Back to Journals » Drug Design, Development and Therapy » Volume 18
The Effect of Ciprofol on Postoperative Delirium in Elderly Patients Undergoing Thoracoscopic Surgery for Lung Cancer: A Prospective, Randomized, Controlled Trial
Authors Liu Z, Jin Y, Wang L, Huang Z
Received 27 September 2023
Accepted for publication 18 January 2024
Published 5 February 2024 Volume 2024:18 Pages 325—339
DOI https://doi.org/10.2147/DDDT.S441950
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Zhaohui Liu, Yi Jin, Lingfei Wang, Zeqing Huang
Department of Anesthesiology, Cancer Hospital of China Medical University, Liaoning Cancer Hospital & Institute, Shenyang, Liaoning, People’s Republic of China
Correspondence: Zeqing Huang, Department of Anesthesiology, Cancer Hospital of China Medical University, Liaoning Cancer Hospital & Institute, No. 44 Xiaoheyan Road, Dadong District, Shenyang, Liaoning, 110042, People’s Republic of China, Tel +86 18900917545, Email [email protected]
Purpose: This study was conducted to assess whether ciprofol vs propofol could affect the incidence of postoperative delirium (POD) in elderly patients with lung cancer after thoracoscopic surgery.
Patients and Methods: In this study, a total of 84 elderly patients undergoing thoracoscopic surgery for lung cancer were recruited and randomized into two groups to receive anesthesia with either ciprofol or propofol. The primary outcome was the incidence of POD within three days after surgery. Secondary outcomes included the Confusion Assessment Method (CAM) score, intraoperative indicators related to mean arterial pressure (MAP), and cerebral tissue oxygen saturation (SctO2). Moreover, MAP- and SctO2-related indicators associated with POD were analyzed.
Results: The incidence of POD was 7.1% and 16.7%, respectively, in the ciprofol group and the propofol group (risk ratio [RR], 0.37; 95% confidence interval [CI], 0.07 to 2.03; risk difference [RD], − 9.6%; 95% CI, − 23.3% to 4.1%; p = 0.178). Compared with those in the propofol group, patients in the ciprofol group had lower CAM scores three days after surgery (13 (12, 15) vs 15 (14, 17); 12 (11, 13) vs 14 (13, 16); 12 (11, 12) vs 13 (12, 14), p< 0.05). Besides, patients in the ciprofol group exhibited higher mean and minimum MAP (88.63 ± 6.7 vs 85 ± 8.3; 69.81 ± 9.59 vs 64.9 ± 9.43, p< 0.05) and SctO2 (77.26 ± 3.96 vs 75.3 ± 4.49, 71.69 ± 4.51 vs 68.77 ± 6.46, p< 0.05) and percentage of time for blood pressure stabilization (0.6 ± 0.14 vs 0.45 ± 0.14, p< 0.05) than those in the propofol group. Furthermore, MAP and SctO2-related indicators were validated to correlate with POD.
Conclusion: Anesthesia with ciprofol did not increase the incidence of POD compared with propofol. The results demonstrated that ciprofol could improve intraoperative MAP and SctO2 levels and diminish postoperative CAM scores.
Keywords: ciprofol, postoperative delirium, thoracoscopic surgery, cerebral tissue oxygen saturation
Introduction
Postoperative delirium (POD) is an acute perioperative fluctuating mental state change, primarily characterized by such symptoms as inattention, impaired consciousness, and disorganized thinking. This condition may induce cognitive impairment and prolonged hospitalization, thus leading to a significantly worse prognosis. Advanced age has been identified as an independent risk factor for the development of POD.1,2 With population aging and increasing environmental pollution, lung cancer exhibits a higher incidence and has become the most prevalent cancer. The median age of patients at the diagnosis of lung cancer is 71 years,3 with the mortality accounting for 19.4% of total cancer-related deaths.4
Video-assisted thoracic surgery (VATS) is the most effective and essential treatment for early-stage lung cancer.5 However, one-lung ventilation (OLV) performed during VATS may impair cerebral oxygen supply-demand balance, leading to POD. Notably, it has been reported that the incidence of intraoperative cerebral tissue oxygen saturation (SctO2) reduction in elderly patients undergoing OLV can reach up to 70%,6 and the incidence of POD ranges from 7% to 23%.7–9 Importantly, there is a significant correlation between the incidence of POD and the reduction in intraoperative blood pressure and SctO2.10–14 Furthermore, it has been established that controlling the blood pressure at a high level contributes to optimal cerebral perfusion, improves the SctO2 level, and diminishes the incidence of POD.15,16
As revealed in previous studies, the choice of sedative medications in the same type of surgery influences the occurrence of POD. For instance, low-dose dexmedetomidine infusion significantly reduces the incidence of POD in elderly patients after non-cardiac surgery within seven days after surgery.17 Conversely, patients sedated with benzodiazepines exhibit a higher incidence of POD compared with those sedated with non-benzodiazepines.18 Ciprofol, a novel intravenous sedative, has the advantages of faster onset of action, quicker awakening, less injection pain, less respiratory and circulatory depression, and an extended safety margin.19–22 It has been indicated that ciprofol leads to a lower incidence of hypotension in patients undergoing prolonged sedation or maintenance of anesthesia compared with propofol.20 Additionally, there is emerging evidence suggesting that ciprofol may exert neuroprotective effects by down-regulating the expression of pro-inflammatory cytokines, enhancing neuronal and mitochondrial functions, up-regulating the expression of nigrostriatal tyrosine hydroxylase,23 and inhibiting the activation of the Wnt/β-catenin signaling pathway.24
Nevertheless, there is no reports on the administration of ciprofol throughout the entire anesthesia process for thoracic surgery in the elderly, and the effect of ciprofol on POD remains inconclusively established. Consequently, this study was designed to explore the effect of ciprofol on POD in elderly patients undergoing thoracoscopic surgery for lung cancer based on a randomized, controlled, double-blind clinical trial.
Materials and Methods
Study Design and Ethics
This prospective, randomized, controlled, double-blind trial was conducted at Liaoning Cancer Hospital, and the trial design was reviewed and approved by the Ethics Committee of Liaoning Cancer Hospital (Ethics Approval No. 202304118). It was registered with the Chinese Clinical Trial Registry (ChiCTR2300075345). The study was conducted according to the principles of the Declaration of Helsinki, and all patients or their legal representatives signed an informed consent.
Patients
From May 1, 2023 to August 31, 2023, a total of 150 patients were enrolled in the trial based on the inclusion criteria. Among them, 66 patients were excluded or dropped out; eventually, 84 patients were included in the trial (Figure 1).
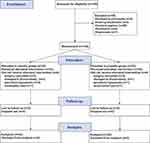 |
Figure 1 CONSORT diagram describing each stage of the randomized trial. |
The inclusion criteria included (1) patients undergoing VATS single lobectomy; (2) patients willing to accept total intravenous anesthesia; (3) patients aged 65 to 80 years with a body mass index (BMI) ranging from 18 kg/m² to 30 kg/m² or classified as American Society of Anesthesiologists (ASA) I or II; (4) patients without a history of surgery in the past three months; (5) patients with preoperative arterial blood gas analysis within the normal range. Those who met all inclusion criteria were enrolled in the study. The exclusion criteria included (1) patients allergic to the anesthesia drugs used in this study; (2) patients with a history of alcohol, sedative-analgesic drugs, psychotropic drugs, or substance abuse and addiction; (3) patients unable to cooperate in completing the Mini-Mental State Examination (MMSE) or scoring below specified thresholds (illiterate ≤ 17, primary school level ≤ 20, secondary school level ≤ 22, and university level ≤ 23);24 (4) patients with a history of stroke, transient ischaemic attack, traumatic brain injury, and moderate-to-severe cerebral stenosis, or patients with preoperative SctO2 < 60%;25 (5) patients with any of the following characteristics: severely impaired respiratory function (forced expiratory volume in 1 second < 50% of the predicted value or vital capacity < 50% of the predicted value), New York Heart Association (NYHA) Classification III–IV, severe hepatic or renal insufficiency (creatinine > 176 μmol/L, blood urea nitrogen > 7.1 mmol/L, albumin < 30 g/L, etc.); (6) patients with skin damage on the forehead; (7) patients refusing to participate in the study.
Randomization and Blinding
Patients were randomized into either the ciprofol or propofol groups at a ratio of 1:1 using a computer-generated random sequence and a sealed envelope method administered by a medical statistician. Specifically, the anesthetist opened the envelope after the patient entered the operating room, retrieved the grouping information, and administered the anesthetic agent accordingly. The anesthesiologist was only responsible for intraoperative anesthesia management and did not participate in the study design, data recording, or analysis. All study personnel, including data recorders, follow-up staff, outcome analysts, and patients, remained unaware of the group assignment except for the anesthesiologist. The group assignment results were unveiled only after the data analysis was completed.
Study Procedures
On the day before surgery, researchers measured the noninvasive blood pressure and heart rate (HR) for patients in stress-free, pain-free, and awake (or mildly sedated) states. The measurement was repeated non-consecutively three times for patients in a lying position on the ward at 07:00, 13:00, and 19:00. Additionally, MMSE was performed to assess the cognitive ability of patients. No patient received pre-anesthetic medication.
These patients were transferred to the operating room in a lying position, and venous access to the upper limb was established. The electrocardiogram (ECG), HR, blood oxygen saturation (SpO2), non-invasive cuff blood pressure (NIBP), and bispectral index (BIS) of patients were monitored continuously. Relevant data were collected every minute by the monitoring equipment. Bilateral prefrontal SctO2 was monitored using a cerebral oxygen monitor, with data recorded every 2 seconds. Before the induction of anesthesia, the radial artery puncture cannulation on the non-operative side was performed under local anesthesia. An arterial pressure transducer was connected for the continuous monitoring of invasive arterial pressure, and the results were calibrated with NIBP. All procedures were performed by the same surgeon team under general anesthesia with double-lumen endotracheal intubation.
The induction of anesthesia involved the intravenous administration of sufentanil at a dose of 0.3–0.4 μg/kg, ciprofol at a dose of 0.3–0.4 mg/kg for the ciprofol group (Group C), and propofol at a dose of 1.5–2.0 mg/kg for the propofol group (Group P). The administration persisted for over 30 seconds. When the eyelash reflex had disappeared and the BIS value was ≤ 60, rocuronium bromide was injected at 0.6 mg/kg. Then, tracheal intubation was performed when sufentanil and muscarinic drugs had taken full effect, and the tube’s precise positioning was confirmed by fibreoptic bronchoscopy.
Mechanical ventilation was conducted in volume-controlled ventilation mode. Tidal volume settings were adjusted to 6–8 mL/kg for two-lung ventilation and 4–6 mL/kg for one-lung ventilation based on the calibrated body weight. The airway pressure was maintained below 30 cmH2O, and the inhaled oxygen concentration (FiO2) was maintained at 100%. The respiratory rate, inspiratory-expiratory time ratio, and positive end-expiratory pressure were adjusted individually to maintain the PaCO2 level within 35–45 mmHg.
The maintenance of anesthesia was achieved through total intravenous anesthesia (TIVA). Patients in Group C received an initial dose of 1 mg•kg−1•h−1 with a maximum permissible rate of 2.4 mg•kg−1•h−1; Those in Group P received an initial dose of 4 mg•kg−1•h−1 with a maximum permissible rate of 12 mg•kg−1•h−1. Remifentanil was intravenously administered at a rate of 8–15 μg•kg−1•h−1. The administration rate was adjusted to maintain the BIS value between 40 and 60. After lying in the lateral position, patients underwent T4 - T6 paravertebral nerve block based on the surgical incision marking. The pumping rate of maintenance drugs was adjusted according to BIS and hemodynamic parameters. If analgesia was not achieved even after remifentanil reached its maximal rate, sufentanil was administered intraoperatively to augment analgesia. Rocuronium bromide was administered to maintain muscle relaxation intermittently. In case of hypotension (the MAP was less than 80% of the baseline blood pressure (BP) for more than 1 min), the anesthetic depth was adjusted or norepinephrine was administered at 0.05–0.10 μg•kg−1•h−1 until the MAP exceeded 80% of the baseline BP. Conversely, the anesthetic depth was adjusted or urapidil (10 mg) was administered in case of hypertension (the MAP exceeded 120% of the baseline BP for more than 1 min).
Sufentanil (0.1–0.2 μg/kg 30 min) was used before the end of the procedure to prevent the occurrence of postoperative discomfort. Rocuronium bromide was no longer given after the restoration of two-lung ventilation. The infusion of intravenous anesthetics was terminated at the end of the procedure. Subsequently, patients were transferred to the Post-Anesthesia Care Unit (PACU). When the train-of-four (TOF) index of patients reached ≥ 2, neostigmine was administered at a dose of 0.04 mg/kg. The tracheal tube was removed once patients could open their eyes, breathe spontaneously, have adequate tidal volume, and achieve circulatory stability.
Patients were assessed through Ramsay sedation score (Ramsay) and Bruggrmann comfort scale (BCS) in the PACU.25,26 The follow-up evaluation was conducted for patients in the ward 24, 48, and 72 hours after surgery. The patient-controlled analgesia infusion pump was primarily responsible for postoperative analgesia after patients were transferred to the ward. The postoperative pain intensity was measured using the visual analog scale (VAS);27 The sedation level was assessed with the Richmond Agitation Sedation Scale (RASS) score, and the incidence of POD was estimated by the confusion assessment method (CAM) if the RASS score exceeded −3, with the scores ≤ 19 indicating no POD, 20–22 indicating suspected POD, and > 22 indicating the presence of POD.28
Outcome Measures
The primary outcome was the incidence of POD within three days after surgery. Secondary outcomes included the CAM score three days after surgery, intraoperative MAP and SctO2, quality of awakening, anesthetic dosage, and vasoactive drug dosage. During surgery, the vital signs of patients were recorded. Additionally, an in-depth analysis was conducted to explore the correlation between the occurrence of POD and the indicators related to MAP and SctO2.
The baseline MAP and HR of patients were determined by taking the mean of three measurements the day before surgery. The NIBP was utilized to calibrate and identify the target range of invasive arterial blood pressure before the induction of anesthesia. The baseline SctO2 was calculated as the mean of left and right SctO2 under the condition that patients were in a conscious and resting state with voluntary respiration. MAP-related measures included the mean MAP, minimum MAP, and stable blood pressure range (intraoperative MAP maintained at 90–110% of the baseline MAP). The percentage of time for blood pressure stabilization during anesthesia was also computed. SctO2-related measures included the mean SctO2, minimum SctO2, absolute decrease (the difference between the baseline and minimum SctO2), relative decrease (the ratio of the absolute decrease to the baseline), incidence of intraoperative SctO2 desaturation (SctO2 maintained below 90% of the baseline for 15s), and the area under the threshold (AUT) (the cumulative area where SctO2 was maintained below 90% of the baseline). The quality of awakening was assessed based on the time taken to awaken, post-extubation Ramsay scores (1: anxious, restless, irritable; 2: quiet, cooperative, orientated; 3: responsive only to commands; 4: asleep but sensitive to stimuli; 5: asleep, slowly response to stimuli; 6: asleep and unable to be awakened), and BCS scores (0: persistent pain; 1: no pain at rest but severe pain with deep breathing or coughing; 2: no pain at rest but slight pain with deep breathing or coughing; 3: deep breathing without pain; 4: no pain symptoms). Additionally, the incidence of postoperative nausea and vomiting (PONV) and delayed awakening (the time from anesthesia discontinuation to achieving OAA/S > 5 exceeded 90 min) were recorded. Intraoperative adverse events included hypoxemia (SpO2 < 90%), hypertension, hypotension, bradycardia (HR less than 80% of the baseline HR for more than 30s), and tachycardia (HR exceeding 120% of the baseline HR for more than 30s).
The observation time points from T0 to T6 were also established (T0: before the induction of anesthesia; T1: after the induction of anesthesia, before intubation; T2: immediately after intubation; T3: at the beginning of surgery; T4: 30 min of OLV; T5: 60 min of OLV; T6: before the end of surgery). MAP, HR, SpO2, SctO2, BIS, and PETCO2 of patients were recorded at T0-T6.
Sample Size Calculation
The sample size required for this study was calculated based on the pre-test results of a POD incidence of 0% in Group C and a POD incidence of 23% in Group P, with a detection rate of 80% and a two-sided significance level of 0.05. With the aid of G*power software, it was determined that each group should consist of 34 subjects, totaling 68 subjects for the study. Given that approximately 20% of patients were excluded, the total number of participants was determined to be 84, with 42 patients allocated to each group.
Statistical Analysis
The statistical analysis was performed by SPSS 26.0. The normality of quantitative data was tested using histograms and the Shapiro–Wilk test. Continuous variables that were normally distributed were presented as mean and standard deviation (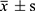 ), and the comparison between groups was performed by the t-test; while those that were not normally distributed were presented as medians and quartiles [M (P25, P75)], and the comparison between groups was performed by the Mann–Whitney U-test. Qualitative data were presented as percentages, and the comparison between groups was performed by the chi-square test. The comparison of indicators at each observation time point was analyzed using repeated measures ANOVA, which applied the Greenhouse-Geisser correction. Bonferroni’s method was employed to correct within-group two-by-two comparisons. The data that were not normally distributed were analyzed through a generalized linear model.
), and the comparison between groups was performed by the t-test; while those that were not normally distributed were presented as medians and quartiles [M (P25, P75)], and the comparison between groups was performed by the Mann–Whitney U-test. Qualitative data were presented as percentages, and the comparison between groups was performed by the chi-square test. The comparison of indicators at each observation time point was analyzed using repeated measures ANOVA, which applied the Greenhouse-Geisser correction. Bonferroni’s method was employed to correct within-group two-by-two comparisons. The data that were not normally distributed were analyzed through a generalized linear model.
Patients were divided into positive (POD) and negative (NPOD) groups according to the presence or absence of POD. Subsequently, the univariate analysis of all independent variables was performed to assess their association with POD. Subsequently, logistic regression analyses were performed on the screened independent variables with differences. To enhance the robustness of the results, sensitivity analyses were carried out by introducing additional covariates, such as age, duration of anesthesia, gender, and the presence of hypertension or diabetes mellitus.
All statistical tests were two-sided, and P < 0.05 was considered statistically significant.
Results
From May 2023 and August 2023, a total of 150 patients who met the inclusion criteria were assessed. Among them, 45 patients were excluded before randomization. Eventually, 105 patients were randomized into Group C (n=53) and Group P (n=52). Among these 105 patients, 21 patients were dropped from the analysis, including 1 patient with incomplete data, 5 with surgery cancellations, 4 converted to thoracotomy due to pleural abnormalities, 3 with persistent hypoxemia, 1 with hemorrhage, and 7 with a loss to follow-up. Eventually, the data of 84 participants were incorporated into the final analysis of this study. Detailed information on these participants is shown in Figure 1.
Participant Characteristics
The baseline characteristics and intraoperative data are depicted in Table 1. The characteristics of participants were well-balanced between both groups.
 |
Table 1 Demographic Characteristics and Intraoperative Data |
Primary Outcome
The overall incidence of POD in this trial was 11.9%, including 3 (7.1%) patients in Group C and 7 (16.7%) patients in Group P (RD, −9.6%; 95% CI, −23.3% to 4.1%; P = 0.178) (Table 2). There was no significant difference in the incidence of POD 1, 2, and 3 days after surgery (P = 0.178, P = 1, P = 1).
 |
Table 2 Primary Outcome |
Secondary Outcomes
The CAM score of patients in Group C was consistently lower than that in Group P within 3 days after surgery, and the differences were statistically significant (13 (12, 15) vs 15 (14, 17), P < 0.001; 12 (11, 13) vs 14 (13, 16), P < 0.001; 12 (11, 12) vs 13 (12, 14), P < 0.01).
In terms of hemodynamics, there was no statistically significant difference in the baseline MAP between both groups. However, patients in Group C exhibited a higher mean MAP (88.63 ± 6.7 vs 85 ± 8.3, P < 0.05), a higher minimum MAP (69.81 ± 9.59 vs 64.9 ± 9.43, P < 0.01), and a larger percentage of the time for blood pressure stabilization (0.6 ± 0.14 vs 0.45 ± 0.14, P < 0.001) compared with those in Group P.
In terms of SctO2-related measures, no statistically significant differences were observed in the baseline SctO2, absolute decrease, relative decrease, or incidence of desaturation between both groups. However, patients in Group C had a higher mean SctO2 (77.26 ± 3.96 vs 75.3 ± 4.49, P < 0.05), a higher minimum SctO2 (71.69 ± 4.51 vs 68.77 ± 6.46, P < 0.05), and a smaller AUT (0 (0, 0) vs 0 (0, 14), P < 0.05) than those in Group P.
In terms of drug dosages, compared with patients in Group P, those in Group C received a significantly lower dosage of sedatives (170.46 ± 9.23 vs 787.56 ± 37.23, P < 0.05) and norepinephrine (160.40 (88.80, 291.05) vs 334.20 (194.20, 573.75), P < 0.05). The dosages of analgesics and muscle relaxants were similar between both groups.
Intraoperative adverse effects and awakening quality did not differ significantly between both groups. Moreover, no patients in either group experienced intraoperative awareness or delayed awakening (Table 3).
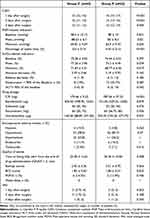 |
Table 3 Secondary Outcomes |
Vital Signs
The changes in various vital signs of patients during surgery are presented in Figure 2. The ANOVA results indicated significant differences in MAP, SctO2, and BIS over time between both groups (P < 0.05). However, there was no significant difference in HR, SpO2, and PETCO2 between both groups at individual time points (P > 0.05). After induction, patients in Group C consistently exhibited higher MAP and SctO2 and lower BIS than those in Group P at all time points. Specifically, MAP was significantly higher in Group C than in Group P at T2 and T3, SctO2 was considerably higher in Group C than in Group P at T2 and T4, and BIS was significantly lower in Group C than in Group P at T1 and T5. However, patients in the two groups had no statistically significant difference in the changes in HR, SpO2, and PETCO2 at various time points during surgery (Figure 2).
The Correlation of MAP and SctO2 with POD
The demographic characteristics and perioperative data of patients in the POD and NPOD groups were compared (Table 4). It was demonstrated that there was no statistically significant difference in age, BMI, preoperative MMSE score, ASA classification, education level, underlying disease, fluid intake and output, baseline MAP, mean MAP, baseline SctO2, and postoperative VAS score between both groups (P > 0.05). However, statistically significant differences were observed in gender, anesthesia time, minimum MAP, percentage of the time for blood pressure stabilization, mean SctO2, minimum SctO2, SctO2 desaturation incidence, absolute decrease, relative decrease, and AUT (P < 0.05).
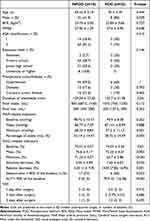 |
Table 4 Demographic Cha Racteristics and Perioperative Data |
Logistic Regression Analysis of POD-Associated Risk Factors
The logistic regression analysis results revealed several intraoperative factors associated with the risk of POD. Lower minimum MAP during surgery (OR=0.838, 95% CI 0.748–0.938, P=0.002), percentage of time to MAP stabilization (OR=0.926, 95% CI 0.875–0.980, P=0.008), mean SctO2 mean SctO2 (OR=0.801, 95% CI=0.618-0.942, P=0.007), and minimum SctO2 (OR=0.55, 95%CI=0.396-0.764, P<0.001) were identified as risk factors related to POD. Conversely, a higher absolute decrease of SctO2 during surgery (OR=1.134, 95% CI 1.002–1.284, P=0.046), relative decrease of SctO2 (OR=1.324, 95% CI 1.121–1.564, P=0.001), and <90% baseline AUT (OR=1.056, 95%CI=1.026-1.087, P<0.001) were also associated with an increased risk of POD. Furthermore, intraoperative SctO2 desaturation (OR=0.199, 95% CI 0.050–0.787, P=0.021) was identified as an additional risk factor for POD. However, the sedative medication was not a risk factor for POD (OR=0.370, 95% CI 0.068–2.025, P=0.252).
Sensitivity Analysis
To assess the robustness of our results, two sensitivity analyses were performed in this study. In the first sensitivity analysis, more covariates, including age, gender, MMSE, and duration of anesthesia, were incorporated. In the second sensitivity analysis, three additional covariates related to the medical history of patients, including hypertension, diabetes, and coronary heart disease, were incorporated. The results of these sensitivity analyses (Table 5) demonstrated that our results remained unchanged, confirming the robustness of these results.
 |
Table 5 Logistic Regression and Sensitivity Analysis Among MAP, SctO2, and POD |
Discussion
To the best of our knowledge, this may be the first prospective randomized controlled study to explore the effect of a novel intravenous sedative drug, ciprofol, on the occurrence of POD in elderly patients undergoing thoracoscopic surgery for lung cancer. These findings revealed several noteworthy outcomes. Specifically, ciprofol did not reduce the incidence of POD within three days after surgery compared with propofol. However, patients receiving ciprofol exhibited lower CAM scores, suggesting a potential improvement in postoperative cognitive function. Besides, ciprofol was associated with the maintenance of higher MAP and SctO2. In addition, the administration of ciprofol resulted in a deeper level of anesthesia while significantly reducing the dosage of sedatives and norepinephrine. Moreover, the correlation of indicators related to MAP and SctO2 with the incidence of POD was also identified. An increase in the minimum MAP, the percentage of time for MAP stabilization, mean SctO2, and minimum SctO2 corresponded to a decreased risk of POD. Conversely, an increase in the absolute decrease, relative decrease, and AUT of SctO2 was associated with a higher risk of POD. Intraoperative SctO2 desaturation events were also linked to an increased risk of POD. These findings revealed the impact of ciprofol and the importance of monitoring MAP and SctO2 in the prevention of POD during thoracoscopic surgery for lung cancer.
The pathophysiological mechanisms of POD still need to be further unraveled. It is widely acknowledged that cerebral ischemia and hypoxia,29 commonly induced by intraoperative hypoxemia and hypotension, may contribute to the development of POD. In this study, the results indicated that there was no significant difference in the incidence of POD between both groups within three days after surgery. Besides, higher MAP and SctO2 levels can be maintained during surgery in Group C. Previous research has revealed a significant correlation between the intraoperative reduction in SctO2 levels and the onset of POD. However, it remains controversial over the precise threshold of SctO2 reduction.30–32 Notably, a prospective cohort study on thoracotomy with one-lung ventilation suggested that a relatively decreased ratio of 10% for SctO2 compared with the baseline value was highly related to POD.33 Based on that, a similar threshold for SctO2 decrease was utilized in this study. It was concluded that the incidence of SctO2 desaturation and the AUT of SctO2 <90% of the baseline were associated with the development of POD, which was consistent with the finding of Fan Cui et al.
The results of this study suggested that sustained intraoperative hypotension may lead to perioperative cerebral ischemia and hypoxia, which can result in damage to hippocampal cells and mitochondrial structural dysfunction, ultimately increasing the risk of POD.34 Some scholars have proposed that maintaining blood pressure levels above 110% of the baseline contributes to a lower incidence of POD. Besides, the perioperative blood pressure within the range of 80% to 90% of the baseline MAP for more than 30 min can influence SctO2 values and the incidence of POD.32 Similarly, this study examined the percentage of time, during which the MAP was maintained within the range of 110% to 90% of the baseline, yielding similar findings.
This result contradicted the established principles of neurovascular coupling and the cerebral vasculature autoregulation confirmed in most studies.35 This suggested that postanesthetic hypotension may not significantly impact cerebral perfusion, indicating that the MAP was maintained above the lower threshold of the cerebrovascular autoregulation threshold (50 mmHg). However, the majority of patients in this trial consisted of elderly patients aged over 65, and the reduced vascular elasticity, cerebral ischemia, chronic hypertension, diabetes mellitus, and congenital cerebral circulatory anomalies of these patients may blunt the normal regulatory mechanisms responsible for maintaining cerebral perfusion.36 For instance, hypertension can elevate the autoregulatory threshold of cerebral blood flow, a condition that persists even after prolonged and effective antihypertensive therapy.37,38 Elderly patients with a history of cardiovascular and cerebrovascular diseases may have a particularly higher risk of immediate or sustained hypotension.
The dosage of propofol not only depresses the circulation but also constricts cerebral blood vessels, thus reducing the cerebral oxygen metabolism rate, cerebral blood flow, and cerebral blood volume in a dose-dependent manner.39,40 It remains unclear about the effects of ciprofol on the cerebrovascular system. However, the results of this study indicated that combining ciprofol with remifentanil for anesthesia maintenance during thoracoscopic surgery in elderly patients can improve intraoperative blood pressure and SctO2, potentially reducing postoperative organ injury risks. Meanwhile, the mean dose of ciprofol used for anesthesia maintenance was only 1/5 to 1/4 of that of propofol. Despite the lower dose, patients in Group C consistently maintained lower intraoperative BIS values than those in Group P. This result further demonstrated that ciprofol had a stronger binding activity to GABAA receptors, allowing patients to achieve adequate anesthesia depth with a much smaller dose compared with propofol. The evidence from an animal study also indicated that ciprofol exhibited a 2.4 times higher therapeutic index and a broader safety profile than propofol.41 The difference may be attributed to the introduction of a ciprofol group to the core structure of propofol, which reduced lipophilicity, broke the original structure’s symmetry, and enhanced the affinity of GABAA receptors.42 There are also some trials reporting that ciprofol is superior to propofol in target selectivity and higher acting strength.43 The special structure of ciprofol conduces to achieving the required surgical anesthesia depth with minimal risks of circulatory depression.
Additionally, the dosage of intraoperative norepinephrine in Group C was much smaller than that in Group P, which may further affect changes in the intraoperative blood pressure and SctO2. It has been demonstrated that the administration of norepinephrine can lead to a sustained decrease in SctO2, which may be attributed to alterations in the cerebral arterial-to-venous blood volume ratio resulting from the reduced cardiac output and arteriolar autoconstriction.44,45
This trial may be the first scientific attempt to compare the effects of ciprofol with propofol on POD. As a derivative of propofol, ciprofol had no analgesic effect. Besides, there was no difference in the dosages of intraoperative analgesia and postoperative pain scores between the two groups in this study. This suggested that ciprofol did not reduce POD by alleviating postoperative pain. However, the results of this study indicated that the low incidence of POD in Group C may be related to the hemodynamically stabilizing properties of ciprofol. In addition, its anti-inflammatory effects, as demonstrated in other experiments,23,24 might be one of the contributing factors. So far, there is no trial to explore the effect of ciprofol on POD compared with other sedatives, which may provide a direction for future research.
Some medical associations, such as the European Society of Anaesthesiology and the American Geriatrics Society, offer evidence-based guidelines for POD management,46 with a focus on the identification of precipitating etiologies and relevant treatment. Our findings provided new insights into the prevention of POD, especially in elderly patients. Compared with propofol, ciprofol applied to thoracic surgery in elderly patients is more likely to maintain higher MAP and SctO2 during surgery, avoiding larger hemodynamic fluctuations or insufficient oxygen supply. Therefore, in addition to preoperative education and pain management, anesthesiologists can choose anesthesia drugs with hemodynamic stabilization and anti-inflammatory effects, such as ciprofol and dexmedetomidine. This conduces to maintaining a relatively high level of MAP and SctO2 during surgery for the treatment of patients at high risks, which may prevent POD at a larger extent.
In this study, the difference in CAM scores was calculated, and significant changes in MAP and SctO2 were identified as risk factors associated with POD. However, there was no significant difference in the incidence of POD between both groups. The overall incidence of POD was 11.9%, lower than that reported in previous studies, possibly due to the following reasons. Firstly, the sample size of this study was insufficient. Although the sample size of the trial was calculated based on pre-test results, a closer examination of outcomes suggested that a much larger cohort, approximately 785 cases, was required to establish a significant clinical difference. Secondly, the majority of subjects in this study consisted of elderly patients without cerebrovascular diseases, characterized by a good preoperative cognitive ability, and the surgical duration was no more than three hours. Favorable patient conditions, shorter surgical duration, and minimally invasive thoracoscopic surgery may avoid some risk factors associated with POD. Thirdly, a single POD assessment method was employed in this study, which may introduce false positives and negatives in the final evaluation. Lastly, only clinically relevant data were monitored in this study and POD-related markers were not investigated at the molecular level. These limitations may induce some bias in the results of this study. Furthermore, the differences in the postoperative quality of recovery and hospitalization length were not analyzed in the short-term study. It is necessary to further explore the long-term effects of ciprofol or its application in different patient populations.
Conclusion
Compared with propofol, the administration of ciprofol did not increase the incidence of POD during general anesthesia in elderly patients undergoing thoracoscopic surgery for lung cancer. Notably, ciprofol exhibited advantages in maintaining superior hemodynamic stability, optimizing cerebral oxygenation, and reducing postoperative CAM scores. The effect of ciprofol on POD still needs to be further confirmed through multicenter trials with different surgical types.
Data Sharing Statement
All data generated or analyzed during this study were included in the published article. Further inquiries about the datasets can be directed to the corresponding author upon reasonable request.
Acknowledgments
The authors would like to acknowledge all staff who assisted this RCT.
Funding
This work was supported by Beijing Medical Award Foundation yxjl-2019-0163-0029.
Disclosure
The authors report no conflicts of interest in this work.
References
1. McCusker J, Cole MG, Dendukuri N, Belzile E. Does delirium increase hospital stay? J Am Geriatr Soc. 2003;51(11):1539–1546. doi:10.1046/j.1532-5415.2003.51509.x
2. Swarbrick CJ, Partridge JSL. Evidence-based strategies to reduce the incidence of postoperative delirium: a narrative review. Anesthesia. 2022;77(1):92–101. doi:10.1111/anae.15607
3. Lung Cancer specialty Committee of Chinese Elderly Health Care Association, Enhanced Recovery after Surgery Specialty Committee of TianJin Medicial and Health Association. Consensus of Chinese Experts on Surgical Treatment of Lung Cancer in the Elderly(2022 Edition). Chin J Lung Cancer. 2023;26(3):177–192. Chinese. doi:10.3779/j.issn.1009-3419.2023.102.09
4. Bade BC, Dela Cruz CS. Lung Cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med. 2020;41(1):1–24. doi:10.1016/j.ccm.2019.10.001
5. Hoy H, Lynch T, Beck M. Surgical treatment of lung cancer. Crit Care Nurs Clin North Am. 2019;31(3):303–313. doi:10.1016/j.cnc.2019.05.002
6. Zhang CJ, Ma JH, Jin F, Li XH, Jia HQ, Mu DL. Effect of one-lung ventilation on the correlation between left and right cerebral saturation. BMC Anesthesiol. 2023;23(1):50. doi:10.1186/s12871-023-02001-7
7. Hayashi K, Motoishi M, Sawai S, Horimoto K, Hanaoka J. Postoperative delirium after lung resection for primary lung cancer: risk factors, risk scoring system, and prognosis. PLoS One. 2019;14(11):e0223917. doi:10.1371/journal.pone.0223917
8. Murakawa K, Kitamura Y, Watanabe S, Hongo S, Shinomiya K, Sendo T. Clinical risk factors associated with postoperative delirium and evaluation of delirium management and assessment team in lung and esophageal cancer patients. J Pharm Health Care Sci. 2015;1:4. doi:10.1186/s40780-014-0002-3
9. Ozyurtkan MO, Yildizeli B, Kuscu K, et al. Postoperative psychiatric disorders in general thoracic surgery: incidence, risk factors and outcomes. Eur J Cardiothorac Surg. 2010;37(5):1152–1157. doi:10.1016/j.ejcts.2009.11.047
10. Wachtendorf LJ, Azimaraghi O, Santer P, et al. Association between intraoperative arterial hypotension and postoperative delirium after noncardiac surgery: a retrospective multicenter cohort study. Anesth Analg. 2022;134(4):822–833. doi:10.1213/ANE.0000000000005739
11. Wang JY, Li M, Wang P, Fang P. Goal-directed therapy based on rScO(2) monitoring in elderly patients with one-lung ventilation: a randomized trial on perioperative inflammation and postoperative delirium. Trials. 2022;23(1):687. doi:10.1186/s13063-022-06654-6
12. Kazan R, Bracco D, Hemmerling TM. Reduced cerebral oxygen saturation measured by absolute cerebral oximetry during thoracic surgery correlates with postoperative complications. Br J Anaesth. 2009;103(6):811–816. doi:10.1093/bja/aep309
13. Hirsch J, DePalma G, Tsai TT, Sands LP, Leung JM. Impact of intraoperative hypotension and blood pressure fluctuations on early postoperative delirium after non-cardiac surgery. Br J Anaesth. 2015;115(3):418–426. doi:10.1093/bja/aeu458
14. Matsuoka T, Ishiyama T, Shintani N, Kotoda M, Mitsui K, Matsukawa T. Changes of cerebral regional oxygen saturation during pneumoperitoneum and Trendelenburg position under propofol anesthesia: a prospective observational study. BMC Anesthesiol. 2019;19(1):72. doi:10.1186/s12871-019-0736-4
15. Fassaert LMM, de Borst GJ, Pennekamp CWA, et al. Effect of phenylephrine and ephedrine on cerebral (Tissue) oxygen saturation during carotid endarterectomy (PEPPER): a randomized controlled trial. Neurocrit Care. 2019;31(3):514–525. doi:10.1007/s12028-019-00749-w
16. Xu X, Hu X, Wu Y, et al. Effects of different BP management strategies on postoperative delirium in elderly patients undergoing Hip replacement: a single center randomized controlled trial. J Clin Anesth. 2020;62:109730. doi:10.1016/j.jclinane.2020.109730
17. Qin C, Jiang Y, Lin C, Li A, Liu J. Perioperative dexmedetomidine administration to prevent delirium in adults after non-cardiac surgery: a systematic review and meta-analysis. J Clin Anesth. 2021;73:110308. doi:10.1016/j.jclinane.2021.110308
18. Iamaroon A, Wongviriyawong T, Sura-Arunsumrit P, Wiwatnodom N, Rewuri N, Chaiwat O. Incidence of and risk factors for postoperative delirium in older adult patients undergoing noncardiac surgery: a prospective study. BMC Geriatr. 2020;20(1):40. doi:10.1186/s12877-020-1449-8
19. Liang P, Dai M, Wang X, et al. Efficacy and safety of ciprofol vs. propofol for the induction and maintenance of general anesthesia: a multicentre, single-blind, randomized, parallel-group, Phase 3 clinical trial. Eur J Anaesthesiol. 2023;40(6):399–406. doi:10.1097/EJA.0000000000001799
20. Qin K, Qin WY, Ming SP, Ma XF, Du XK. Effect of ciprofol on induction and maintenance of general anesthesia in patients undergoing kidney transplantation. Eur Rev Med Pharmacol Sci. 2022;26(14):5063–5071. doi:10.26355/eurrev_202207_29292
21. Wei A, Yang L, Ma S, Jin G, Yang M, Zhou J. A case report of ciprofol overdose during anesthesia/analgesia and literature review: clinical presentation, blood pressure, and management. J Int Med Res. 2022;50(11):3000605221132466. doi:10.1177/03000605221132466
22. Ding YY, Long YQ, Yang HT, Zhuang K, Ji FH, Peng K. Efficacy and safety of ciprofol for general anesthesia induction in elderly patients undergoing major noncardiac surgery: a randomized controlled pilot trial. Eur J Anaesthesiol. 2022;39(12):960–963. doi:10.1097/EJA.0000000000001759
23. Yang Y, Xia Z, Xu C, Zhai C, Yu X, Li S. Ciprofol attenuates the isoproterenol-induced oxidative damage, inflammatory response and cardiomyocyte apoptosis. Front Pharmacol. 2022;13:1037151. doi:10.3389/fphar.2022.1037151
24. Shang PP, Liu XN, Fan JC, et al. Neuroprotective effect cyclophenol on Parkinson’s disease rats through Wnt/β- catenin signaling pathway. Drug Ang Clinic. 2022;37(3):453–457.
25. Rasheed AM, Amirah MF, Abdallah M, Parameaswari PJ, Issa M, Alharthy A. Ramsay sedation scale and Richmond agitation sedation scale: a cross-sectional study. Dimens Crit Care Nurs. 2019;38(2):90–95. doi:10.1097/DCC.0000000000000346
26. Ying Y, Fei S, Zeng Z, Qu X, Cao Z. Comparative study of dezocine and ketorolac tromethamine in patient-controlled intravenous analgesia of laparoscopic cholecystectomy. Front Surg. 2022;9:881006. doi:10.3389/fsurg.2022.881006
27. Faiz KW. VAS--visual analog scale. Tidsskr Nor Laegeforen. 2014;134(3):323. doi:10.4045/tidsskr.13.1145
28. Ho MH, Nealon J, Igwe E, et al. Postoperative delirium in older patients: a systematic review of assessment and incidence of postoperative delirium. Worldviews Evid Based Nurs. 2021;18(5):290–301. doi:10.1111/wvn.12536
29. Hshieh TT, Inouye SK, Oh ES. Delirium in the Elderly. Clin Geriatr Med. 2020;36(2):183–199. doi:10.1016/j.cger.2019.11.001
30. Srinivasan MA, LaMotte RH. Tactile discrimination of shape: responses of slowly and rapidly adapting mechanoreceptive afferents to a step indented into the monkey fingerpad. J Neurosci. 1987;7(6):1682–1697. doi:10.1523/JNEUROSCI.07-06-01682.1987
31. Shen L, Chen JQ, Yang XL, et al. Flurbiprofen used in one-lung ventilation improves intraoperative regional cerebral oxygen saturation and reduces the incidence of postoperative delirium. Front Psychiatry. 2022;13:889637. doi:10.3389/fpsyt.2022.889637
32. Tang L, Kazan R, Taddei R, Zaouter C, Cyr S, Hemmerling TM. Reduced cerebral oxygen saturation during thoracic surgery predicts early postoperative cognitive dysfunction. Br J Anaesth. 2012;108(4):623–629. doi:10.1093/bja/aer501
33. Cui F, Zhao W, Mu DL, et al. Association between cerebral desaturation and postoperative delirium in thoracotomy with one-lung ventilation: a prospective cohort study. Anesth Analg. 2021;133(1):176–186. doi:10.1213/ANE.0000000000005489
34. Nguyen DN, Huyghens L, Parra J, Schiettecatte J, Smitz J, Vincent JL. Hypotension and a positive fluid balance are associated with delirium in patients with shock. PLoS One. 2018;13(8):e0200495. doi:10.1371/journal.pone.0200495
35. Meng L, Gelb AW, McDonagh DL. Changes in cerebral tissue oxygen saturation during anaesthetic-induced hypotension: an interpretation based on neurovascular coupling and cerebral autoregulation. Anesthesia. 2013;68(7):736–741. doi:10.1111/anae.12254
36. Zhang Y, Tan J, Li P, et al. The perioperative application of continuous cerebral autoregulation monitoring for cerebral protection in elderly patients. Ann Palliat Med. 2021;10(4):4582–4592. doi:10.21037/apm-21-707
37. Strandgaard S, Paulson OB. Cerebral blood flow and its pathophysiology in hypertension. Am J Hypertens. 1989;2(6 Pt 1):486–492. doi:10.1093/ajh/2.6.486
38. Koh JL, Levin SD, Chehab EL, Murphy GS. Neer Award 2012: cerebral oxygenation in the beach chair position: a prospective study on the effect of general anesthesia compared with regional anesthesia and sedation. J Shoulder Elbow Surg. 2013;22(10):1325–1331. doi:10.1016/j.jse.2013.01.035
39. Ruzman T, Simurina T, Gulam D, Ruzman N, Miskulin M. Sevoflurane preserves regional cerebral oxygen saturation better than propofol: randomized controlled trial. J Clin Anesth. 2017;36:110–117. doi:10.1016/j.jclinane.2016.10.010
40. Engelhard K, Werner C. Inhalational or intravenous anesthetics for craniotomies? Pro inhalational. Curr Opin Anaesthesiol. 2006;19(5):504–508. doi:10.1097/01.aco.0000245275.76916.87
41. Liao J, Li M, Huang C, et al. Pharmacodynamics and pharmacokinetics of HSK3486, a Novel 2,6-disubstituted phenol derivative as a general anesthetic. Front Pharmacol. 2022;13:830791. doi:10.3389/fphar.2022.830791
42. Lu M, Liu J, Wu X, Zhang Z. Ciprofol: a novel alternative to propofol in clinical intravenous anesthesia? Biomed Res Int. 2023;2023:7443226. doi:10.1155/2023/7443226
43. Qin L, Ren L, Wan S, et al. Design, synthesis, and evaluation of novel 2,6-disubstituted phenol derivatives as general anesthetics. J Med Chem. 2017;60(9):3606–3617. doi:10.1021/acs.jmedchem.7b00254
44. Moerman AT, Vanbiervliet VM, Van Wesemael A, Bouchez SM, Wouters PF, De Hert SG. Assessment of cerebral autoregulation patterns with near-infrared spectroscopy during pharmacological-induced pressure changes. Anesthesiology. 2015;123(2):327–335. doi:10.1097/ALN.0000000000000715
45. Poterman M, Vos JJ, Vereecke HE, et al. Differential effects of phenylephrine and norepinephrine on peripheral tissue oxygenation during general anesthesia: a randomized controlled trial. Eur J Anaesthesiol. 2015;32(8):571–580. doi:10.1097/EJA.0000000000000247
46. Vlisides P, Avidan M. Recent advances in preventing and managing postoperative delirium. F1000Res. 2019;F1000:1.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

