Back to Journals » Research and Reports in Urology » Volume 12
The Effect of Exogenous Superoxide Dismutase (SOD) on Caspase-3 Activation and Apoptosis Induction in Pc-3 Prostate Cancer Cells
Authors Ismy J , Sugandi S, Rachmadi D, Hardjowijoto S, Mustafa A
Received 16 July 2020
Accepted for publication 8 September 2020
Published 27 October 2020 Volume 2020:12 Pages 503—508
DOI https://doi.org/10.2147/RRU.S271203
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jan Colli
Jufriady Ismy,1 Suwandi Sugandi,2 Dedi Rachmadi,3 Sunaryo Hardjowijoto,4 Akhmad Mustafa2
1Department of Urology, Faculty of Medicine Universitas Syiah Kuala, Zainoel Abidin General Hospital, Banda Aceh, Indonesia; 2Department of Urology, Faculty of Medicine Universitas Padjadjaran, Hasan Sadikin Hospital, Bandung, Indonesia; 3Department of Pediatric, Faculty of Medicine Universitas Padjadjaran, Hasan Sadikin Hospital, Bandung, Indonesia; 4Department of Urology, Faculty of Medicine Universitas Airlangga, Dr. Soetomo General Hospital, Surabaya, Indonesia
Correspondence: Jufriady Ismy Jl. Tgk Daud Beureueh No. 108, Banda Aceh, Indonesia
Email [email protected]
Background: This study aimed to assess the effects of exogenous SOD administration on prostate cancer cell line (PC-3) apoptosis via the intrinsic pathway by examining the expression of manganese superoxide dismutase (MnSOD), caspase-3, and apoptosis index of the PC-3 cell line.
Methods: We used the prostate cancer cells from secondary prostate cancer cell lines (PC-3) derived from castration refractory prostate cancer (CRPC), cell differentiation grade IV, and had metastasized to the bone from the American Type Culture Collection (ATCC, Rockville, MD, USA). Superoxide dismutase (SOD) is derived from extracts of melon seeds and wheat gliadin biopolymer, and divided into 62.5 mg/mL, 83 mg/mL, 125 mg/mL, and 250 mg/mL doses. Expression of MnSOD was measured by immunohistochemistry (IHC). Expression of caspase-3 was measured using Western Blot method. Apoptotic index is calculated based on the reaction introduction 3OH end of fragmentation of DNA by the enzyme terminal transferase in preparations with TUNEL staining reagents. A one-way ANOVA test and Pearson correlation test were used to determine the relationship between SOD with expression of caspase-3 and apoptotic index.
Results: SOD extract significantly increased the expression of caspase-3 (P=0.016) and the apoptotic index (P=0.000) (P< 0.05). There was a correlation between the increased doses of SOD extract and the apoptosis index (P=0.015; r=0.679) and between the increased caspase-3 expression and the apoptosis index (P=0.015; r=0.682).
Conclusion: Administration of superoxide dismutase (SOD) increased apoptosis in a prostate cancer cell line (PC-3) through the increased expression of caspase-3. Superoxide dismutase (SOD) can be considered as a therapy for late-stage prostate cancer that had been progressed to hormone resistant and metastasized and promote apoptosis in those prostate cancer cells.
Keywords: apoptosis, prostate cancer, superoxide dismutase
Introduction
Prostate cancer is one of the most common cancers in men.1 Various factors, such as suspected genetic and environmental factors, are important for prostate cancer development. Until now, hormonal therapy strategy for managing locally advanced and metastatic prostate cancer is mainly based on the theory that prostate cells are sensitive to androgens.2–4
Various studies suggest that the chronic inflammatory process contributes to the progression of cancers in many organs including the prostate. The inflammatory process itself generates reactive oxygen species (ROS) that can damage mitochondria and nuclear DNA. Damage in DNA and anti-oncogene will damage the normal apoptosis process in tissues and increase the progression towards cancer.2–6
Nowadays, the development of anti-cancer drugs has been shifted towards cell cycle transduction signal, growth factors, DNA repair, and apoptosis. Apoptosis specifically has an important role in carcinogenesis and cancer therapy. Apoptosis can occur via two pathways: the extrinsic pathway and the intrinsic pathway. The intrinsic apoptosis pathway occurs when mitochondria are damaged. In the intrinsic pathway of apoptosis activation, caspase 9 plays a role as an initiator, while caspase 3 plays a role as an “executor” enzyme. Thus, an increase of caspase 3 will leads to an increase of apoptosis.7 Also, altered caspase 3 expression may represent an additional mechanism of apoptotic resistance to androgen deprivation.8
Superoxide dismutase (SOD) is group of antioxidant enzyme that have a significant role in encountered oxidative stress in cells. Manganese superoxide dismutase (MnSOD) is a primary SOD antioxidant enzyme present in mitochondria that plays an important role in detoxifying ROS.9 MnSOD is often decreased or missing in cancer cells. In some studies, decreased MnSOD activity correlates to increasing cancer cell progression, migration, and invasion.10 In this study we aim to investigate the effect of exogenous SOD on prostate cancer cell caspase-3 activation and cell apoptosis as novel anti-cancer treatment for hormone resistant, bone metastasized prostate cancer cell line (PC-3).
Materials and Methods
Sample Characteristics
In this study we were using the prostate cancer cell line PC-3 that was derived from a patient with hormone therapy-resistant prostate cancer with ISUP grade 4 and metastasized to the bone. These cell lines were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA), with inclusion criteria uncontaminated PC-3 cell lines and confluent cell culture.
The growing medium used was Ham’s F12 containing 2 mM L-glutamine, 1.5 g/L sodium bicarbonate and supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. Cell multiplication is done by the subculture through cell dissociation from the previous culture. The number of cells used in each treatment was calculated using a hemocytometer.
Superoxide Dismutase Preparation
Exogenous superoxide dismutase (SOD) was derived from the extracts of melon seeds and the wheat gliadin biopolymer (GliSODin®, Kalbe Pharmaceutical, tbk.). The drugs were then diluted with aquadest and separated into four doses: 62.5 mg/mL, 83 mg/mL, 125 mg/mL, and 250 mg/mL to find an optimal dose for cell apoptosis.
Study Design
This study was an in vitro experimental study with a completely randomized design. There were five experimental groups in this study (four treatment groups and one control group) with three repetitions per group. The extract of superoxide dismutase (GliSODin®) was given to each treatment group.
Expression of MnSOD
MnSOD expression was determined using immunohistochemistry (IHC) using rabbit antibody for MnSOD (Abcam, ab68155). MnSOD was measured in three high power fields (HPF) by an experienced pathologist.
Expression of Caspase-3
Caspase-3 activity was determined by Western blotting. Primary rabbit polyclonal antibody to caspase-3 (Abcam, ab32351) and secondary goat polyclonal antibody to rabbit IgG H & L (Abcam ab6271) were used. Caspase-3 cleavage was measured by detecting the products at different masses in kilodaltons.
Apoptotic Index
The proportion of types of apoptotic cells was calculated based on the reaction at the 3ʹOH end of DNA fragments by the terminal transferase enzyme and TUNEL staining reagents.
Statistical Analysis
Statistical analysis was preceded by a normality test of the data using the one-sample Kolmogorov–Smirnov test. If the data were normally distributed, then the data were analyzed using a parametric ANOVA (analysis of variance). Significance results were followed by a post-hoc test to determine which treatment group showed the most significant results. The Pearson correlation test was conducted to show the correlation between each variable. This study was conducted after obtaining ethical approval from the Research Ethics Committee Faculty of Medicine Universitas Padjadjaran No.719/UN6.C1.3.2/KEPK/PN/2016. All authors contributed to the data analysis, drafting, or revising of the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Result of the Study
MnSOD Expression in Prostate Cancer Cell Lines (PC-3)
We measured the expression of MnSOD in PC-3 cell lines on each dosage using immunohistochemistry method. We found that there was an increase of MnSOD on each dosage group, with a peak increase of MnSOD expression on 250 mg/mL dosage (154.23 intensity/mm2) compared to the control group (63.35 intensity/mm2) of high power field (HPF). There was a significant increase of MnSOD expression on each dosage compared to negative control and control with antibody for MnSOD (P=0.0001) (Tables 1 and 2, Figure 1)
 |
Table 1 Intensity of MnSOD Expression on PC-3 Cell Line |
 |
Table 2 Post Hoc Analysis of MnSOD Expression |
 |
Figure 1 Intensity of MnSOD expression on PC-3 cell line. |
Caspase 3 Expression in PC-3 Cell Line
From Table 2, we found a progressive increase of caspase 3 expression in PC-3 cell lines, with a maximum increase of caspase 3 expression at a 125 mg dose. We found a significant difference in caspase 3 expression in PC-3 cell lines compared to the control group (P=0.016) (Tables 3 and 4, Figure 2). Table 4 shows that there was no significant difference between the concentration dose of 62.5 mg in increasing caspase-3 activity, as compared to the control group (P>0.05). However, significant differences were found on the concentration dose group of 83 mg, 125 mg, and 250 mg (P<0.05).
 |
Table 3 Caspase 3 Expression in PC-3 Cell Line |
 |
Table 4 Post Hoc Analysis of Caspase 3 Expression |
 |
Figure 2 Caspase 3 expression in PC-3 cell line. |
Apoptosis Index in PC-3 Cell Line
From Table 3, we found an increase of apoptosis index in PC-3 cell lines, with a maximum increase of apoptosis index at 125 mg dose (85.2%). We found a significant difference in apoptosis index in PC-3 cell lines compared to the control group (P=0.0001) (Tables 5 and 6, Figure 3). Table 6 shows that there was no significant difference between the concentration dose of 62.5 mg in increasing apoptosis activity, as compared to the control group (P>0.05). However, significant differences were found on the concentration dose groups of 83 mg, 125 mg, and 250 mg (P<0.05).
 |
Table 5 Apoptosis Index Expression in PC-3 Cell Line |
 |
Table 6 Post Hoc Analysis of Apoptosis Index |
 |
Figure 3 Apoptosis index expression in PC-3 cell line. |
Correlation Analysis Between MnSOD, Caspase 3, and Apoptosis Index in PC-3 Cell Line
We performed correlation analysis between exogenous SOD dose, MnSOD expression, caspase 3, and apoptosis index. From Table 7, there were no significant correlations between increasing exogenous SOD dosage with MnSOD and Caspase 3 expression. On the other hand, there was a strong correlation between increasing exogenous SOD dosage with increasing apoptosis index in PC-3 cell lines (P=0.015, r=0.679). Other analysis showed that there was a strong correlation between increasing caspase 3 expression with apoptosis index (P=0.015, r=0.682).
 |
Table 7 Correlation Between SOD Extract Dose, MnSOD Expression, Caspase-3 Expression, and Apoptotic Index in a Prostate Cancer Cell Line (PC-3) |
Discussion
Superoxide dismutase (SOD) is a primary antioxidant enzyme found in the mitochondria. Among the SOD enzymes, the Manganese Superoxide Dismutase (MnSOD) is a predominant enzyme of the mitochondria. On 2005, Venkataraman et al11 have reported that an increase in MnSOD levels on breast and liver tissues will inhibit cancer cell growth. The MnSOD is one of the few SOD enzymes that are often lacking or nonexistent in tumor cells.
The study was conducted in vitro on prostate cancer cell lines (PC-3). This prostate cancer tissue culture is of a 62 year-old male with a poorly differentiated, bone-metastasized, stage IV prostate cancer on a refractory period after hormonal therapy. Later, the tissue was treated by different doses of SOD and observed through multiple periods.
The study revealed that, as compared to other test groups, the mean MnSOD enzyme levels are low in the prostate cancer cell line PC-3 tissue culture (negative control group). This is in accordance with previous literature regarding cancerous cells where they discovered reduced antioxidant enzyme levels, especially MnSOD, compared to those of normal cells. After the delivery of 62.5 mg, 83 mg, and 250 mg SOD, mean increases in the MnSOD expressions are apparent as compared to control groups with P=0.000 (P<0.05). This proves that low levels of the MnSOD enzyme on prostate cancer cell line PC-3 could be supplemented by exogenous SOD delivery (from outside of the body). This study also revealed that SOD supplementation (62.5 mg, 83 mg, 125 mg, and 250 mg) to every test group showed significant differences compared to the control group. However, there was no significant difference between the different dose concentrations despite an average increase of MnSOD expressions, directly proportional to dosage increments of up to 250 mg SOD.
Increased MnSOD enzymes can serve as tumor suppressor genes on prostate cancer cells. Tumor suppressor genes are specialized proteins tasked to induce apoptosis and DNA repair. Apoptosis can be initiated through two pathways, the extrinsic or the intrinsic (mitochondrial) pathway. Caspase is a mediatory enzyme responsible for cellular apoptosis; thus increased expressions of caspase are indicators of an ongoing apoptosis process among cancer cells.12 Numerous protein/enzymes mediate cell death through apoptosis via the proteolytic activity initiator and caspase executor by the intrinsic pathway (caspase-9) and caspase executor (caspase-3).13 A study by Zhang et al14 showed that caspase-3 was a key component in the apoptotic signaling associated with the induction of inter nucleosomal DNA fragmentation. A study by Yu et al15 showed increased expression of caspase-3, −8, and −9 that was associated with increased apoptosis in cancer cells.
This study has shown an average increase of caspase-3 expressions on all concentrated doses with significant differences compared to the control group, P=0.016 (P<0.05), hence expressing a directly proportional relationship between the increment of MnSOD enzyme and the increase of caspase-3 activity of PC-3 cancer cells in the apoptosis process.
Table 4 has shown that there was no significant difference between the concentration dose of 62.5 mg in increasing caspase-3 activity, as compared to the control group (P>0.05). However, significant differences were found on the concentration dose group of 83 mg, 125 mg, and 250 mg (P<0.05). This research has also shown that there is no significant difference between the groups 125 mg and 250 mg in increasing the expression of Caspase-3.
The increase of caspase-3 can activate specific proteins in cells which will further induce the apoptosis process. The increase of caspase-3 enzyme expression will increase the turnover of prostate cancer cell line PC-3 in vitro through the intrinsic pathway. The reason for this is, theoretically, the enzyme MnSOD is present at the mitochondria, which is also mediating the intrinsic pathway of apoptosis. Thus, this research adds to the bulk of evidence stating that the pathway of SOD in increasing the apoptosis rate of prostate cancer cell line PC-3 in vitro is through the intrinsic pathway (mitochondrial pathway). This research presented similar results to those of Palchaudhuri et al,16 who stated that small natural molecules with anti-cancer properties might induce the intrinsic pathway of apoptosis (mitochondrial pathway) (Figure 4).
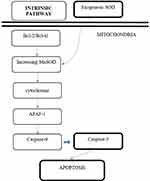 |
Figure 4 Proposed mechanism of induction of PC-3 cell line apoptosis by exogenous SOD via intrinsic pathway. |
This research also observed the impact of the increase of apoptosis on the prostate cancer cell line PC-3. The results have shown that the average increase of apoptosis expression occurs after exposure of various concentrations of SOD, P=0.000 (P<0.05). This research also proves that there is no difference between the SOD concentration of 62.5 mg in increasing the apoptosis rate as compared to the control group. However, several significant differences are present between the dosages of 83 mg, 125 mg, and 250 mg in increasing the apoptosis rate of PC-3, as compared to control groups (P<0.05), but no significant difference was found between dosage 125 mg and 250 mg in increasing apoptosis (P>0.05).
Hence, relating the effects of Superoxide Dismutase (SOD) to the increase of Manganese Superoxide Dismutase (MnSOD) in inducing the intrinsic pathway of apoptosis by observing the increase of caspase-3, we can gather that the best concentration doses are 125 mg and 250 mg. The supplementation of exogenous SOD.
Conclusion
Administration of superoxide dismutase (SOD) increased apoptosis in a prostate cancer cell line (PC-3) through the increased expression of caspase-3. Superoxide dismutase (SOD) can be considered as a therapy for late-stage prostate cancer that had been progressed to hormone resistant and metastasized and promote apoptosis in those prostate cancer cells.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012(45):152–156. doi:10.1093/jncimonographs/lgs035
2. Kumar V, Cotran R, Robbin S. Pathologic Basic of Disease. Philadelphia, PA: Elsevier; 2003.
3. Stephenson AJ. Prostate cancer etiology, natural history and prevention of prostate cancer. In: Campbell’s Urology.
4. Heidenreich A. Identification of high-risk prostate cancer: role of prostate-specific antigen, PSA doubling time, and PSA velocity. Eur Urol. 2008;54:976–977. doi:10.1016/j.eururo.2008.06.077
5. Mottet N, et al. Guidelines on Prostate Cancer. EAU Guidelines; 2016.
6. Venkataraman S, Jiang X, Weydert C, et al. Manganese superoxide dismutase overexpression inhibits the growth of androgen-independent prostate cancer cells. Oncogene. 2005;24:77–89. doi:10.1038/sj.onc.1208145
7. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi:10.1080/01926230701320337
8. O’Neill AJ, Boran SA, O’Keane C, et al. Caspase 3 expression in benign prostatic hyperplasia and prostate carcinoma. Prostate. 2001;47(3):183–188. doi:10.1002/pros.1061
9. Holley AK, Bakthavatchalu V, Velez-Roman JM, St Clair DK. Manganese superoxide dismutase: guardian of the powerhouse. Int J Mol Sci. 2011;12(10):7114–7162. doi:10.3390/ijms12107114
10. Fan JJ, Hsu WH, Hung HH, et al. Reduction in MnSOD promotes the migration and invasion of squamous carcinoma cells. Int J Oncol. 2019;54:1639–1650.
11. Venkataraman S, Xiaohong J, Christine W, et al. Manganese superoxide dismutase overexpression inhibits the growth of cancer cells. Nat Publ Group. 2005;24:77–89.
12. Fiandalo MV, Kyprianou N. Caspase control: protagonists of cancer cell apoptosis. Exp Oncol. 2012;34(3):165–175.
13. Jiao C, Chen W, Tan X, et al. Ganoderma lucidum spore oil induces apoptosis of breast cancer cells in vitro and in vivo by activating caspase-3 and caspase-9. J Ethnopharmacol. 2020;247:112256. doi:10.1016/j.jep.2019.112256
14. Zhang X, Steiner MS, Rinaldy A, Lu Y. Apoptosis induction in prostate cancer cells by a novel gene product, pHyde, involves caspase-3. Oncogene. 2001;20:5982–5990. doi:10.1038/sj.onc.1204831
15. Yu CX, Zhang X-Q, Kang L-D, et al. Emodin induces apoptosis in human prostate cancer cell LNCaP. Asian J Androl. 2008;10:625–634. doi:10.1111/j.1745-7262.2008.00397.x
16. Palchaudhuri R, Lambrecht MJ, Botham RC, et al. A small molecule that induces intrinsic pathway apoptosis with unparalleled speed. Cell Rep. 2015;13(9):2027–2036. doi:10.1016/j.celrep.2015.10.042
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
