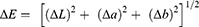Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 14
The Effect of Loading Time on Color Stability of Various Restorative Materials Bonded to Silver Diamine Fluoride-Treated Demineralized Dentin
Authors Aldosari MM, Al-Sehaibany FS
Received 8 March 2022
Accepted for publication 4 May 2022
Published 16 May 2022 Volume 2022:14 Pages 123—130
DOI https://doi.org/10.2147/CCIDE.S365478
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Christopher E. Okunseri
Mohammed M Aldosari, Fares S Al-Sehaibany
Department of Pediatric Dentistry and Orthodontics, College of Dentistry, King Saud University, Riyadh, Saudi Arabia
Correspondence: Fares S Al-Sehaibany, Department of Pediatric Dentistry and Orthodontics, College of Dentistry, King Saud University, P.O. Box 60169, Riyadh, 11545, Saudi Arabia, Email [email protected]
Aim: To investigate the effect of immediate versus one week later loading time on color stability of resin-based composite (RBC), resin-modified glass ionomer cement (RMGIC) and glass ionomer cement (GIC) restorative materials bonded to silver diamine fluoride (SDF)-treated demineralized dentin.
Materials and Methods: Ninety extracted premolars were randomly assigned to the following groups (n = 30 each): group-I (loaded with RBCs), group-II (loaded with RMGIC), and group-III (loaded with GIC). Each group was divided randomly into the following subgroups (n = 10): subgroup-A (control specimens) consisted of sound dentin and the restorative material was immediately loaded; subgroup-B consisted of demineralized dentin, underwent a SDF treatment and the restorative material was immediately loaded; subgroup-C consisted of demineralized dentin, underwent a SDF treatment and the restorative material was loaded 1 week later. The color difference (ΔE) and visual color changes (L*a*b*) of each specimen were calculated using a spectrophotometer. Data were analyzed using one-way ANOVA and post hoc Tukey’s tests to compare the ΔE values and a paired t test to compare the mean of L* a* b* coordinates.
Results: The highest color stability was observed for GIC, followed by RMGIC, and the lowest color stability was observed for the RBC group. Significant differences within groups were noted in RBC and RMGIC (p < 0.001). Regarding the loading time of the restorative material, in RBCs, a significantly lower ΔE value was observed in the delayed loading group than in the immediate loading group (p = 0.035). In the RMGIC and GIC groups, there were no significant differences.
Conclusion: Delaying the loading time of the restorative material for 1 week following the application of SDF resulted in greater color stability than that of immediate loading. The caries arresting potential of SDF was revealed by the dark staining, which could be improved with the subsequent delayed restoration.
Keywords: silver diamine fluoride, restorative materials, color stability
Introduction
Over the last century, the significance of preserving natural tooth structure and biological adaptation to dental caries management has led to the evolution and recognition of dental treatments that involve maximum interception and are minimally invasive.1 One such approach is the phenomenon of arresting dental caries with silver diamine fluoride (SDF) by utilizing its profound bactericidal property, which was first introduced by Nishino et al in 1969.2 The use of SDF in arresting dental caries, particularly in primary dentition3–5 and root surface caries in permanent dentition6–10 has shown promising results in several randomized clinical trials and systematic reviews. Recent advancements with the use of SDF along with the atraumatic restorative treatment or Hall technique, also known as silver-modified atraumatic restorative treatment (SMART) restoration, stops the progression of dental caries due to the sealed carious lesion.11,12
One of the greatest drawbacks of the clinical application of SDF is the discoloration it imparts to the arrested carious lesion.13 The formation of various silver compounds, such as silver phosphate precipitate, silver chloride and silver thiocyanate, following SDF application has been examined as the cause of discoloration.14 Clinicians consider this treatment not so widely accepted by parents and patients for aesthetic reasons.13 However, it has been proposed that educating clinicians of its use and gaining informed consent from parents by showing photographs of the resultant staining, particularly for younger children and preferably in posterior teeth, may be beneficial.13 Moreover, during the COVID-19 pandemic, it may become standard to use SDF applications, as they provide an important means of minimum intervention dentistry and a nonaerosol-producing adjunct to caries management.15
The effectiveness of topical SDF application has been attributed to the ability of the silver component to result in dentin sclerosis, the silver nitrate component that has germicidal properties and the well-established remineralizing potential of fluoride.14,16 As SDF has been advocated to be an effective cariostatic agent and diagnostic indicator17 that is cost-effective and simple to use, many researchers have tried to explore ways to minimize discoloration. The use of various concentrations of potassium iodide following SDF application showed a reduction in black staining in several studies.18–20 However, the application of potassium iodide with SDF has been reported to reduce the anticaries effect of SDF.17,20
The best approach of caries management is to identify risk factors in the early stages and stabilize the oral environment, allowing remineralization, followed by placement of minimal permanent restorations, if required as a result of cavitation.21 Arresting and reversing an initial carious lesion along with adequate alterations to lifestyle and behavioral risk factors should be the most prudent treatment option before committing to restorative procedures.1 Eliminating the cavitation by placing restorations not only removes the protected environment for the plaque to thrive but also restores tooth function and helps the patient achieve adequate plaque control.22
In aesthetic dentistry, the color stability of tooth-colored dental restorations is one of the most important clinical parameters for understanding the durability of the treatment.23 A clear-cut value of ΔE < 3.3 is considered to be accepted as color stability in clinical situations. This means that any increase in the ΔE value greater than 3.3 can be described as less color stability.24 In an attempt to mask, the permanent staining caused by SDF applications, various tooth-colored restorative materials and their time of loading following SDF application should be further explored, as they offer alternative options. The primary aim of the present study was to investigate the effect of an immediate loading time versus loading one week later on the color stability of resin-based composite (RBC), resin-modified glass ionomer cement (RMGIC) and glass ionomer cement (GIC) restorative materials bonded to SDF-treated demineralized dentin. The null hypothesis tested was that SDF treated demineralized dentin did not affect color stability of the restorative materials with regard to the loading time.
Materials and Methods
This study was approved by the Institutional Review Board and College of Dentistry Research Center (PR 0109) at King Saud University on July 2020 and was conducted from December 2020 to November 2021.
The sample of this in vitro study consisted of ninety extracted sound (caries-free) premolar teeth, which were extracted for orthodontic reasons and were collected from dental clinics in Riyadh, Saudi Arabia. Parental informed consent was obtained prior to premolar teeth extraction. The inclusion criteria of the study were sound (caries-free) teeth. The exclusion criteria were teeth with carious lesions or restorations. The teeth were cleaned under tap water, placed in fresh 0.5% chloramine-T and stored at 4–7 °C until use. A simple random sampling technique was used to assign the teeth randomly into three groups (n=30) as follows: Group I was loaded with RBC (Neo Spectra ST LV; Dentsply Sirona, Pennsylvania, USA), group II was loaded with RMGIC restorative material (Fuji II LC CAPSULE; GCC, Tokyo, Japan) and group III was loaded with GIC restorative material (Fuji IX; GCC, Tokyo, Japan). The shade of the three restorative materials was A1.
Each group was divided randomly into three subgroups (n=10) as follows: subgroup A (control specimens), which consisted of sound dentin and the restorative material was immediate loaded; subgroup B, which consisted of demineralized dentin, involved a SDF treatment and the restorative material was immediately loaded (immediate); and subgroup C, which consisted of demineralized dentin, involved a SDF treatment and the restorative material was loaded 1 week later (delayed).
The occlusal enamel of the teeth was removed using a slow speed cutting machine (IsoMet; Buehler, Illinois, USA). The dentin surface of the specimens was assessed using a stereomicroscope (SM80; Swift microscope, California, USA) to ensure that the enamel was completely removed. The specimens in the control subgroup for the three groups were loaded immediately with RBC, RMGIC and GIC restorative materials. The specimens in subgroups B and C were demineralized for 7 days with the pH adjusted to 5.0 at 37 °C as described by Lippert et al.25
The occlusal surface of demineralized dentin specimens in subgroups B and C for the three groups was treated with an application of one drop of 38% SDF (Advantage Arrest; Elevate Oral Care, Florida, USA) using a microbrush applicator. The specimens in subgroup B for the three groups were loaded immediately after SDF was dried, and by using a custom-made mold, it was not necessary to rinse with RBC, RMGIC and GIC (5 mm diameter and 2 mm height). The specimens were compressed occlusally with a mylar strip to ensure good adaptation. The specimens in subgroup C for the three groups were treated with SDF similar to subgroup B and then loaded one week later after being stored at 37 °C in distilled water with RBC, RMGIC and GIC restorative materials similar to those for subgroup B.
The color difference (ΔE value) of each specimen was calculated using the ΔELab equation:24
A ΔE value >3.3 was considered significant.25 The ΔE value of the specimens in subgroup A for all groups was measured twice after 1 day (T1) and then after 7 days (T7) for RBC, RMGIC and GIC bonded to sound dentin at the center of the occlusal surface of the restoration after being maintained in distilled water at 37 °C. The ΔE values were recorded using a spectrophotometer device relative to the standard D65 (LabScan XE; Virginia, USA) and recorded in a three-dimensional CIELAB color space system (frequently denoted as L*a*b*) as follows: (L*) represents brightness ranging from dark (0) to bright (100), (a*) describes red (+1 to +100) or green (−1 to −100), and (b*) represents yellow (+1 to +100) or blue (−1 to −100). The ΔE values were repeated three times for each specimen at different angles, and the mean values were recorded.
Similarly, the ΔE values of RBC, RMGIC and GIC bonded to demineralized dentin in subgroups B and C for the three groups were measured as for subgroup A. Data in this study were analyzed utilizing the Statistical Package for Social Sciences, version 20 (SPSS Inc; Chicago, IL, USA). One-way ANOVA and Tukey’s post-hoc test were performed to compare the ΔE values. A paired t test was used to compare the mean of L* a* b* coordinates. The significance level was set at p<0.05.
Results
The mean and standard deviation of the color differences (ΔE) of the three groups are presented in Table 1. With regard to the restorative materials used, a significantly higher color stability was observed with GIC followed by RMGIC, and the least color stability was observed for the RBC group (p<0.001).
 |
Table 1 Mean and Standard Deviation (SD) of the ΔE Values Based on the Type of Restorative Material |
Multiple comparisons were performed between the three groups and their subgroups, as shown in Table 2. There were significant color differences between the RBC and RMGIC groups, irrespective of the loading time (p <0.001 for both). No significant color changes were observed in the GIC group (p = 0.335).
 |
Table 2 Mean and Standard Deviation (SD) of the ΔE Values for Each Group |
As shown in Table 3, there was a significant decrease in the lightness (L* value) in all the B & C groups when comparing the two time intervals in which the color analysis was performed, such as after the first day of restorative material loading and after 7 days of restorative material loading. Among all subgroups, the highest lightness value was observed with the GIC group compared to the RBC group and RMGIC group. There was a decreasing trend in the color change to a darker version when comparisons were made after the first day and after 7 days following the restorative loading in all groups.
 |
Table 3 Mean and Standard Deviation (SD) Values of L* a* b* Coordinates for All Groups (N=90) |
With regard to the red and green chromaticity (a* value), all groups showed an inclination towards green (decrease in the a* value). Except for the immediate loading subgroup of RBC and GIC loading, there were significant differences in the a* value in all groups based on the color deviation in the time intervals tested. RBC-loaded groups showed an increase in the a* value, whereas RMGIC- and GIC-loaded groups showed a decline in the a* value.
The yellow and blue chromaticity (b* value) were found to be statistically significant in the RMGIC-loaded groups between the time intervals of restorative material loading points after 1 day and after 7 days. There was a significant shift to the blue-colored component (decrease in the b* value) in RMGIC subgroups B and C.
Discussion
The effect of immediate loading versus loading one week later on the color stability of three different tooth colored restorative materials, RBC, RMGIC and GIC, when bonded to SDF-treated demineralized dentin was investigated in this study. Based on the results of this in vitro study, significant color differences were observed with the restorations following SDF applications. When the restorative materials were loaded after 1 week of SDF application, there was less discoloration noted compared to that of the immediate loading, thus, the null hypothesis has been rejected.
The use of a colorimetric device, such as a spectrophotometer and the CIE L*a*b* color coding system, was determined to be an appropriate tool for objectively identifying color changes that cannot be visualized by the naked eye. The color changes could be studied effectively because of the numeric data generated from the sound and lesion affected areas.26 In this study, a spectrophotometer based on the CIE L*a*b* system was used to measure the color changes in restorative materials loaded onto sound dentin and at two time intervals, including immediately and 1 week after SDF application on demineralized dentin.
It has been proposed by the American Academy of Pediatric Dentistry guidelines that the treated and arrested cavitated caries lesions by SDF may be restored to improve esthetics.27 The timing of the restoration loading is an important factor, and in this study, a delayed restoration after 1 week of SDF application had better color stability to mask the dark staining of the SDF-treated demineralized dentin. A restoration to mask the permanent staining caused by SDF may be delayed up to a week, and the findings are in accordance with a similarly designed in vitro study in which the authors proposed delaying the restorations for two weeks to reduce the discoloration caused by SDF.28
The waiting period of one week has limitations, such as patient compliance, the possibility of diminishing the anti-caries effect of SDF following its application, and the role of the oral microbiome;29 in addition, food habits and poor oral hygiene may hinder the ability of SDF to sustain caries arrest. However, it has been recommended that an annual application of SDF is more effective and feasible than applying sodium fluoride varnish four times a year.30 It is likely that the staining caused by SDF, which should be anticipated, may result in the patient seeking further treatment, but there is also the possibility that the patient may not return for the second appointment if the dentist does not educate the patient or guardian appropriately about the consequences. Thus, gaining informed consent and explaining the risks and benefits with the use of SDF and the possible need for restorations following arrest of the caries is extremely important.
The limitations of this study are its in vitro nature, which may not justify the in vivo conditions, such as changes in salivary parameters, abundant oral microbiomes and the oral environmental factors that might impact the use of SDF and restorative treatments in caries management. Furthermore, in vitro studies may not reveal the actual mode of color stability but may only rank the materials or techniques. Therefore, caution is advised when interpreting the results. The time that the hard, stained dentin appeared following application of SDF was not taken into account as a baseline reading to compare the changes in discoloration after 7 days. The time of appearance of the black staining was investigated by Patel et al31 to be within 2 minutes of SDF application, and it increased in value over time, irrespective of the concentration of SDF used (12% or 38%) or the length of SDF application. Long-term clinical studies are necessary to obtain evidence of color changes through taking digital photography of the specimens that are being used and can be considered in future research.
This study may be a valuable addition to SDF research in which the staining potential of SDF has become more evident, and the use of tooth-colored restorative materials after one week of SDF application to mitigate the unesthetic stains may be a logical alternative treatment approach. A delay of one week after SDF application to perform the restoration would give ample time for the activity of arresting SDF caries to take place. This approach needs to be explored in patients to understand its effectiveness and acceptance as a treatment modality. The use of SDF in this current and post-COVID-19 pandemic period has been advocated as one of the most sought-after biological treatment alternatives in caries control, particularly in pediatric dentistry.32 A wider acceptance of the use of SDF is needed, especially due to the need for a second appointment for the minimally aerosol generating procedure that involves a conventional restoration, once caries has been arrested.
The use of SDF and its effect on the bond strength of various restorative materials have been studied.33,34 In vitro studies revealed that restorations following SDF application created a better resistance to secondary caries as well as greater induction of tertiary dentin than that of other treatments.35 Such evidence-based applications of SDF along with restorative treatments need to be highlighted and could result in a paradigm shift in caries management in high caries risk individuals.
Conclusion
Delaying the loading time of the restorative material for 1 week following the application of SDF resulted in greater color stability than that of immediate loading. The GIC showed greater color stability following SDF application than that of RBC and RMGIC. Long-term clinical studies with follow-up analysis are necessary to determine the color stability of the various restorative materials following SDF application.
Acknowledgment
The authors thank College of Dentistry Research Center and the Deanship of Scientific Research at King Saud University, Saudi Arabia, for supporting this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mount GJ, Ngo H. Minimal intervention: a new concept for operative dentistry. Quintessence Int. 2000;31(8):527–533.
2. Nishino M, Yoshida S, Sobue S, Kato J, Nishida M. Effect of topically applied ammoniacal silver fluoride on dental caries in children. J Osaka Univ Dent Sch. 1969;9:149–155.
3. Mabangkhru S, Duangthip D, Chu CH, Phonghanyudh A, Jirarattanasopha V. A randomized clinical trial to arrest dentin caries in young children using silver diamine fluoride. J Dent. 2020;99:103375. doi:10.1016/j.jdent.2020.103375
4. Jabin Z, Vishnupriya V, Agarwal N, Nasim I, Jain M, Sharma A. Effect of 38% silver diamine fluoride on control of dental caries in primary dentition: a systematic review. J Family Med Prim Care. 2020;9(3):1302–1307. doi:10.4103/jfmpc.jfmpc_1017_19
5. Vollu AL, Rodrigues GF, Rougemount Teixeira RV, et al. Efficacy of 30% silver diamine fluoride compared to atraumatic restorative treatment on dentine caries arrestment in primary molars of preschool children: a 12-months parallel randomized controlled clinical trial. J Dent. 2019;88:103165. doi:10.1016/j.jdent.2019.07.003
6. Hiraishi N, Sayed M, Takahashi M, Nikaido T, Tagami J. Clinical and primary evidence of silver diamine fluoride on root caries management. Jpn Dent Sci Rev. 2022;58:1–8. doi:10.1016/j.jdsr.2021.11.002
7. Mitchell C, Gross AJ, Milgrom P, Mancl L, Prince DB. Silver diamine fluoride treatment of active root caries lesions in older adults: a case series. J Dent. 2021;105:103561. doi:10.1016/j.jdent.2020.103561
8. Grandjean ML, Maccarone NR, McKenna G, Muller F, Srinivasan M. Silver Diamine Fluoride (SDF) in the management of root caries in elders: a systematic review and meta-analysis. Swiss Dent J. 2021;131(5):417–424.
9. Silver Diamine GJ. Fluoride may prevent and arrest root caries in older adults. J Evid Based Dent Pract. 2019;19(2):186–188. doi:10.1016/j.jebdp.2019.05.009
10. Subbiah GK, Gopinathan NM. Is silver diamine fluoride effective in preventing and arresting caries in elderly adults? A systematic review. J Int Soc Prev Community Dent. 2018;8(3):191–199. doi:10.4103/jispcd.JISPCD_99_18
11. Ballikaya E, Unverdi GE, Cehreli ZC. Management of initial carious lesions of hypomineralized molars (MIH) with silver diamine fluoride or silver-modified atraumatic restorative treatment (SMART): 1-year results of a prospective, randomized clinical trial. Clin Oral Investig. 2021;26(2):2197–2205. doi:10.1007/s00784-021-04236-5
12. Seifo N, Robertson M, MacLean J, et al. The use of silver diamine fluoride (SDF) in dental practice. Br Dent J. 2020;228(2):75–81. doi:10.1038/s41415-020-1203-9
13. Seifo N, Cassie H, Radford J, Innes N. “It’s really no more difficult than putting on fluoride varnish”: a qualitative exploration of dental professionals’ views of silver diamine fluoride for the management of carious lesions in children. BMC Oral Health. 2020;20(1):257. doi:10.1186/s12903-020-01243-y
14. Mei ML, Lo ECM, Chu CH. Arresting dentine caries with silver diamine fluoride: What’s behind it? J Dent Res. 2018;97(7):751–758. doi:10.1177/0022034518774783
15. Hamdy D, Giraki M, Abd Elaziz A, Badran A, Allam G, Ruettermann S. Laboratory evaluation of the potential masking of color changes produced by silver diamine fluoride in primary molars. BMC Oral Health. 2021;21(1):337. doi:10.1186/s12903-021-01697-8
16. Chibinski AC, Wambier LM, Feltrin J, Loguercio AD, Wambier DS, Reis A. Silver diamine fluoride has efficacy in controlling caries progression in primary teeth: a systematic review and meta-analysis. Caries Res. 2017;51(5):527–541. doi:10.1159/000478668
17. Nguyen V, Neill C, Felsenfeld J, Primus C. Potassium iodide. The solution to silver diamine fluoride discoloration? Adv Dent Oral Health. 2017;5(1):555–655.
18. Detsomboonrat P, Thongmak P, Lertpayab P, Aiemsri W, Sooampon S. Optimal concentration of potassium iodide to reduce the black staining of silver diamine fluoride. J Dent Sci. 2022;17(1):300–307. doi:10.1016/j.jds.2021.03.014
19. Vennela E, Sharada J, Hasanuddin S, Suhasini K, Hemachandrika I, Singh PT. Comparison of staining potential of silver diamine fluoride versus silver diamine fluoride and potassium iodide under tooth-colored restorations: an in vitro study. J Indian Soc Pedod Prev Dent. 2021;39(1):47–52. doi:10.4103/jisppd.jisppd_533_20
20. Zhao IS, Mei ML, Burrow MF, Lo EC-M, Chu C-H. Effect of silver diamine fluoride and potassium iodide treatment on secondary caries prevention and tooth discolouration in cervical glass ionomer cement restoration. Int J Mol Sci. 2017;18(340):1–12.
21. Walsh LJ, Brostek AM. Minimum intervention dentistry principles and objectives. Aust Dent J. 2013;58(s1):3–16. doi:10.1111/adj.12045
22. Fejerskov O, Nyvad B, Kidd EAM. Dental Caries: The Disease and Its Clinical Management.
23. Samra APB, Pereira SK, Delgado LC, Borges CP. Color stability evaluation of aesthetic restorative materials. Braz Oral Res. 2008;22(3):205–210. doi:10.1590/S1806-83242008000300003
24. Ruyter I, Nilner K, Möller B. Color stability of dental composite resin materials for crown and bridge veneers. Dent Mater. 1987;3(5):246–251. doi:10.1016/S0109-5641(87)80081-7
25. Lippert F, Lynch R, Eckert G, Kelly S, Hara AT, Zero DT. In situ fluoride response of caries lesions with different mineral distributions at baseline. Caries Res. 2011;45(1):47–55. doi:10.1159/000323846
26. Kim HE, Kim B-I. An in vitro comparison of quantitative light-induced fluorescence-digital and spectrophotometer on monitoring artificial white spot lesions. Photodiagnosis Photodyn Ther. 2015;12(3):378–384. doi:10.1016/j.pdpdt.2015.06.006
27. Crystal YO, Marghalani AA, Ureles SD, et al. Use of silver diamine fluoride for dental caries management in children and adolescents, including those with special health care needs. Pediatr Dent. 2017;39(5):135E–145E.
28. Alsagob E, Sawan N, Aladhyan S, Alsalem N, Alshami A, Albluwi S. Silver diamine fluoride with delayed restoration reduces tooth discoloration. Saudi J Biol Sci. 2021;29:1434–1438.
29. Paul B, Sierra MA, Xu F, et al. Microbial population shift and metabolic characterization of silver diamine fluoride treatment failure on dental caries. PLoS One. 2021;16(3):e0242396. doi:10.1371/journal.pone.0242396
30. Lo E, Chu C, Lin H. A community-based caries control program for pre-school children using topical fluorides: 18-month results. J Dent Res. 2001;80(12):2071–2074. doi:10.1177/00220345010800120901
31. Patel J, Anthonappa RP, King NM. Evaluation of the staining potential of silver diamine fluoride: in vitro. Int J Paediatr Dent. 2018;28(5):514–522. doi:10.1111/ipd.12401
32. Al‑Halabi M, Salami A, Alnuaimi E, Kowash M, Hussein I. Assessment of paediatric dental guidelines and caries management alternatives in the post COVID‑19 period. A critical review and clinical recommendations. Eur Arch Paediatr Dent. 2020;21(5):543–556. doi:10.1007/s40368-020-00547-5
33. Favaro JC, De Mello Peixoto YCT, Geha O, et al. Can silver diamine fluoride or silver nanoparticle-based anticaries agents to affect enamel bond strength? Restor Dent Endod. 2021;46(1):e7. doi:10.5395/rde.2021.46.e7
34. Jiang M, Mei ML, Wong MCM, Chu CH, Lo ECM. Effect of silver diamine fluoride solution application on the bond strength of dentine to adhesives and to glass ionomer cements: a systematic review. BMC Oral Health. 2020;20(1):40. doi:10.1186/s12903-020-1030-z
35. Zhao IS, Mei ML, Burrow MF, Lo EC, Chu CH. Prevention of secondary caries using silver diamine fluoride treatment and casein phosphopeptide-amorphous calcium phosphate modified glass-ionomer cement. J Dent. 2017;57:38–44. doi:10.1016/j.jdent.2016.12.001
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.