissection, as well as heart transplant patients, were excluded from the study. Data were collected by trained chart reviewers using specialized forms. We extracted the following variables from medical records: demographic and preoperative variables including age, sex, previous cardiac surgery, dyslipidemia, hypertension, chronic obstructive lung disease (COPD), organ failure, angina, prior myocardial infarction (MI), active endocarditis, CARE score, pulmonary hypertension, NYHA class, right ventricular function, underweight, diabetes, renal failure, preoperative arrhythmias; surgical factors including the priority of surgery (elective, urgent), off-pump technique, the weight of procedures. We used the Canadian Cardiovascular Society (CCS) and New York Heart Association (NYHA) classification systems to identify angina and CHF status of patients, respectively. Preoperative echocardiography was performed in all patients.
LVEF was derived from transthoracic echocardiography (TTE) results. All patients in the study had standard TTE as part of pre-operative evaluation. LVEF was calculated using Simpson’s formula and LVEF < 30% was considered as poor in accordance with EuroSCORE II classification. Outcome for the current study was postoperative mortality, defined as any death event that occurred within 30 days after procedure or until discharge, whichever is sooner.
Data were stored in a database and analyzed using SPSS ver 26 (IBM Corp., Armonk, NY). Categorical variables are presented as numbers (percent), whereas numerical variables are presented as mean ± standard deviation (SD) or as median (interquartile range). We performed univariate analysis using independent Student’s t-test or Mann Whitney U-test when appropriate for numerical data; or χ2 test for categorical data. Variables with P < 0.25 in univariate analysis were considered for inclusion in the multivariate analysis. Logistic regression was performed to identify independent predictors of mortality. We considered variables with p-values of < 0.05 as significant with 95% confidence intervals.
Ethical Clearance
The study received ethical clearance from the Medical and Health Research Ethics Committee of Universitas Gadjah Mada (ref no KE/FK/1277/EC/2019), as well as approval from participating centers. Informed consent waiver was approved by the ethics committee due to its retrospective design based on patient records. We performed the study in accordance with the Declaration of Helsinki.
Results
Baseline Characteristics
Out of 4789 patients who underwent cardiac surgery in participating centers during the study period, 189 subjects (3.9%) had poor preoperative LVEF, defined as LVEF < 30%, with a mean LVEF of 58.33±13.43 and range 14–90%. The mean age of our patients was 50.35±13.49 years. A large proportion of our subjects was male (3014 patients, 62.9%). Most of the subjects had a CARE score of 2 (3533 patients, 73.8%). Half of the subjects had NYHA class II (2401 patients, 50.1%). CABG procedure was the most common procedure performed in the study, in which 2042 subjects (42.6%) had CABG-only procedures. Demographic characteristics of the subject population are presented in Table 1.
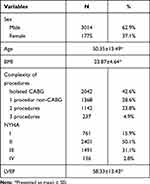 |
Table 1 Demographic Data |
From all cardiac surgery patients in the study, 298 subjects died during the postoperative period, translated into a postoperative mortality rate of 6.2%. Bivariate analysis using the chi-square test found that poor LVEF was associated with mortality. Only 3.7% (167 subjects) of those who survived had poor LVEF, compared to 9% (27 subjects) in subjects who dead (unadjusted OR 2.538, 95% CI 1.648–3.908, p < 0.001). Other preoperative variables associated with postoperative mortality were: age > 65 years, non-elective surgery, more complex procedures, CPB, previous cardiac surgery, organ failure, infective endocarditis, higher CARE score, pulmonary hypertension, NYHA class ≥ III, and right ventricular function (Table 2).
 |
Table 2 Bivariate Analysis |
We performed a subgroup analysis to assess the association between LVEF and mortality according to the type of surgery. We analyzed subjects who only had one procedure, either CABG, valve, or congenital surgery. Results suggested that LVEF ≤ 30% had a significant association with mortality only in CABG patients. In these patients, LVEF ≤ 30% was associated with a threefold higher risk for mortality (OR 3.165, 95% CI 1.765–5.666, p <0.001). However, we did not find similar results for valve surgery patients. As for patients in the congenital surgery group, we could not draw a definitive conclusion since only one patient in this group had poor LVEF (Table 3).
 |
Table 3 Association Between Poor LVEF and Outcome Based on Type of Surgery |
We performed a logistic regression as multivariate analysis to identify variables that are independently associated with postoperative mortality. Poor LVEF had a significant and independent association with postoperative mortality. Patients with poor LVEF have almost three times higher risk for postoperative mortality (adjusted OR 2.761, 95% CI 1.763–4.323, p < 0.001). Other significant independent predictors of in-hospital mortality included age more than 65 years old, non-elective surgery, the complexity of procedures, history of cardiac surgery, organ failure, CARE score ≥ 3, NYHA class ≥ III, and poor right ventricular function. Results of multivariate analysis is presented in Table 4.
 |
Table 4 Multivariate Analysis |
Discussion
LVEF is the percentage of blood volume ejected during each cardiac cycle.15 It reflects the efficiency of the ventricle in emptying itself.1 LVEF has been considered a better measurement of LV function than stroke volume. The study confirmed that poor LVEF remains a challenge in cardiac surgery, even after significant improvements in the field of cardiac surgery. While we found poor LVEF in only 3.9% of the subjects in our study, it had a significant association with mortality. However, looking deeper into specific types of surgery, LVEF only showed a significant association with mortality in patients who only had CABG surgery. We did not find a significant association between LVEF and mortality in valve surgery patients, and we could not draw a definitive conclusion of the association in congenital surgery patients. There are only a few studies investigating the effect of LVEF in valve replacement surgery. Previous studies found a significant association between lower LVEF and mortality in valve surgery patients.16–18 However, only 28 (2.8%) of valve surgery patients in the current study had poor LVEF. This relatively small number of valve surgery patients with poor LVEF may affect the results. However, our results emphasized the possibility of the different impacts of LVEF between different types of surgery and should be further studied, especially in valve surgery patients.
Low EF is an independent risk factor for postoperative mortality. Christakis et al19 reported a mortality rate of 9.8% in patients with EF < 20%, and another study by Carr et al20 found a mortality rate of 11% in patients with EF between 10–20%. Di Carli et al reported a 30-day postoperative mortality rate of 9.3% in patients with EF < 40%.21 However, a more recent report suggested a lower postoperative mortality rate. A review of 55,515 patients form a New York State Database who underwent CABG procedures between 1997 and 1999 reported a mortality rate of 4.6% in patients with EF ≤ 20%.3 In another study, immediate mortality in patients with EF < 35% was 10.5%.22 A study that included patients with valve disease reported a mortality rate of 5.6% in patients with LVEF ≤ 40%.7 The decrease in mortality over time represents a significant improvement in the surgical outcome of patients with low EF. We could not determine if there is a similar trend in our study since we did not have mortality data from an older database as a baseline.
Other risk factors of postoperative mortality in our study were age > 65 years, non-elective surgery, the complexity of procedures, history of cardiac surgery, organ failure, CARE score ≥ 3, NYHA class ≥ III, and poor right ventricular function. Mahesh et al23 reported a postoperative mortality rate of 2.9%. Predictors of mortality in their study were age ≥ 70 years, female [OR = 1.97 (95% CI: 1.3–3.1); P = 0.003], hypertension [OR = 1.6 (95% CI: 1–2.6); P = 0.046], LVEF < 50%, neurological dysfunction [OR = 3.7 (95% CI: 1.7–7.9); P = 0.001], previous cardiac surgery [OR = 3.1 (95% CI: 1.7–5.6); P < 0.001], emergency procedure [OR = 2.9 (95% CI: 1.2–6.8); P = 0.017], and 3 surgical procedures [OR = 4.4 (95% CI: 1.6–12.2); P = 0.005]. Age was an important determinant of postoperative mortality in their study. Using age 50–59 years as reference, OR of mortality for age group of 70–79 years was 5 (95% CI: 1.7–15, P = 0.004), and for age group ≥80 years was 10.9 (95% CI: 3.4–34, P < 0.001). Advanced left ventricular dysfunction was a predictor of poor outcome, with moderate, poor, and very poor left ventricular function having 2, 5, and 10 times higher risk, respectively, than those with normal LV function.
The study showed the importance of preoperative LVEF measurement in cardiac surgery patients. LVEF is considered a better measurement than stroke volume as a marker of LV function. However, it may not appropriately reflect the status of the circulation in certain situations.2 LVEF has several limitations, most importantly it has been associated with data variability and poor reproducibility. EF results varied among different imaging techniques. LVEF is also dependent on heart rate, thus atrial fibrillation and rapid ventricular response may lead to artificially low LVEF.24
The study has several limitations that should be considered. First, due to its retrospective design, we could not control how LVEF was measured. Different techniques and operators’ skills may influence the result and classification of LVEF. Second, we could not exclude the effect of other potential variables that were not included in the analysis.
Conclusion
Moderate to severe left ventricular dysfunctions are common in the general cardiac surgery population. Patients with a poor LVEF undergoing cardiac surgery have a high risk of postoperative complications and mortality, but surgery can proceed with a relatively lower mortality rate. A significant association between poor LVEF and mortality was found in CABG patients, while valve surgery and congenital surgery still need further studies. Preoperative evaluation and accurate risk stratification are essential in cardiac surgery and careful perioperative management is mandatory.
Acknowledgments
The authors would like to thank dr. Teddy Ferdinand, SpAn, KAKV, dr. Heru Dwijatmiko, SpAn, KAKV, and dr. Widya Istanto, SpAn, KAKV for their contribution in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Arroyo JP, Schweickert AJ. Fluid movement in the body. In: Back to Basics in Physiology. Elsevier; 2013:43–66. doi:10.1016/B978-0-12-407168-1.00003-4
2. Marwick TH. Ejection fraction pros and cons. J Am Coll Cardiol. 2018;72(19):2360–2379. doi:10.1016/j.jacc.2018.08.2162
3. Topkara VK, Cheema FH, Kesavaramanujam S, et al. Coronary artery bypass grafting in patients with low ejection fraction. Circulation. 2005;112(9_supplement). doi:10.1161/CIRCULATIONAHA.104.526277
4. Mariscalco G, Biancari F, Zanobini M, et al. Bedside tool for predicting the risk of postoperative atrial fibrillation after cardiac surgery: the POAF score. JAHA. 2014;3(2). doi:10.1161/JAHA.113.000752
5. Allou N, Bronchard R, Guglielminotti J, et al. Risk factors for postoperative pneumonia after cardiac surgery and development of a preoperative risk score. Crit Care Med. 2014;42(5):1150–1156. doi:10.1097/CCM.0000000000000143
6. Bove T, Calabrò MG, Landoni G, et al. The incidence and risk of acute renal failure after cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18(4):442–445. doi:10.1053/j.jvca.2004.05.021
7. Pieri M, Belletti A, Monaco F, et al. Outcome of cardiac surgery in patients with low preoperative ejection fraction. BMC Anesthesiol. 2016;16(1):97. doi:10.1186/s12871-016-0271-5
8. Hundley WG, Bluemke DA, Finn JP, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on expert consensus documents. Circulation. 2010;121(22):2462–2508. doi:10.1161/CIR.0b013e3181d44a8f
9. Habash-Bseiso DE, Rokey R, Berger CJ, Weier AW, Chyou PH. Accuracy of noninvasive ejection fraction measurement in a large community-based clinic. Clin Med Res. 2005;3(2):75–82. doi:10.3121/cmr.3.2.75
10. Pellikka PA, She L, Holly TA, et al. Variability in ejection fraction measured by echocardiography, gated single-photon emission computed tomography, and cardiac magnetic resonance in patients with coronary artery disease and left ventricular dysfunction. JAMA Netw Open. 2018;1(4):e181456. doi:10.1001/jamanetworkopen.2018.1456
11. ElBardissi AW, Aranki SF, Sheng S, O’Brien SM, Greenberg CC, Gammie JS. Trends in isolated coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons adult cardiac surgery database. J Thorac Cardiovasc Surg. 2012;143(2):273–281. doi:10.1016/j.jtcvs.2011.10.029
12. Hickey GL, Grant SW, Murphy GJ, et al. Dynamic trends in cardiac surgery: why the logistic EuroSCORE is no longer suitable for contemporary cardiac surgery and implications for future risk models. Eur J Cardio Thorac Surg. 2013;43(6):1146–1152. doi:10.1093/ejcts/ezs584
13. Khaled S, Kasem E, Fadel A, et al. Left ventricular function outcome after coronary artery bypass grafting, King Abdullah Medical City (KAMC)- single-center experience. Egypt Heart J. 2019;71(1):2. doi:10.1186/s43044-019-0002-6
14. Elassy S, El-Bawab H, Abd El-Fatah M. Early outcome of coronary artery bypass surgery in patients with poor left ventricular function. J Egypt Soc Cardiothorac Surg. 2012;20(3–4):125–131.
15. Bamira D, Picard MH. Imaging: echocardiology—assessment of cardiac structure and function. In: Encyclopedia of Cardiovascular Research and Medicine. Elsevier; 2018:35–54. doi:10.1016/B978-0-12-809657-4.10953-6
16. Halkos ME, Chen EP, Sarin EL, et al. Aortic valve replacement for aortic stenosis in patients with left ventricular dysfunction. Ann Thorac Surg. 2009;88(3):746–751. doi:10.1016/j.athoracsur.2009.05.078
17. Murashita T, Schaff HV, Suri RM, et al. Impact of left ventricular systolic function on outcome of correction of chronic severe aortic valve regurgitation: implications for timing of surgical intervention. Ann Thorac Surg. 2017;103(4):1222–1228. doi:10.1016/j.athoracsur.2016.09.004
18. Bohbot Y, de Meester de Ravenstein C, Chadha G, et al. Relationship between left ventricular ejection fraction and mortality in asymptomatic and minimally symptomatic patients with severe aortic stenosis. JACC Cardiovasc Imaging. 2019;12(1):38–48. doi:10.1016/j.jcmg.2018.07.029
19. Christakis GT, Weisel RD, Fremes SE, et al. Coronary artery bypass grafting in patients with poor ventricular function. Cardiovascular Surgeons of the University of Toronto. J Thorac Cardiovasc Surg. 1992;103(6):
20. Carr JA, Haithcock BE, Paone G, Bernabei AF, Silverman NA. Long-term outcome after coronary artery bypass grafting in patients with severe left ventricular dysfunction. Ann Thorac Surg. 2002;74(5):1531–1536. doi:10.1016/S0003-4975(02)03944-9
21. Di Carli MF, Maddahi J, Rokhsar S, et al. Long-term survival of patients with coronary artery disease and left ventricular dysfunction: implications for the role of myocardial viability assessment in management decisions. J Thorac Cardiovasc Surg. 1998;116(6):997–1004. doi:10.1016/S0022-5223(98)70052-2
22. Soliman Hamad MA, van Straten AH, Schönberger JP, et al. Preoperative ejection fraction as a predictor of survival after coronary artery bypass grafting: comparison with a matched general population. J Cardiothorac Surg. 2010;5(1):29. doi:10.1186/1749-8090-5-29
23. Mahesh B, Peddaayyavarla P, Ong LP, Gardiner S, Nashef SAM. Cardiac surgery improves survival in advanced left ventricular dysfunction: multivariate analysis of a consecutive series of 4491 patients over an 18-year period. Eur J Cardiothorac Surg. 2016;50(5):857–866. doi:10.1093/ejcts/ezw134
24. Lupón J, Bayés-Genís A. Left ventricular ejection fraction in heart failure: a clinician’s perspective about a dynamic and imperfect parameter, though still convenient and a cornerstone for patient classification and management: editorial. Eur J Heart Fail. 2018;20(3):433–435. doi:10.1002/ejhf.1116
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
