Back to Journals » OncoTargets and Therapy » Volume 13
The effect of allicin on cell proliferation and apoptosis compared to blank control and cis-platinum in oral tongue squamous cell carcinoma
Authors Guo Y , Liu H, Chen Y, Yan W
Received 29 June 2018
Accepted for publication 20 March 2019
Published 29 December 2020 Volume 2020:13 Pages 13183—13189
DOI https://doi.org/10.2147/OTT.S178718
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Federico Perche
Yanjun Guo,1 Hongli Liu,2 Yong Chen,1 Wei Yan1
1Department of Oral and Maxillofacial Surgery, Cangzhou Central Hospital, Cangzhou, People’s Republic of China; 2School of Stomatology, Cangzhou Medical College, Cangzhou, People’s Republic of China
Correspondence: Yanjun Guo
Department of Oral and Maxillofacial Surgery, Cangzhou Central Hospital, 16 West Xinhua Road, Cangzhou 061001, People’s Republic of China
Tel +86 317 207 5544
Fax +86 317 207 5544
Email [email protected]
Background: Oral tongue squamous cell carcinoma (OTSCC) has aggressive clinical behavior with poor prognosis. Allicin plays a tumor-suppressive role in various cancers, although the role of allicin in OTSCC is unknown. We aimed to investigate the effect of allicin on cell proliferation and apoptosis compared to blank control and cis-platinum in OTSCC.
Methods: Tca-8113 and SCC-25 cells were treated with non-stimulated control, 12.5 μg/mL allicin, 25 μg/mL allicin, 50 μg/mL allicin, and 40 μg/mL cis-platinum, which were divided into blank control, allicin 12.5 μg/mL, allicin 25 μg/mL, allicin 50 μg/mL, and cis-platinum 40 μg/mL groups, respectively. Cell proliferation was determined by the Cell Counting Kit-8 assay. Cell apoptosis was detected by annexin V/propidium iodide and Western blot assays.
Results: In Tca-8113 and SCC-25 cells, cell proliferation was inhibited by 40 μg/mL cis-platinum, 12.5 μg/mL allicin, 25 μg/mL allicin, and 50 μg/mL allicin. Cell apoptosis was promoted by 40 μg/mL cis-platinum, 12.5 μg/mL allicin, 25 μg/mL allicin, and 50 μg/mL allicin, while compared to 40 μg/mL cis-platinum, it was increased by 50 μg/mL allicin. Western blot assay revealed that expression of pro-apoptosis protein Bax and C-Caspase 3 increased, but apoptosis-inhibitory protein Bcl-2 expression decreased with 40 μg/mL cis-platinum, 12.5 μg/mL allicin, 25 μg/mL allicin, and 50 μg/mL allicin, while compared to 40 μg/mL cis-platinum, Bax and C-Caspase 3 expression was increased by 50 μg/mL allicin.
Conclusion: Allicin was shown to have good efficacy in repressing cell proliferation as well as facilitating cell apoptosis in OTSCC.
Keywords: allicin, cell proliferation, cell apoptosis, cis-platinum, OTSCC
Introduction
Oral cancer, a malignant neoplasia which arises on the lip or oral cavity, is considered the eighth most common carcinoma worldwide, with an estimated 354,862 new cases and 177,384 deaths occurring during 2018 in 185 countries.1 As the most common type of oral cancer, oral tongue squamous cell carcinoma (OTSCC) is characterized by fast growth, strong infiltration, high rates of metastasis, and aggressive clinical behavior, with a relatively poor prognosis, and the global incidence of this disease is continuing to increase.2,3 In clinical practice, chemotherapy has been widely applied in OTSCC patients. Cis-platinum-based chemotherapy is frequently utilized for OTSCC treatment and has great efficacy in killing cancer cells and inhibiting tumor growth to improve therapeutic outcomes; however, several adverse effects are related to cis-platinum-based chemotherapy (including pain, nausea, and vomiting), which decrease the quality of life and result in poor outcomes in some OTSCC patients.4 In addition, some OTSCC patients have been reported to gradually develop drug resistance to cis-platinum, which directly leads to worse therapy outcomes, high recurrence rates, or even death.5 According to a previous study, tumor biomarkers are associated with the tumorigenesis and progression of various cancers, such as breast cancer.6 Therefore, there is a need to explore additional and convincing treatment drugs or tumor biomarkers to improve the efficacy of therapy in OTSCC patients.
Allicin, a natural diallyl trisulfide compound isolated from traditional food and medicinal garlic, is widely known for its multiple pharmacological effects, especially its anti-tumor effect7 Several studies suggest that allicin can inhibit cell proliferation, hinder cell migration, and enhance cell apoptosis in various cancers, including colorectal cancer, liver cancer, and human renal clear cell carcinoma, while the role of allicin in OTSCC cells is scarcely known.7–9 Therefore, we aimed to investigate the effect of allicin on cell proliferation and cell apoptosis compared to a blank control and cis-platinum in OTSCC.
Methods
Cell culture
The Tca-8113 cell line was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, People's Republic of China). The SCC-25 cell line was a gift from Shanghai Jiao Tong University School of Medicine (Shanghai, People's Republic of China). Tca-8113 was from tongue squamous cell carcinoma and established from the biopsy of a squamous cell carcinoma of the tongue, and SCC-25 cells were from squamous cell carcinoma and established from the biopsy of a squamous cell carcinoma of the tongue of a 70-year-old man. Both of these cell lines were the third generation when they were utilized. After resuscitation, Tca-8113 cells were cultured in 80% Roswell Park Memorial Institute (RPMI) 1640 (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) medium that was supplemented with 1.5 g/L NaHCO3, 2.5 g/L glucose, 0.11 g/L sodium pyruvate, and 20% FBS (Gibco). SCC-25 cells were maintained in a 1:1 mixture of 90% DMEM (Gibco) and Ham’s F12 medium (Gibco) containing 1.2 g/L sodium bicarbonate, 2.5 mM L-glutamine, 15 mM HEPES, and 0.5 mM sodium pyruvate, and supplemented with 400 ng/mL hydrocortisone as well as 10% FBS. All cells were maintained in a humid incubator at 37°C with 5% CO2. The use of the cell lines in this experiment was approved by the Chinese Academy of Sciences (Shanghi, China) (no. 2017-021-01).
Treatment of Tca-8113 cells and SCC-25 cells
Allicin was bought from Sigma (St Louis, MO, USA). After weighing, allicin was dissolved to the desired concentration in DMSO, and the final concentration of ethanol in the culture medium was 0.1%. According to several previous studies, the concentration of allicin was 50 µg/mL, and the concentrations were multiplied or divided exponentially by 2.10,11 Therefore, Tca-8113 cells and SCC-25 cells were treated with non-stimulated control, 12.5 µg/mL allicin, 25 µg/mL allicin, 50 µg/mL allicin, and 40 µg/mL cis-platinum, which were divided into five groups and labeled as blank control, allicin 12.5 µg/mL, allicin 25 µg/mL, allicin 50 µg/mL, and cis-platinum 40 µg/mL groups, respectively. Cell proliferation was determined by the Cell Counting Kit-8 (CCK-8) (Abcam, Cambridge, MA, USA) assay at 0, 24, and 48 hours. The cell apoptosis rate was detected by an annexin V (AV) apoptosis detection kit with propidium iodide (PI) (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) at the time-point of 48 hours. Pro-apoptotic proteins and anti-apoptotic protein expression, including Bax, Bcl-2, C-Caspase 3, and β-actin, were evaluated by Western blot assay at 48 hours.
CCK-8 assay
Cells were seeded in 96-well tissue culture plates at a density of 3×104 cells per well. Cells were added to 90 µL medium supplemented with 10 µL CCK-8, and then incubated at 37°C with 5% CO2. The cell proliferation ability was represented by the optical density (OD), which was detected using a microplate reader (BioTek, Winooski, VT, USA). The cellular inhibition rate was computed by the following formula: Cellular inhibition rate = (OD Blank control – OD Experimental group)/OD Blank control.
AV/PI assay
After digestion, washing, and collection, cells were suspended in 100 µL binding buffer supplemented with 2 µL AV (Invitrogen) and incubated at room temperature for 15 minutes in the dark. Subsequently, 2 µL PI (Invitrogen) was added to the cell suspension and incubated for 5 minutes. Apoptotic cells were detected and analyzed by flow cytometry (BD FACSVerse™; BD Biosciences, Heidelberg, Germany).
Western blot
Cells were seeded in six-well tissue culture plates at a density of 2×105 cells per well. Cells were lysed using a radio-immunoprecipitation assay (RIPA buffer) (Thermo Fisher Scientific) supplemented with 1% protease inhibitor cocktail as well as 1% phenylmethanesulfonyl fluoride, and then centrifuged. Subsequently, the concentration of the protein samples was measured by a Pierce BCA Protein Assay Kit (Pierce Biotechnology, Thermo Scientific, Rockford, IL, USA). Then, the protein samples were separated by SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The PVDF membranes were blocked with skim milk at room temperature for 2 hours, and incubated with primary antibodies including Bax, BCL-2, C-Caspase 3, and β-actin (Cell Signaling Technology, Danvers, MA, USA). Following this, the PVDF membranes were incubated with the horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology) and finally visualized by an ECL advanced Western Blotting Detection Kit (GE Healthcare, Piscataway, NJ, USA). Western blot images were quantified according to the following steps: after obtaining the gray level of indicated proteins by the ChemiDocTM MP imaging system, the gray value of indicated protein was computed as: Gray value = Gray level of indicated protein/Gray level of corresponding β-actin. All experiments were independently repeated three times. The detailed information and catalogue numbers for BAX, BCL-2, CASP3, and β-actin antibodies are as follows: Bax (D2E11) rabbit monoclonal antibody (mAb) #5023; Bcl-2 (D55G8) rabbit mAb (human specific) #4223; cleaved Caspase-3 (Asp175) (5A1E) rabbit mAb #9664; Caspase-3 (D3R6Y) rabbit mAb #14,220; and β-actin (13E5) rabbit mAb #4970.
Statistics
All experiments were independently performed three times. SPSS version 21.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 5.01 (GraphPad Software, La Jolla, CA, USA) were used for statistical analysis. Data are shown as mean ± standard error. Comparisons between two groups were made using the independent sample t-test. p<0.05 was considered significant.
Results
Effect of allicin on cell proliferation and cell apoptosis rate in Tca-8113 cells and SCC-25 cells
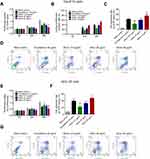
In Tac-8113 cells, cell proliferation was inhibited by 40 μg/mL cis-platinum (p=0.025), 25 μg/mL allicin (p=0.018), and 50 μg/mL allicin (p=0.012) at 24 hours, and repressed by 40 μg/mL cis-platinum (p=0.009), 12.5 μg/mL allicin (p=0.035), 25 μg/mL allicin (p=0.008), and 50 μg/mL allicin (p=0.007) at 48 hours compared to the blank control. In addition, cell proliferation was suppressed by 50 μg/mL allicin compared to 40 μg/mL cis-platinum at 48 hours (p=0.044) (Figure 1A). The cell proliferation inhibition rate after treatment with 40 μg/mL cis-platinum, 12.5 μg/mL allicin, 25 μg/mL allicin, and 50 μg/mL allicin was 26.2±5.38%, 16.5±2.2%, 22.7±3.9%, and 35.4±2.6%, respectively, at 24 hours, and 38.4±3.6%, 24.3±1.8%, 36.3±2.0%, and 51.5±1.4%, respectively, at 48 hours (Figure 1B). Compared to the 40 μg/mL cis-platinum group (38.4±3.6%), the cell proliferation inhibition rate was higher in the 50 μg/mL allicin group (51.5±1.4%) at 48 hours (p=0.032). Apoptotic cells were detected and analyzed by flow cytometry, which revealed that cell apoptosis was increased in the 40 μg/mL cis-platinum (p<0.001), 12.5 μg/mL allicin (p=0.003), 25 μg/mL allicin (p<0.001), and 50 μg/mL allicin (p<0.001) groups compared to the blank control group at 48 hours (Figure 1C, D). Compared to the 40 μg/mL cis-platinum group, cell apoptosis was decreased in the 12.5 μg/mL allicin group (p=0.005) but increased in the 50 μg/mL allicin group (p=0.033) (Figure 1C, D). In SCC-25 cells, cell proliferation was inhibited by 40 μg/mL cis-platinum (p=0.022), 25 μg/mL allicin (p=0.020), and 50 μg/mL allicin (p=0.009) at 24 hours, and reduced by 40 μg/mL cis-platinum (p<0.001), 25 μg/mL allicin (p=0.006), and 50 μg/mL allicin (p<0.001) at 48 hours compared to the blank control. Also, cell proliferation was enhanced by 12.5 μg/mL allicin (p=0.039) and suppressed by 50 μg/mL allicin (p=0.005) compared to 40 μg/mL cis-platinum at 48 hours (Figure 1E). In addition, cell apoptosis was stimulated in the 40 μg/mL cis-platinum (p<0.001), 12.5 μg/mL allicin (p=0.002), 25 μg/mL allicin (p<0.001), and 50 μg/mL allicin (p<0.001) groups compared with the blank control group at 48 hours (Figure 1F, G). Compared with the 40 μg/mL cis-platinum group, cell apoptosis was repressed in the 12.5 μg/mL allicin group (p=0.004) (Figure 1F, G). Together, these results indicate that allicin hindered cell proliferation and induced cell apoptosis, and 50 μg/mL allicin was more effective in reducing cell proliferation and triggering apoptosis than 40 μg/mL cis-platinum in both Tca-8113 cells and SCC-25 cells.
Effect of allicin on cell proliferation in HUVEC cells
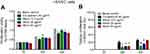
Further validation was performed in a normal oral cell line. Compared to the lank control, cell proliferation was inhibited by 40 μg/mL cis-platinum (p=0.027) at 24 hours and hindered by 40 μg/mL cis-platinum (p=0.002), 25 µg/mL allicin (p=0.019), and 50 µg/mL allicin (p=0.011) at 48 hours. Also, cell proliferation ability was increased in the 12.5 µg/mL allicin group compared with the 40 μg/mL cis-platinum group (p=0.037) at 24 hours (Figure 2A). In addition, the cell proliferation inhibition rate was decreased in the 12.5 µg/mL allicin (p=0.021), 25 µg/mL allicin (p=0.029), and 50 μg/mL allicin (p=0.022) groups compared to the 40 μg/mL cis-platinum group at 24 hours, and in the 12.5 µg/mL allicin (p=0.004) and 25 µg/mL allicin (p=0.042) groups at 48 hours (Figure 2B).
Effect of allicin on apoptosis markers in Tca-8113 cells
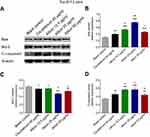
Compared to the blank control group, expression of the pro-apoptosis protein Bax (Figure 3A, B) and C-Caspase 3 (Figure 3A, 3D) was elevated in 40 μg/mL cis-platinum (p=0.005 for Bax and p=0.004 for C-Caspase 3), 12.5 μg/mL allicin (p<0.001 for Bax and C-Caspase 3), 25 μg/mL allicin (p<0.001 for Bax and C-Caspase 3), and 50 μg/mL allicin groups (p<0.001 for Bax and p=0.006 for C-Caspase 3), while expression of the apoptosis-inhibitory protein Bcl-2 (Figure 3A, C) was reduced in 40 μg/mL cis-platinum (p=0.027), 12.5 μg/mL allicin (p=0.041), 25 μg/mL allicin (p<0.001), and 50 μg/mL allicin (p=0.007) groups at 48 hours. Bax expression was higher in the 12.5 μg/mL allicin (p=0.001), 25 μg/mL allicin (p<0.001) and 50 μg/mL allicin (p=0.049) groups compared to the 40 μg/mL cis-platinum group (Figure 3B). C-Caspase 3 expression was also increased in the 12.5 μg/mL allicin (p=0.002) and 25 μg/mL allicin (p=0.002) groups compared with the 40 μg/mL cis-platinum group (Figure 3D). Bcl-2 expression declined in both the 25 μg/mL allicin (p=0.003) and 50 μg/mL allicin (p=0.021) groups compared to the 40 μg/mL cis-platinum group (Figure 3C). Hence, allicin promoted pro-apoptotic proteins but inhibited anti-apoptotic protein, and 50 μg/mL allicin was more effective than 40 μg/mL cis-platinum in elevating pro-apoptotic proteins in Tca-8113 cells.
Discussion
In this study, we found that 1) allicin inhibited cell proliferation and promoted cell apoptosis in OTSCC cells; and 2) 50 μg/mL allicin more effectively repressed cell proliferation and triggered cell apoptosis compared to 40 μg/mL cis-platinum in OTSCC cells.
OTSCC, one of the most common subgroups of oral cancers, is related to modifiable behaviors (including frequent tobacco use and excessive alcohol consumption), chronic infections caused by fungi, bacteria, or viruses (due to poor oral hygiene, ill-fitting dentures, and other rough surfaces on the teeth), and poor nutrition.4 Surgery is commonly recommended as treatment for tumor removal in patients with OTSCC in the early stage, while for advanced-stage OTSCC patients who may not be able to undergo surgery, various therapies (including chemotherapy, radiotherapy, and targeted therapy) have been widely used. Despite improvements in the management of disease in the past few decades, there are still some adverse effects and drug resistance caused by the different therapies, leading to poorer treatment outcomes in OTSCC patients.5,12 Therefore, the discovery of other therapeutics for OTSCC is necessary to improve treatment outcomes in OTSCC patients.
Allicin is a major ingredient of crushed garlic in traditional medicine. It has multiple pharmacological effects and contributes to a wide spectrum of anti-cancer activities. For instance, allicin hinders cell proliferation in colon cancer cells by depleting the intracellular glutathione level.8 Allicin also triggers apoptosis in gastric carcinoma cells through the p38 mitogen-activated protein kinase/caspase-3 signaling pathway.13 Moreover, allicin enhances cell apoptosis in human renal clear cell carcinoma cells by suppressing the hypoxia-inducible factor-1α pathway.9 Therefore, these previous discoveries suggest that allicin has a good effect in killing cancer cells and controlling tumor growth in various carcinomas, particularly in digestive cancer. However, limited information was found on the effect of allicin on OTSCC cells, with just one previous study demonstrating that allicin has cytotoxic effects on cell line SCC-15.11 Considering that, in the previous study,11 there was only one human tongue squamous carcinoma cell line (SCC-15), there was no positive control, and the assessment of apoptosis was performed using a single test, there is a great need for additional study with more cancer cell lines and multiple tests. In the present experiment, we used two human tongue squamous carcinoma cell lines (Tca-8113 cells and SCC-25 cells) with multiple tests to explore the effect of allicin on cell proliferation and apoptosis compared to a blank control and cis-platinum in OTSCC, and we observed that allicin inhibited cell proliferation and promoted cell apoptosis in OTSCC. To verify the effect of allicin in OTSCC, in vitro experiments were repeated in another OTSCC cell line (SCC-25 cells), and similar results were also observed, indicating that allicin may be a novel and effective compound for the treatment of OTSCC, by inhibiting cancer progression and improving treatment outcomes through its effects on cell proliferation and apoptosis.
Cis-platinum is a common chemotherapy drug, which was approved by the US Food and Drug Administration in 1978, and has great benefits in reducing tumor growth and inhibiting distant metastasis to control disease progression and improve prognosis in patients with carcinomas, including OTSCC.14 However, a proportion of OTSCC patients still experiences adverse effects caused by cis-platinum, including pain, nausea, and vomiting, which directly decrease their quality of life and worsen therapeutic outcomes. In addition, the majority of OTSCC patients after long-term or large-dose cis-platinum treatment will gradually develop acquired chemoresistance, leading to poor therapeutic outcomes, high recurrence rates, or even death.14,15 Although several studies have demonstrated the role of cis-platinum in OTSCC, few studies have compared the efficacy of cis-platinum and other drug compounds in OTSCC. In the current study, we carried out cell assays to compare the effects of allicin and cis-platinum on cell proliferation and apoptosis in OTSCC cells, and found that 50 μg/mL allicin was more effective in repressing cell proliferation and promoting cell apoptosis compared to 40 μg/mL cis-platinum, suggesting that high-dose allicin may be more effective than cis-platinum in the treatment of OTSCC patients. Taking all the results together, this experiment may not only provide a more solid foundation for the effect of allicin in killing tumor cells, but also offer additional evidence for the effect of allicin on normal cells, thereby providing a convincing perspective for the clinical application of allicin in the future.
Conclusion
Allicin has good efficacy in repressing cell proliferation as well as facilitating cell apoptosis in OTSCC.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
2. Almangush A, Heikkinen I, Makitie AA, et al. Prognostic biomarkers for oral tongue squamous cell carcinoma: a systematic review and meta-analysis. Br J Cancer. 2017;117(6):856–866. doi:10.1038/bjc.2017.244
3. Ng JH, Iyer NG, Tan MH, Edgren G. Changing epidemiology of oral squamous cell carcinoma of the tongue: a global study. Head Neck. 2017;39(2):297–304. doi:10.1002/hed.24589
4. Srinivasprasad V, Dineshshankar J, Sathiyajeeva J, Karthikeyan M, Sunitha J, Ragunathan R. Liaison between micro-organisms and oral cancer. J Pharm Bioallied Sci. 2015;7(Suppl 2):S354–60. doi:10.4103/0975-7406.163451
5. Chen B, Xue J, Meng X, Slutzky JL, Calvert AE, Chicoine LG. Resveratrol prevents hypoxia-induced arginase II expression and proliferation of human pulmonary artery smooth muscle cells via Akt-dependent signaling. Am J Physiol Lung Cell Mol Physiol. 2014;307(4):L317–25. doi:10.1152/ajplung.00285.2013
6. Shuang C, Weiguang Y, Zhenkun F, et al. Toll-like receptor 5 gene polymorphism is associated with breast cancer susceptibility. Oncotarget. 2017;8(51):88622–88629. doi:10.18632/oncotarget.20242
7. Cha JH, Choi YJ, Cha SH, Choi CH, Cho WH. Allicin inhibits cell growth and induces apoptosis in U87MG human glioblastoma cells through an ERK-dependent pathway. Oncol Rep. 2012;28(1):41–48. doi:10.3892/or.2012.1910
8. Hirsch K, Danilenko M, Giat J, et al. Effect of purified allicin, the major ingredient of freshly crushed garlic, on cancer cell proliferation. Nutr Cancer. 2000;38(2):245–254. doi:10.1207/S15327914NC382_14
9. Song B, Shu Y, Cui T, Fu P. Allicin inhibits human renal clear cell carcinoma progression via suppressing HIF pathway. Int J Clin Exp Med. 2015;8(11):20573–20580.
10. Zhang Q, Yang D. Allicin suppresses the migration and invasion in cervical cancer cells mainly by inhibiting NRF2. Exp Ther Med. 2019;17(3):1523–1528. doi:10.3892/etm.2019.7348
11. Szychowski KA, Binduga UE, Rybczynska-Tkaczyk K, Leja ML, Gminski J. Cytotoxic effects of two extracts from garlic (Allium sativum L.) cultivars on the human squamous carcinoma cell line SCC-15. Saudi J Biol Sci. 2018;25(8):1703–1712. doi:10.1016/j.sjbs.2016.10.005
12. Su YY, Chen CH, Chien CY, Lin WC, Huang WT, Li SH. Mitochondrial assembly receptor expression is an independent prognosticator for patients with oral tongue squamous cell carcinoma. J Renin Angiotensin Aldosterone Syst. 2017;18(3):1470320317717904. doi:10.1177/1470320317717904
13. Zhang X, Zhu Y, Duan W, Feng C, He X. Allicin induces apoptosis of the MGC-803 human gastric carcinoma cell line through the p38 mitogen-activated protein kinase/caspase-3 signaling pathway. Mol Med Rep. 2015;11(4):2755–2760. doi:10.3892/mmr.2014.3109
14. Gibson MK, Li Y, Murphy B, et al. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern cooperative oncology group. J Clin Oncol. 2005;23(15):3562–3567. doi:10.1200/JCO.2005.01.057
15. Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–1883. doi:10.1038/onc.2011.627
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.