Back to Journals » International Journal of Nanomedicine » Volume 15
The Effect of Cerium Oxide on Lung Tissue in Lower Extremity Ischemia Reperfusion Injury in Sevoflurane Administered Rats
Authors Tuncay A , Sivgin V, Ozdemirkan A , Sezen SC , Boyunaga H, Kucuk A , Gunes I , Arslan M
Received 28 May 2020
Accepted for publication 21 August 2020
Published 6 October 2020 Volume 2020:15 Pages 7481—7489
DOI https://doi.org/10.2147/IJN.S263001
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Aydin Tuncay,1 Volkan Sivgin,2 Aycan Ozdemirkan,2 Saban Cem Sezen,3 Hakan Boyunaga,4 Aysegul Kucuk,5 Isin Gunes,6 Mustafa Arslan2
1Faculty of Medicine, Department of Cardiovascular Surgery, Erciyes University, Kayseri, Turkey; 2Faculty of Medicine, Department of Anesthesiology and Reamination, Gazi University, Ankara, Turkey; 3Faculty of Medicine, Department of Histology and Embryology, Kırıkkale University, Kırıkkale, Turkey; 4Faculty of Medicine, Department of Medical Biochemistry, Kırıkkale University, Kırıkkale, Turkey; 5Faculty of Medicine, Department of Physiology, Kütahya Health Science University, Kütahya, Turkey; 6Faculty of Medicine, Department of Anesthesiology and Reamination, Erciyes University, Kayseri, Turkey
Correspondence: Mustafa Arslan Gazi University, Faculty of Medicine
Department of Anesthesiology and Reanimation, Ankara 06510, Turkey
Tel +90 533 422 85 77
Email [email protected]
Introduction: We aimed to investigate the effects of cerium oxide, applied before the sevoflurane anesthesia, on lung tissue in rats with lower extremity ischemia-reperfusion (IR).
Materials and Methods: A total of 30 rats were randomly divided into five groups as; control (C), IR, cerium oxide-IR (CO-IR), IR-sevoflurane (IRS), and cerium oxide-IR-sevoflurane (CO-IRS). In the CO-IR group, 30 minutes after the injection of cerium oxide (0.5 mg/kg, intraperitoneal (i.p)), an atraumatic microvascular clamp was placed on the infrarenal abdominal aorta for 120 minutes. Then, the clamp was removed and reperfused for 120 minutes. Sevoflurane was applied in 100% oxygen at a rate of 2.3% at 4 L/min during IR. The blood samples were taken for biochemical analysis and the lung tissue samples were taken for histological analysis.
Results: Neutrophil infiltration/aggregation was significantly higher in the IR group than in the C and CO-IRS groups. The alveolar wall thickness and total lung injury scores were significantly higher in the IR group than in the C, IRS, CO-IR and CO-IRS groups.
Discussion: We determined that the administration of 0.5 mg/kg dose of cerium oxide with sevoflurane reduces the oxidative stress and corrects IR-related damage in lung tissue. Our results show that the administration of cerium oxide before IR and the administration of sevoflurane during IR have a protective effect in rats.
Keywords: cerium oxide, sevoflurane, ischemia reperfusion, biochemical analysis, histopathological analysis
Introduction
Temporary interruption of blood flow by clamping the infrarenal abdominal aorta such as abdominal aortic aneurysm surgery is a common surgical procedure. The reperfusion period, which defines the re-supply of blood flow and oxygen by termination of the interruption, worsens the damage at the cellular level occurring in the ischemic period. This phenomenon is known as ischemia reperfusion (IR) injury.1,2
It is known that application of a cross-clamp during infrarenal abdominal aortic surgery and then removal of the clamp results in the development of aortic IR injury.3 The formation of free oxygen radicals, systemic vasoconstrictive mediators, neutrophil activation, lipid peroxidation and systemic inflammatory response after the aortic IR injury cause distant organ damage.3,4 Nowadays, many treatment strategies have been developed to prevent and reduce the IR damage. Despite the developments in cardiovascular surgery techniques and early postoperative follow-up applications, IR damage after aortic interventions is a serious problem affecting postoperative morbidity and mortality. Tthe most appropriate strategy is still a matter of debate.
Various drugs, including sevoflurane, isoflurane, desflurane, dexmedetomidine, and ketamine, are used to prevent IR damage in anesthesia.5,6 Volatile agents are an important member of perioperative medication and almost all patients receiving general anesthesia will meet volatile agents. In previous studies with rats and mice, volatile agents have been shown to reduce inflammation and necrosis and protect from IR injury.7,8 Cerium oxide nanoparticles are effective scavengers of reactive oxygen species.9 It has a wide range of applications such as: solar cells, fuel cells, gas sensors, oxygen pumps, and also used as a fuel additive.10 This rare-earth metal has been listed as one of five nanomaterials under investigation by the US Environmental Protection Agency. There is also growing interest in the medical field due to its potential antioxidant capabilities.11,12 Nonetheless, there are few studies about in vitro and in vivo inflammatory effects associated with cerium oxide, especially how it might alter systemic inflammatory responses.13 Also numerous studies have indicated the potential use of cerium oxide nanoparticles on cardiomyopathy,12 stroke,14 ovarian cancer,15 sepsis,16 obesity,17 hepatic IR,18 lower extremity IR,19 and intestinal IR.20 However, in these studies the effect of cerium oxide was investigated alone.
In this study, we aimed to study the combined effects of sevoflurane and cerium oxide on lung tissue of IR injury with the help of biochemical and histopathological analysis.
Materials and Methods
Animals and Experimental Protocol
This study was approved by the Gazi University Ethics Committee. (Ethic number: G.U.ET-19-044). All of the procedures were performed according to accepted standards of Guide for the Care and Use of Laboratory Animals. At the beginning of experimental procedures all rats were anesthetized with ketamine (50 mg/kg, i.p) and romphun (20 mg/kg, i.p.). During the surgical procedure rats were placed on a heating pad in order to maintain the body temperature. Rats were kept in a temperature-controlled (21±1°C) and humidity-controlled (45–55%) room, which was maintained on a 12/12 reversed light cycle. Animals were fed with a standard pellet and allowed to drink water ad libitum.
A total of 30 Wistar Albino rats weighing between 200 and 250 grams were used. Animals were equally divided into five equal groups (n: 6); control (C), ischemia-reperfusion (IR), cerium oxide-IR (CO-IR), IR-sevoflurane (IRS), and cerium oxide-IR-sevoflurane (CO-IRS).
Control group (C): Rats were only subjected to midline laparotomy.
Ischemia reperfusion group (IR): Rats were only subjected to midline laparotomy. An atraumatic microvascular clamp was placed on the infrarenal abdominal aorta for 120 minutes, then the clamp was removed and reperfused for 120 minutes. Sodium heparin (500 IU/kg) was administered through the peripheral vein in the tail for the maintenance of reperfusion after occlusion.21
Cerium oxide-ischemia reperfusion (CO-IR): Rats were only subjected to midline laparotomy. An atraumatic microvascular clamp was placed on the infrarenal abdominal aorta during 120 minutes, then the clamp was removed and reperfused for 120 minutes. Cerium oxide (Sigma Aldrich®, Cerium oxide, aqueous nanoparticle dispersion, 100 mL) was given (0.5 mg.kg−1, i.p) 30 minutes before the ischemia period.16,19,22,23
Ischemia reperfusion-sevoflurane (IRS): Rats were only subjected to midline laparotomy. An atraumatic microvascular clamp was placed on the infrarenal abdominal aorta for 120 minutes then the clamp was removed and reperfused for 120 minutes. Anesthetic gas vaporizers were calibrated and set a minimum alveolar concentration (MAC) of 1 sevoflurane (2.3%). The anesthesia procedure was conducted on the rats in a transparent plastic box (40 x 40 x 70 cm). Sevoflurane was applied with the 2.3% inspiratory concentration at a rate of 4 L.min−1 in 100% O2 for 4 hours.
Cerium oxide-ischemia reperfusion-sevoflurane (CO-IRS): Rats were only subjected to midline laparotomy. An atraumatic microvascular clamp was placed on the infrarenal abdominal aorta for 120 minutes, then the clamp was removed and reperfused for 120 minutes. Cerium oxide was given (0.5 mg.kg−1, i.p) 30 minutes before the ischemia period. Anesthetic gas vaporizers were calibrated and set a MAC of 1 sevoflurane (2.3%). The anesthesia procedure was conducted on the rats in a transparent plastic box (40 x 40 x 70 cm). Sevoflurane was applied with the 2.3% inspiratory concentration at a rate of 4 L.min−1 in 100% O2 for 4 hours.
At the end of the experiments rats were sacrificed under anesthesia. Then the blood samples were taken for biochemical analysis and the lung tissues were excised for histopathological analysis.
Biochemical Analysis
Malondialdehyde (MDA), and nitric oxide (NO) concentrations were measured. Lipid peroxidation was measured using the Esterbauer method. First of all, MDA reacted with thiobarbituric acid at 90°C–95°C and thereby yielded pink chromogranin. Specimens were rapidly cooled and absorbances were then read at 532 nm spectrophotometrically. Results were presented as nmol/g tissue protein.24 Stable oxidative NO metabolites (NO2- vs NO3-) were measured in the serum, and to determine, NO production was measured. The Griess reaction was used in order to measure the nitrite concentration.25
Histopathological Analysis
Lung tissue samples were removed and fixed in 10% neutral formalin solution. Then the lungs were examined with light microscopy by the same pathologist, who was blinded to the study. A total of 10 random areas were evaluated with 200–400 times magnified microscopy in hematoxylin and eosin (H&E) stained sections. Stained slides were examined under a light microscope. Neutrophil infiltration and alveolar thickness are measured in each specimen for exposing the degree of lung injury area. Each parameter was scored as any (0 point), only a little (1 point), medium amount (2 points), or severe (3 points); The two scores were added and noted as last lung injury score.26
Statistical Analysis
Statistical Package for the Social Sciences v. 20.0 (SPSS, IBM Corp., Armonk, NY, USA) for Windows was used. Each categorical variables were analyzed by Kolmogorov–Smirnov test. MDA levels, NO activities and histopathological parameters were tested by using the Kruskal–Wallis test, Bonferroni Correction test and Mann–Whitney U-test. A statistical value of less than 0.05 was considered significant. All values were expressed as mean± standard deviation (Mean ± SD).
Results
Biochemical Results
Significant differences were found in serum MDA levels among the groups (p=0.001). MDA levels were similar in the C, IRS, IRSO and IRSOS groups (Table 1). However the levels of MDA were notably lower in C, IRS, CO-IR, and CO-IRS groups compared to the IR group (p<0.0001, p=0.015, p=0.001, p<0.0001, respectively).
 |
Table 1 MDA and NO Levels [Mean ± SD] |
Serum NO activity between the groups was significantly different (p=0.022). NO activity in the C, IRS, CO-IR and CO-IRS groups were similar (Table 1), but the activity of NO was higher in the IR group than C, CO-IR and CO-IRS groups (p=0.003, p=0.019, p=0.005, respectively).
Histopathological Results
According to neutrophil infiltration/aggregation there were significant differences between the groups (p=0.010). Neutrophil infiltration/aggregation was significantly higher in the IR group compared to the C and CO-IRS groups (p=0.001, p=0.020, respectively), (Table 2 and Figures 1–5). The alveolar wall thickness was significantly higher in the IR group than in the C, IRS, CO-IR and CO-IRS groups (p<0.0001, p=0.004, p=0.001, p=0.001, respectively), (Table 2 and Figures 1–5). Also total lung injury scores were significantly higher in the IR group compared to the C, IRS, CO-IR and CO-IRS groups (p<0.0001, p=0.005, p=0.001, p<0.0001, respectively), (Table 2 and Figures 1–5).
 |
Table 2 Histopathological Data of Lung Tissue [Mean ± SD] |
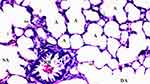 |
Figure 1 Normal-structural lung tissue parenchyma in the control group, HEx100. |
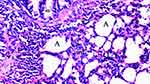 |
Figure 2 Severe neutrophilic infiltration and increased alveolar wall thickness in IR group, HEx100. |
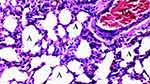 |
Figure 3 Mild neutrophilic infiltration and increased alveolar wall thickness in IRS group, HEx100. |
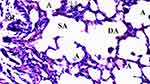 |
Figure 4 Mild neutrophilic infiltration and increased alveolar wall thickness in CO-IR group, HEx100. |
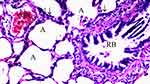 |
Figure 5 Mild neutrophilic infiltration and increased alveolar wall thickness in CO-IRS group, HEx100. |
Discussion
Lungs are the target organs in distant organ damages especially after the lower limb IR, In aortic surgery hypoxemia, pulmonary hypertension, decreased lung compliance and nonhydrostatic pulmonary edema emerges as a sign of lung injury caused by lower extremity IR can lead to a serious condition ranging from a temporary subclinical course to an acute distressed respiratory syndrome.27
It is known that lung damage occurs because of the systemic inflammatory response after IR. The primary effects of oxygen radicals released into the medium on the lungs include vascular endothelial damage, increased permeability and consequently pulmonary edema.28,29 Although many studies in the literature use different parameters, lung damage has been evaluated especially with light microscopic findings, histopathological staining and biochemical parameters. In our study we aimed to investigate the effects of cerium oxide, that applied before the sevoflurane anesthesia, on lung tissue in rats with lower extremity IR.
MDA is a stable end product formed as a result of peroxidation of polyacited fatty acids and is considered as a marker of cell wall peroxidation. Plasma and tissue levels of MDA are considered to be an indicator of systemic response following oxidative stress and IR.30 Increased lipid peroxidation causes the release of proteolytic lysosomal enzymes and mitochondrial matrix enzymes into the plasma. This is one of the important causes of cellular damage.31
NO is one of the significant markers in the formation of lung injury caused by lower extremity IR. Acute ischemia of the lower extremities also leads to an increase in NO production in the lung. NO interacts with free oxygen radicals (such as the superoxide anion), while creating molecules such as peroxynitrite, on the other hand, it inhibits neutrophils from adhesion, accumulating and emptying their contents. Endothelial cells activate genes that increase the number of endothelial nitric oxide synthetase (iNOS) enzymes that provide NO synthesis during reperfusion and thus try to prevent damage with increased NO amount.32,33
A series of studies in the literature including sevoflurane and desflurane, has previously shown that the volatile anesthetic agents reduce inflammation values, thus protecting against IR damage.8,34 Numerous studies both in experimental animals35 and in patients undergoing cardiovascular surgery,36 mention that the application of volatile anesthetics, immediately before or after ischemic conditions has a healing effect. Sevoflurane pre-medication has also been shown to protect heart,37 lung38 and kidneys34,38 against IR injury. Lee et al8 studied the protective effects of volatile anesthetics, but it has not been fully clarified at which stage (ischemia, reperfusion or both ischemia and reperfusion stage).
Xu et al39 evaluated the damage of lung after liver IR by measuring MDA levels. They demonstrated that MDA levels increased after IR, but decreased after sevoflurane administration. Oncul et al40 compared the effects of propofol and sevoflurane and they reported that lipid peroxidation could be suppressed better in the sevoflurane group than in the propofol group during the early reperfusion period.
Kalb et al38 investigated the effects of pre- or post-conditioning with sevoflurane showing pulmonary neutrophil accumulation after IR injury of the aorta. They demonstrated that pre-conditioning, but not post-conditioning, with sevoflurane reduced pulmonary neutrophil accumulation after IR injury. As a result they concluded that neutrophil accumulation played a major role in the pathophysiology of acute lung injury.38
Kosucu et al41 focused on the effects of sevoflurane anesthesia combined with epidural anesthesia on IR injury in patients undergoing surgical revascularization due to aorto iliac occlusive disease. They measured the plasma MDA and ischemia modified albümin (IMA) levels and demonstrated that serum levels of MDA and IMA were lower in the study group compared to the control group. As a consequence, they concluded that the sevoflurane anesthesia combined with epidural anesthesia might decrease the IR injury in aorto iliac occlusive disease.41
PCO2, dry/wet lung ratio and permeability outcomes were compared and the protective effects of sevoflurane on lung IR damage were demonstrated by Li et al.42 Similarly Liu et al43 compared pulmonary vascular resistance, microvascular permeability, wet/dry lung ratios and the changes in parameters such as lactate dehydrogenase (LDH), NO and tumor necrosis factor-alpha (TNF-α). As a result they reported that administration of sevoflurane before ischemia decreased IR injury. In another study sevoflurane decreases IR damage by making changes in oxygen values (PO2/FiO2) and miRNA expression.44 Luo et al45 showed that sevoflurane exerts its effect by decreasing mast cell activation and oxidative damage in the indirect lung damage occurring after intestinal IR injury.45
In the literature, numerous studies have tested the effects of cerium oxide nanoparticles in animal models of tissue damage. Following radiation=induced damage in mice, intravenous injection of cerium oxide nanoparticles has been shown to ameliorate intestinal injury.15 After systemic bacterial infection, intraperitoneal injection of cerium oxide nanoparticles has been shown to attenuate acute kidney injury in rats.46 Although systemic administration of these nanoparticles in mice is well tolerated,47 there is evidence for potential toxicity effects of nanoceria. For example, aerosol delivery of cerium oxide nanoparticles can promote pulmonary inflammation.48 Yokel et al49 showed that cerium oxide nanoparticles remain in the circulation for a short period of time such as t1/2 of 7.5 minute upon intravenous injection.49 Oxidative stress increases upon reperfusion and 1 hour prior to ischemia the administration of cerium oxide nanoparticles would result in bioaccumulation of cerium oxide nanoparticles in liver.2,50 In another study, cerium oxide nanoparticles have been shown to reduce IR-induced cell death and recommend their use for prophylaxis against liver damage associated with graft failure. They concluded that cerium oxide nanoparticles may be a new therapeutic approach for hepatic IR injury.
One of the methods to show IR damage in the lung is to examine histopathological preparations with light microscopy after staining with H&E.32 In our study, neutrophil infiltration/aggregation in lung tissue, alveolar wall thickness and total damage score were evaluated quantitatively in light microscopy with H&E staining.
In our study, it was seen that the COS-IR group had an important effect in preventing neutrophil infiltration/aggregation compared to the IR group. In addition, it was found that the alveolar wall thickness and total lung injury scores were significantly decreased in cerium oxide and sevoflurane groups. With infrarenal aortic occlusion and subsequent lower limb reperfusion, IR damage occurred in the lung. The combined administration of sevoflurane and cerium oxide reduces IR damage in the lung. Significant reduction in MDA levels suggests that the damage may be associated with lipid peroxidation. Administration of cerium oxide and sevoflurane decreased the IR damage by changing lipid peroxidation. Also histopathological results support the biochemical data and show that co-administration of cerium oxide and sevoflurane is more effective in preventing lung damage.
As a result the examination of cerium oxide on IR-related lung injury showed that pretreatment with 0.5 mg/kg intraperitoneal cerium oxide effectively decreased lung injury in sevoflurane-administered rats. However different and repetitive doses would be helpful for clarifying these effects.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Xu SQ, Li YH, Hu SH, Chen K, Dong LY. Effects of Wy14643 on hepatic ischemia reperfusion injury in rats. World J Gastroenterol. 2008;14:6936–6942. doi:10.3748/wjg.14.6936
2. Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, Piña E, Geller DA. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res. 2008;147:153–159. doi:10.1016/j.jss.2007.06.015
3. Joyce M, Kelly C, Winter D, Chen G, Leahy A, Bouchier-Hayes D. Pravastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, attenuates renal injury in an experimental model of ischemia-reperfusion. J Surg Res. 2001;101:79–84. doi:10.1006/jsre.2001.6256
4. Gelman S. The pathophysiology of aortic cross-clamping and unclamping. Anesthesiology. 1995;82:1026–1060. doi:10.1097/00000542-199504000-00027
5. Bedirli N, Ofluoglu E, Kerem M, et al. Hepatic energy metabolism and the differential protective effects of sevoflurane and isoflurane anesthesia in a rat hepatic ischemia-reperfusion injury model. Anesth Analg. 2008;106(3):830–837. doi:10.1213/ane.0b013e3181616fc9
6. Guzmán-de La Garza FJ, Cámara-Lemarroy CR, Ballesteros-Elizondo RG, Alarcón-Galván G, Cordero-Pérez P, Fernández-Garza NE. Ketamine reduces intestinal injury and inflammatory cell infiltration after ischemia/reperfusion in rats. Surg Today. 2010;40:1055–1062. doi:10.1007/s00595-009-4177-4
7. Lee HT, Kim M, Kim M, et al. Isoflurane protects against renal ischemia and reperfusion injury and modulates leukocyte infiltration in mice. Am J Physiol Renal Physiol. 2007;293:F713–22.
8. Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. Differential protective effects of volatile anesthetics against renal ischemia–reperfusion injury in vivo. Anesthesiology. 2004;101:1313–1324. doi:10.1097/00000542-200412000-00011
9. Córdoba-Jover B, Arce-Cerezo A, Ribera J, et al. Cerium oxide nanoparticles improve liver regeneration after acetaminophen-induced liver injury and partial hepatectomy in rats. J Nanobiotechnology. 2019;17(1):112. doi:10.1186/s12951-019-0544-5
10. Health Effects Institute. Evaluation of human health risk from cerium added to diesel fuel. Communication. 2001;9.
11. Colon J, Herrera L, Smith J, et al. Protection from radiation-induced pneumonitis using cerium oxide nanoparticles. Nanomedicine. 2009;5:225–231. doi:10.1016/j.nano.2008.10.003
12. Niu J, Azfer A, Rogers LM, Wang X, Kolattukudy PE. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc Res. 2007;73:549–559. doi:10.1016/j.cardiores.2006.11.031
13. Wingard CJ, Walters DM, Cathey BL, et al. Mast cells contribute to altered vascular reactivity and ischemia reperfusion injury following cerium oxide nanoparticle instillation. Nanotoxicology. 2011;5:531–545. doi:10.3109/17435390.2010.530004
14. Estevez AY, Pritchard S, Harper K, et al. Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Radic Biol Med. 2011;51(6):1155–1163. doi:10.1016/j.freeradbiomed.2011.06.006
15. Giri S, Karakoti A, Graham RP, et al. Nanoceria: a rare-earth nanoparticle as a novel anti-angiogenic therapeutic agent in ovarian cancer. PLoS One. 2013;8(1):e54578. doi:10.1371/journal.pone.0054578
16. Manne ND, Arvapalli R, Nepal N, et al. Cerium oxide nanoparticles attenuate acute kidney injury induced by intra-abdominal infection in Sprague-Dawley rats. J Nanobiotechnology. 2015;13:75. doi:10.1186/s12951-015-0135-z
17. Rocca A, Moscato S, Ronca F, et al. Pilot in vivo investigation of cerium oxide nanoparticles as a novel anti-obesity pharmaceutical formulation. Nanomedicine. 2015;11(7):1725–1734. doi:10.1016/j.nano.2015.05.001
18. Manne NDPK, Arvapalli R, Graffeo VA, et al. Prophylactic treatment with cerium oxide nanoparticles attenuate hepatic ischemia reperfusion injury in Sprague Dawley rats. Cell Physiol Biochem. 2017;42(5):1837–1846. doi:10.1159/000479540
19. Tatar T, Polat Y, Çomu FM, et al. Effect of cerium oxide on erythrocyte deformability in rat lower extremity ischemia reperfusion injury. Bratisl Med J. 2018;119:441–443. doi:10.4149/BLL_2018_080
20. Gubernatorova EO, Liu X, Othman A, et al. Europium-doped cerium oxide nanoparticles limit reactive oxygen species formation and ameliorate intestinal ischemia-reperfusion injury. Adv Healthc Mater. 2017;6:14. doi:10.1002/adhm.201700176
21. Sezen ŞC, Kucuk A, Özer A, et al. Assessment of the effects of levosimendan and thymoquinone on lung injury after myocardial ischemia reperfusion in rats. Drug Des Devel Ther. 2018;12:1347–1352. doi:10.2147/DDDT.S160092
22. Arya A, Sethy NK, Singh SK, Das M, Bhargava K. Cerium oxide nanoparticles protect rodent lungs from hypobaric hypoxia-induced oxidative stress and inflammation. Int J Nanomedicine. 2013;8:4507–4520. doi:10.2147/IJN.S53032
23. Hegazy MA, Maklad HM, Samy DM, Abdelmonsif DA, El Sabaa BM, Elnozahy FY. Cerium oxide nanoparticles could ameliorate behavioral and neurochemical impairments in 6-hydroxydopamine induced Parkinson’s disease in rats. Neurochem Int. 2017;108:361–371. doi:10.1016/j.neuint.2017.05.011
24. Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421.
25. Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem. 1990;36(8 Pt 1):1440–1443. doi:10.1093/clinchem/36.8.1440
26. Peng CK, Huang KL, Wu CP, et al. Glutamine protects ischemia-reperfusion induced acute lung injury in isolated rat lungs. Pulm Pharmacol Ther. 2011;24:153–161. doi:10.1016/j.pupt.2010.07.002
27. Alacam B, Ek RO, Yıldız Y, et al. The effect of carnosine on lung injury induced by abdominal aortic ischemia-reperfusion. ADÜ Tıp Fakültesi Dergisi. 2010;11:41–47.
28. Gokalp O, Yurekli I, Kiray M, et al. Assessment of protective effects of pheniramine maleate on reperfusion injury in lung after distant organ ischemia: a rat model. Vasc Endovascular Surg. 2013;47:219–224. doi:10.1177/1538574413475885
29. Werns SW, Shea MJ, Lucchesi BR. Free radicals and myocardial injury: pharmacologic implications. Circulation. 1986;74:1–5. doi:10.1161/01.CIR.74.1.1
30. Baltalarli A, Ozcan V, Bir F, et al. Ascorbic acid (vitamin C) and iloprost attenuate the lung injury caused by ischemia/reperfusion of the lower extremities of rats. Ann Vasc Surg. 2006;20:49–55. doi:10.1007/s10016-005-9284-0
31. Likidlilid A, Patchanans N, Peerapatdit T, Sriratanasathavorn C. Lipid peroxidation and antioxidant enzyme activities in erythrocytes of type 2 diabetic patients. J Med Assoc Thai. 2010;93:682–693.
32. Tassiopoulos A, Carlin RE, Gao Y. Role of nitric oxide and tumor necrosis factor on lung injury caused by ischemia/reperfusion of the lower extremities. J Vasc Surg. 1997;26(4):647–656. doi:10.1016/S0741-5214(97)70065-X
33. Vural KM, Öz MC. Endothelial adhesivity, pulmonary hemodynamics and nitric oxide synthesis in ischemiareperfusion. Eur J Cardiothorac Surg. 2000;18:348–352. doi:10.1016/S1010-7940(00)00492-9
34. Lee HT, Kim M, Jan M, Emala CW. Anti-inflammatory and antinecrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. Am J Physiol Renal Physiol. 2006;291(1):F67–78. doi:10.1152/ajprenal.00412.2005
35. Obal D, Dettwiler S, Favoccia C, Scharbatke H, Preckel B, Schlack W. The influence of mitochondrial KATP-channels in the cardioprotection of preconditioning and postconditioning by sevoflurane in the rat in vivo. Anesth Analg. 2005;101(5):1252–1260. doi:10.1213/01.ANE.0000181336.96511.32
36. De Hert SG, Van der Linden PJ, Cromheecke S, et al. Cardioprotective properties of sevoflurane in patients undergoing coronary surgery with cardiopulmonary bypass are related to the modalities of its administration. Anesthesiology. 2004;101(2):299–310. doi:10.1097/00000542-200408000-00009
37. Li H, Wang JK, Zeng YM, et al. Sevoflurane post-conditioning protects against myocardial reperfusion injury by activation of phosphatidylinositol-3-kinase signal transduction. Clin Exp Pharmacol Physiol. 2008;35(9):1043–1051. doi:10.1111/j.1440-1681.2008.04952.x
38. Kalb R, Schober P, Schwarte LA, Weimann J, Loer SA. Preconditioning, but not postconditioning, with sevoflurane reduces pulmonary neutrophil accumulation after lower body ischaemia/reperfusion injury in rats. Eur J Anaesthesiol. 2008;25(6):454–459. doi:10.1017/S0265021508003682
39. Xu G, Wang X, Xiong Y, Ma X, Qu L. Effect of sevoflurane pretreatment in relieving liver ischemia/reperfusion-induced pulmonary and hepatic injury. Acta Cir Bras. 2019;34(8):e201900805. doi:10.1590/s0102-865020190080000005
40. Oncul S, Karabiyik L, Coskun E, Kadioglu E, Gulbahar O. Comparisons of the effects of the sevoflurane and propofol on acute ischemia reperfusion and DNA damages in rabbits. Braz J Anesthesiol. 2017;67:35–41. doi:10.1016/j.bjan.2016.10.001
41. Kosucu M, Ulusoy H, Topbas M, Pulathan Z, Mentese A, Karahan C. The effect of combination of epidural anaesthesia and general anaesthesia on ischemia-reperfusion injury in vascular surgery. Turk J Anaesthesiol Reanim. 2012;40:310–314. doi:10.5152/TJAR.2012.012
42. Li XH, Liu ZH, Ma HB, et al. Effect of sevoflurane on tissue permeability of lung ischemia-reperfusion injury in rats. Asian Pac J Trop Med. 2014;7:276–279. doi:10.1016/S1995-7645(14)60037-7
43. Liu R, Ishibe Y, Ueda M. Isoflurane-sevoflurane adminstration before ischemia attenuates ischemia-reperfusion-induced injury in isolated rat lungs. Anesthesiology. 2000;92:833–840. doi:10.1097/00000542-200003000-00027
44. Otsuki T, Ishikawa M, Hori Y, Goto G, Sakamoto A. Volatile anesthetic sevoflurane ameliorates endotoxin-induced acute lung injury via microRNA modulation in rats. Biomed Rep. 2015;3:408–412. doi:10.3892/br.2015.428
45. Luo C, Yuan D, Zhao W, et al. Sevoflurane ameliorates intestinal ischemia-reperfusion-induced lung injury by inhibiting the synergistic action between mast cell activation and oxidative stress. Mol Med Rep. 2015;12(1):1082–1090. doi:10.3892/mmr.2015.3527
46. Colon J, Hsieh N, Ferguson A, et al. Cerium oxide nanoparticles protect gastrointestinal epithelium from radiationinduced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomedicine. 2010;6:698–705. doi:10.1016/j.nano.2010.01.010
47. Hirst SM, Karakoti A, Singh S, et al. Biodistribution and in vivo antioxidant effects of cerium oxide nanoparticles in mice. Environ Toxicol. 2013;28:107–118. doi:10.1002/tox.20704
48. Aalapati S, Ganapathy S, Manapuram S, Anumolu G, Prakya BM. Toxicity and bio-accumulation of inhaled cerium oxide nanoparticles in CD1 mice. Nanotoxicology. 2014;8:786–798. doi:10.3109/17435390.2013.829877
49. Yokel RA, Hussain S, Garantziotis S, Demokritou P, Castranova V, Cassee FR. The Yin: an adverse health perspective of nanoceria: uptake, distribution, accumulation, and mechanisms of its toxicity. Environ Sci Nano. 2014;1(5):406–428. doi:10.1039/C4EN00039K
50. Xu Z, Yu J, Wu J, et al. The effects of two anesthetics, propofol and sevoflurane, on liver ischemia/reperfusion injury. Cell Physiol Biochem. 2016;38:1631–1642. doi:10.1159/000443103
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
