Back to Journals » ClinicoEconomics and Outcomes Research » Volume 16
The Economic Burden of Diabetic Retinopathy in Jordan: Cost Analysis and Associated Factors
Authors Al-Dwairi RA , Aleshawi A, Abu-zreig L, Al-Shorman W , Al Beiruti S, Alshami AO, Allouh MZ
Received 30 December 2023
Accepted for publication 5 March 2024
Published 15 March 2024 Volume 2024:16 Pages 161—171
DOI https://doi.org/10.2147/CEOR.S454185
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Samer Hamidi
Rami A Al-Dwairi,1 Abdelwahab Aleshawi,1 Laith Abu-zreig,1 Wafa Al-Shorman,1 Seren Al Beiruti,1 Ali Omar Alshami,1 Mohammed Z Allouh2
1Department of Special Surgery, Division of Ophthalmology, Faculty of Medicine, Jordan University of Science & Technology, Irbid, 22110, Jordan; 2College of Medicine and Health Sciences, United Arab Emirates University, Al Ain, 15551, United Arab Emirates
Correspondence: Rami A Al-Dwairi, Department of Special Surgery, Division of Ophthalmology, Faculty of Medicine Jordan University of Science and Technology, P. O. Box: 3030, Irbid, 22110, Jordan, Tel +962795355056, Fax +962 2 7201064, Email [email protected] Mohammed Z Allouh, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain, 15551, United Arab Emirates, Email [email protected]
Objective: Diabetic retinopathy (DR) is the leading cause of visual loss worldwide in patients with diabetes mellitus (DM). The aims of our study are to describe the costs associated with (DR) and to evaluate its economic impact in Jordan.
Methods: Retrospectively, we included all patients with DM and classified them according to the severity of DR. Data regarding medical history, ophthalmic history, stage of DR, presence of DME, and the ophthalmic procedures and operations were collected. The total DR-related cost was measured as a direct medical cost for the outpatient and inpatient services.
Results: Two hundred and twenty-nine patients were included in the study. Only 49.7% of the patients presented without DR, and 21% presented with diabetic macular edema (DME) unilaterally or bilaterally. The DR-related cost was significantly associated with insulin-based regimens, longer duration of DM, higher HbA1c levels, worse stage of DR at presentation, the presence of DME at presentation, the presence of glaucoma, and increased mean number of intravitreal injections, laser sessions, and surgical operations. Multivariate analysis should the presenting stage of DR, presence of DME, and the presence of DME be the independent factors affecting the DR-related cost.
Conclusion: This study is the first study to be conducted in Jordan and encourages us to establish a screening program for DR for earlier detection and treatment. DM control and treatment compliance will reduce the heavy costs of the already exhausted healthcare and financial system.
Keywords: diabetic retinopathy, economic burden, intravitreal injections, diabetic macular edema
Introduction
Diabetic retinopathy (DR) is a type of microangiopathy caused by diabetes mellitus (DM). DR is a leading cause of vision impairment in 25–74 years of age.1–4 In Jordan, the prevalence of DR has been estimated at 34.1%. Similarly, the prevalence of DR around the world is 34.6% (93 million people).5,6 DR progresses over chronological pattern. The first stage is non-proliferative diabetic retinopathy (NPDR), which may be accompanied by diabetic macular edema (DME). Then, proliferative diabetic retinopathy (PDR) progressively develops, which if not treated aggressively, may lead to advanced PDR and permanent visual loss.7 The prevalence of PDR in Jordan is 9.6%.5 Worldwide, the prevalence rate has been estimated at 10.2%.6,8 Treatment of DR is challenging, individualized, and multidisciplinary. Based on the stage of DR, intravitreal anti-vascular endothelial growth factor (VEGF) injection, panretinal photocoagulation argon laser, and surgical procedures can be adopted. DM control is crucial for all patients.9
Irreversible damage can result from DME or from complicated PDR.10 The progressive stages and the eventual irreversible damage can, however, be significantly postponed or prevented. Screening is the most effective method for the earliest possible diagnosis of DR, along with precise and accurate treatment.11 A direct, low-cost, and highly sensitive screening method is needed. This guarantees the best possible success while ensuring cost-efficiency by preventing or delaying a very high incidence of blindness and related costs. The total financial DR-related cost in the United States (US) residents aged 40 years or older in 2004 was estimated at 493 million US dollar, and the average annual total cost per DR patient was about 629 US dollar.12 On the other hand, the cost of screening of DR by one modality was much less in US, which was reported by Kirkizlar et al.13
In this study, we investigate the burden of DR in Jordan, a country in the Middle East where the DM prevalence is high, and the control is poor. In addition, this study evaluates the factors that affect the DR-related costs with the related visual outcome.
Patients and Methods
Patients and Data
After obtaining the approval of the Institutional Review Board (IRB) of Jordan University of Science and Technology (37/137/2021), this cross-sectional study was conducted at a tertiary care center located in Jordan affiliated with the aforementioned university. Retrospectively, all diabetic patients being treated or screened for diabetic retinopathy (DR) from June 2021 to June 2023 were included for attributed cost estimation and factor analysis. The hospital medical electronic records were used to extract patients’ data. Demographic (age and sex) data were collected. In addition, medical status (DM duration, type of DM treatment, HbA1c, the co-existence of diabetic nephropathy and lower limb ischemia); method of presentation; and number of follow-up visits. In addition, details of procedures such as the number of intravitreal injections, number of laser sessions, and number and type of ophthalmic operations were included. Moreover, clinical ophthalmic and visual data were retrieved.
The study population included all diabetic patients older than 18 years of age, have either type 1 or type 2 DM, who presented to our clinics for either screening of DR, or receiving treatment for DR. Exclusion criteria included diagnoses other than DR for which the treatment was conducted (retinal vein occlusion, uveitis, age-related macular degeneration, central serous chorioretinopathy). Moreover, pregnant patients and patients who lost follow-up were excluded.
Duration of DM was measured from the diagnosis of DM in years. The treatment of DM was divided into either oral hypoglycemic agents (OHGA) or insulin-based regimens. The last Hb1Ac reading was recorded for all patients. The method of presentation included either screening for DR in patients who were not known to have DR, presenting with symptoms of blurry vision, or being referred from secondary centers as a case of advanced DR for management. Other presentation methods included treatment for other abnormalities such as refractive errors, cataracts, or incidental findings.
The International Clinical Diabetic Retinopathy (ICDR) classification severity scale was utilized as a base for our classification of DR and diabetic macular edema (DME).14 The findings were assessed at the first presentation visit, 6-month-visit after the presentation, and at the last follow-up visit. DME was studied separately and was diagnosed by optical coherence tomography (OCT). DME was classified as no DME was detected, the presence of DME in one eye, and the presence of DME in both eyes. DR was categorized into four classes. The first category is patients without evidence of clinical DR. The second category is non-proliferative DR (NPDR), comprising mild, moderate, and severe NPDR cases. The third group is the low-risk proliferative DR (PDR), which includes PDR cases that are not sufficient to meet the criteria of high-risk PDR or patients who achieved stabilization of the PDR status after successful treatment. The last category is the advanced PDR, which is defined by the presence of disc neovascularization greater than third disc area, any disc neovascularization with vitreous hemorrhage (VH), any retinal neovascularization greater than half disc area with VH, and the presence of advanced surgical features such as non-resolving VH and tractional retinal detachment (TRD). DR in the worst affected eye was used for retinopathy grading.
The procedures that were applied were intravitreal injections, retinal laser photocoagulation, and surgical interventions. The mean number for intravitreal injections was calculated for each eye and included anti-vascular endothelial growth factor injections (ranibizumab and aflibercept) and intravitreal dexamethasone implants. The number of retinal laser photocoagulation sessions was also quantified for each eye. The surgical operations were divided into two groups: major operations, which included pars plana vitrectomy and glaucoma surgery, and minor operations, which included the rest of operations, such as cataract operations. The presence of glaucoma in both eyes was also studied and documented. Both primary open-angle glaucoma and neovascular glaucoma were included.
The visual outcome was assessed in LogMAR and analyzed at the first visit, 6-month-visit after the presentation, and at the last follow-up visit. Schulze-Bonsel et al study was referenced for patients with visual acuity of counting fingers, hand motion, light perception, or “no light perception”.15 Number of follow-up visits and the duration of follow-up were evaluated.
The total cost that was analyzed is the total cost that the hospital expends on treating DR regardless of whether the patient is “non‑paying” or “paying.” The cost analysis addressed only resources related to DR (not included treatment for other DM-related systemic complications). Medical, inpatient, and outpatient direct costs were gathered from patient charts through the hospital record system. The medical costs included consultations, investigations (such as OCT and angiography), and medical interventions like lasers, intravitreal injections, and surgery. Prescription fees (such as anti-glaucoma) were added. These values were derived and adhered to a uniform value system for pricing in Jordan regardless of the type of insurance. Medication costs were taken from the Jordanian Food and Drug Administration and included all relevant medications for DR. The indirect expenses, such as disability, sick leave, blindness allowances, and early retirement due to DR, were not assessed. The total cost was analyzed in Jordanian Dinar (JOD). The total cost included treatment for both eyes within the entire period of treatment and follow-up.
Setting
A single vitreoretinal consultant surgeon performed clinical diagnosis and treatment plans. The ophthalmic examination was conducted by well-trained residents and confirmed by the consultant vitreoretinal surgeon for suspicious cases. According to the international guidelines, similar protocols were adopted for intravitreal injections and were given by well-trained senior residents. Through argon laser, pan-retinal photocoagulation (PRP) laser treatment was performed after fully dilating the pupil. The surgical operations were performed by a consultant vitreoretinal surgeon.
Statistical Analysis
Raw data were entered into a spreadsheet and analyzed using the IBM statistical package for the social sciences (SPSS) v.26 (Armonk, New York, NY, USA). In brief, data were expressed as frequency (percentage) or mean ± standard error of the mean (SEM). Statistical significance is determined using the chi-square test for categorical variables and the ANOVA test for continuous variables. A simple linear regression test is applied to study the relation between two continuous variables, and the B coefficient with standard error is used to express the relation. Multiple logistic regression analyses were performed to investigate the confounding effects of different variables. P≤0.05 was considered to be statistically significant.
Results
Patients’ Characteristics
The study population included 229 diabetic patients with 458 eyes who were enrolled and investigated. More than half of the patients were male (54.6%) and the mean age of the patients was 60.1 years. The mean duration of DM was 12.4 years. OHGA was utilized by 115 (50.2%) patients, while the other half received insulin within their regimens. The mean HbA1c percent was 8.50%. Seventeen patients (8.2%) have a range of diabetic nephropathy and 8 (3.8%) patients complain of diabetic foot.
Regarding the mode of presentation, 82 (35.8%) patients presented for screening of DR, 78 (34.1%) presented with symptoms of blurry vision, 12 (5.2%) were referred from a primary center as a case of advanced DR, and the remaining patients were presented seeking ophthalmic advice for other indications. Table 1 summarizes the patients’ characteristics of the population.
 |
Table 1 General Demographics and Characteristics |
At the first presentation visit, 16 (7%) patients had DME in one eye, and 32 (14%) had DME in both eyes. In addition, 56 (24.5%) patients had NPDR at the presentation, 32 (14%) had low-risk PDR, and 27 (11.8%) had advanced PDR. Only 114 (49.7%) patients presented without DR. The mean best corrected visual acuity (BCVA) at the presentation was 0.5022 LogMAR. At the last follow-up visit, 20 (9.1%) patients had DME in one eye, and 18 (8.1%) had DME in both eyes. Furthermore, 59 (25.8%) patients had NPDR, 52 (22.7%) had low-risk or stable PDR, and 19 (8.3%) had advanced PDR. The mean BCVA at the last follow-up visit was 0.4923 LogMAR. Table 2 summarizes the clinical and visual outcomes at the presentation, the 6-month follow-up visit, and the last follow-up visit.
 |
Table 2 Visual and Clinical Outcome |
The mean number of intravitreal injections provided during the study period was 4.57 (mean of 2.39 injections for the right eye and 2.18 injections for the left eye). The mean number of retinal photocoagulation sessions was 1.35 (mean of 0.71 for the right eye and 0.64 for the left eye). The right eye underwent 26 major operations and 65 minor operations. The left eye underwent 24 major operations and 53 minor operations. It is important to notice that 26 (11.4%) patients have bilateral glaucoma, and 8 (3.5%) patients have unilateral glaucoma.
The mean follow-up duration was 37.3 months, and the mean number of follow-up visits was 8.83 visits. The mean total medical and non-medical DR-related cost was 1233.9 Jordanian dinars (JOD).
Cost Evaluation and Factors Analysis
Age, sex, and the presence of diabetic feet were not associated with DR-related costs. The insulin-based regimen was associated with higher DR-related costs than OHGA (1611.6 JOD for the insulin-based regimen versus 840.4 JOD for the OHGA). Also, the longer duration of DM was associated with higher DR-related costs (for each one-year longer duration of DM, the cost increased by 69.1 JOD). In addition, the higher HbA1c level was related to higher DR-related costs (For each unit increase in the Hb1Ac, the cost increased by 239.1 JOD). Furthermore, the presence of diabetic nephropathy was associated significantly with higher DR-related costs.
Patients who presented for screening of DR were associated with the lowest possible DR-related costs. However, referral cases were associated with the highest burden expenses on the system. Moreover, the presence of DME in one eye or two eyes at presentation was associated with higher costs. In addition, the worse the DR stage, the higher the DR-related cost. Patients with advanced PDR were associated with the highest costs. Furthermore, BCVA was negatively associated with DR-related cost. The worse the BCVA, the higher the DR-related cost (for each LogMAR unit increase in the BCVA, the cost increased by 813.6 JOD).
As expected, the mean numbers of intravitreal injections and laser sessions were associated with higher DR-related costs. Moreover, undergoing major operations was associated with significantly higher DR-related costs. Furthermore, the presence of glaucoma was related to higher DR-related costs.
On multiple regression analysis, the worse the stage of DR at presentation, the presence of DME at presentation, and the presence of glaucoma were the independent and most effective burden factors that increased the cost on the healthcare system. Table 3 summarizes the factors affecting the DR-related cost.
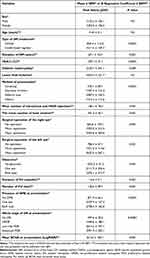 |
Table 3 Factors Affecting Total Direct Medical DR-Related Cost |
Factors Affecting the Mean BCVA at the Last Follow-Up Visit
According to Table 4, the mean BCVA had a similar pattern for the cost. Age, sex, and the presence of diabetic foot were not associated with the mean of BCVA. However, insulin-based regimen, the longer the duration of DM, the higher the HbA1c level, and the presence of diabetic nephropathy were associated with worse BCVA at the last follow-up visit. Additionally, the advanced PDR stage of DR, the presence of DME at presentation, and the presence of glaucoma were associated with worse BCVA. Moreover, patients who received higher numbers of intravitreal injections, laser sessions, and major operations were related to worse BCVA at the last follow-up visit.
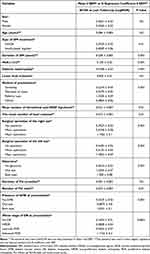 |
Table 4 Factors Affecting the BCVA at the Last Follow-Up Visit |
Similar to the cost analysis, on multiple regression analysis, the worse the stage of DR at presentation, the presence of DME at presentation, and the presence of glaucoma were the independent and most crucial burden factors associated with worse BCVA at the last follow-up visit.
Discussion
Currently, most studies about the economic burden and cost analyses of DR have been reported from the Western world.16–19 Despite the high prevalence of DM and DR, similar data have not been published in Jordan. This study is the first to investigate the burden and cost analysis of DR in Jordan and the Middle East, where this region is complaining of a high DM prevalence, poor DM control, and relatively low community resources.20–23 This study highlights that DM control parameters are associated directly with increasing the DR-related cost. The longer the duration of DM, the higher the HbA1c level, and the insulin-based regimen were related to the rising cost of DR. In addition, the stage of DR at presentation, the presence of DME at presentation, and the presence of glaucoma were the independent risk factors for DR-related costs. Subsequently, increasing numbers of intravitreal injections, laser sessions, and ocular operations were associated with higher DR-related costs.
In most cases, DR progresses in an orderly arranged to more advanced stages and it is essential to detect the stages earlier which in turn can prevent/delay the rate of severe vision loss up to 90%.4 Many studies have demonstrated a promising effect of intravitreal anti-VEGF injections in cases of DME and even PDR cases.24–26 The previous Diabetic Retinopathy Study examined the utilization of a PRP and found that a PRP laser reduced visual loss by 50% compared to no treatment.27 PRP laser aims to ablate the ischemic areas and reduce the intravitreal VEGF levels. In DR, surgery is indicated for severe active FVM or for persistent vitreous hemorrhage. One study demonstrated that at 4 years follow-up, the final visual acuity of 20/40 or better was achieved by 44% of the early vitrectomy cases and 28% of the conventional management cases.28 Although the visual outcome may be variable, tractional retinal detachment with recent macular involvement is a well-established indication for vitrectomy surgery.29 In our study, 50 eyes underwent major vitrectomy surgery for advanced DR stages, which is costly and has a burden on the healthcare system. In addition, the mean number of laser sessions in this study was 1.3 sessions, meaning that at least all patients underwent slightly more than one session of laser.
Direct DR-related costs increased as the stages of the disease progress, with higher costs increasing between severe NPDR and PDR. This is consistent with reports from other Asian countries, Singapore and India.30,31 In Singapore’s study by Zhang et al, they revealed that the presence and severity of DR was associated with increased direct medical costs in a multi-ethnic Asian diabetic patient. The total cost for PDR patients was 3.79 times the non-DR patients.30 A study from India by Orji et al demonstrated that treatment of DR benefits, but the DR-related cost increases with disease stages and visual impairment. They demonstrated that a nearly 3‑fold difference in DR-related cost per eye for subjects with severe visual loss (<3/60).31 In a USA study, after adjustment for demographic characteristics, the mean direct DR-related cost is significantly higher in patients with DR than those without DR.19 Within the DR subgroups, PDR cases had twice DR-related cost over the NPDR cases.19 In Germany, the ratio of DR-related costs for mild, moderate, and severe NPDR and PDR relative to no DR were 1.2, 2.5, 3.2, and 7.4, respectively.16 In our study, patients presented with advanced PDR had a total cost of about 6.2-times more than patients without DR. Moreover, patients presented with bilateral DME had a total cost of 3.1-fold of patients presented without DME.
DM costs the United States an estimated $327 billion annually, with $237 billion coming from direct medical costs and $90 billion coming from indirect cost related to disability. Patients with DM are at risk for ophthalmic sequela other than DR, including glaucoma, cataracts, and serious infections. Nearly 30% of patients with DM suffer from diabetic retinopathy worldwide. Additionally, patients with DR have noticeably higher medical costs than those with other diabetes-related conditions. Fortunately, early detection and treatment can reduce the risk of blindness from DR by 95%.32
DR can be avoided with early detection. The traditional methods available for this purpose are taking longer and the prediction accuracy is low. If the problem is identified in advanced stages, the chance of efficient treatment for recovery is low. Hence, early detection with high accuracy plays a major role in DR. Deep learning and artificial intelligence are technologies that are shown to be efficient in DR screening.33–37
Our study is not without limitations. First, the sample size is relatively small. Second, the subdivision of the total cost was not achieved. The total cost for DR is composed of direct medical and non-medical (transportation, relocation, and informal care) costs, indirect costs (disability), and intangible costs (psychological pain, discomfort, and distress related to diabetes).38 In this study, the directed medical costs were only investigated. Patients with DR may have a higher proportion of indirect and intangible costs. Third, patients in tertiary medical centers, like our center, tend to have more complicated DR stages than primary centers, which may affect the cost.
Conclusions
Jordanian patients tend to have poor DM control, which was reflected in the cost and visual outcome. Less than half of the patients presented without DR and a fifth of them had DME at presentation. Worse stage of DR, presence of DME, presence of glaucoma, and increased mean number of intravitreal injections, laser sessions, and surgical operations were associated with higher DR-related cost. Screening programs to detect DR at earlier stages should be promoted to reduce the total cost in a country where resources are limited.
Data Sharing Statement
The datasets generated and analyzed during the current study are available from the corresponding author.
Ethical Approval
This study has been performed in accordance with the 1964 Declaration of Helsinki and its later amendments. This study has obtained ethical approval from the IRB at Jordan University of Science and Technology and King Abdullah University Hospital, Irbid, Jordan (38/137/2021). The authors confirm that the patients’ privacy was saved, and the data was anonymized and kept confidential. The IRB waived the need for consent due to the study’s retrospective nature.
Funding
The authors have not declared any grant for this work from any funding authority.
Disclosure
The authors have no conflicts of interest to disclose for this work.
References
1. Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14(4):179–183. doi:10.1080/09286580701396720
2. Klein R, Klein BE, Moss SE. Visual impairment in diabetes. Ophthalmology. 1984;91(1):1–9. doi:10.1016/S0161-6420(84)34337-8
3. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi:10.1016/S0140-6736(09)62124-3
4. Al-Dwairi R, Rwashdeh H, Otoom M. The Influence of COVID-19 Lockdown in Jordan on Patients with Diabetic Retinopathy: a Case-Control Study. Therapeutics Clin Risk Management. 2021;17:1011–1022. doi:10.2147/TCRM.S316265
5. Al-Amer RM, Khader Y, Malas S, Abu-Yaghi N, Al-Bdour M, Ajlouni K. Prevalence and risk factors of diabetic retinopathy among Jordanian patients with type 2 diabetes. Digital j Ophthalmol. 2008;14:42–49. doi:10.5693/djo.01.2008.013
6. Kempen JH, O’Colmain BJ, Leske MC, et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122(4):552–563.
7. Matz H, Falk M, Göttinger W, Kieselbach G. Cost-benefit analysis of diabetic eye disease. Ophthalmologica J Int. 1996;210(6):348–353. doi:10.1159/000310742
8. Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304(6):649–656. doi:10.1001/jama.2010.1111
9. Early Treatment Diabetic Retinopathy Study Research Group. Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report no. 19. Arch Ophthalmol. 1995;113(9):1144–1155. doi:10.1001/archopht.1995.01100090070025
10. Drummond MF, Davies LM, Ferris FL. Assessing the costs and benefits of medical research: the diabetic retinopathy study. Soc sci med. 1992;34(9):973–981. doi:10.1016/0277-9536(92)90128-D
11. Kohner EM, Porta M. Protocols for screening and treatment of diabetic retinopathy in Europe. Eur j Ophthalmol. 1991;1(1):45–54. doi:10.1177/112067219100100109
12. Rein DB, Zhang P, Wirth KE, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124(12):1754–1760. doi:10.1001/archopht.124.12.1754
13. Kirkizlar E, Serban N, Sisson JA, Swann JL, Barnes CS, Williams MD. Evaluation of telemedicine for screening of diabetic retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120(12):2604–2610. doi:10.1016/j.ophtha.2013.06.029
14. Wilkinson CP, Ferris FL, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. doi:10.1016/S0161-6420(03)00475-5
15. Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual Acuities “Hand Motion” and “Counting Fingers” Can Be Quantified with the Freiburg Visual Acuity Test. Invest Ophthalmol Visual Sci. 2006;47(3):1236–1240. doi:10.1167/iovs.05-0981
16. Happich M, Reitberger U, Breitscheidel L, Ulbig M, Watkins J. The economic burden of diabetic retinopathy in Germany in 2002. Graefe’s Arch Clin Exp Ophthalmol. 2008;246(1):151–159. doi:10.1007/s00417-007-0573-x
17. Schmitt-Koopmann I, Schwenkglenks M, Spinas GA, Szucs TD. Direct medical costs of type 2 diabetes and its complications in Switzerland. Eur j Public Health. 2004;14(1):3–9. doi:10.1093/eurpub/14.1.3
18. Schmier JK, Covert DW, Lau EC, Matthews GP. Medicare expenditures associated with diabetes and diabetic retinopathy. Retina. 2009;29(2):199–206. doi:10.1097/IAE.0b013e3181884f2d
19. Lee LJ, Yu AP, Cahill KE, et al. Direct and indirect costs among employees with diabetic retinopathy in the United States. Curr Med Res Opin. 2008;24(5):1549–1559. doi:10.1185/030079908X297303
20. Khattab M, Khader YS, Al-Khawaldeh A, Ajlouni K. Factors associated with poor glycemic control among patients with type 2 diabetes. J diabet complicat. 2010;24(2):84–89. doi:10.1016/j.jdiacomp.2008.12.008
21. Al-Rasheedi AA. Glycemic Control among Patients with Type 2 Diabetes Mellitus in Countries of Arabic Gulf. Int j Health Sci. 2015;9(3):345–350. doi:10.12816/0024701
22. Noureddine H, Nakhoul N, Galal A, Soubra L, Saleh M. Level of A1C control and its predictors among Lebanese type 2 diabetic patients. Therapeutic Adv Endocrinology Metab. 2014;5(3):43–52. doi:10.1177/2042018814544890
23. Janghorbani M, Amini M. Patterns and predictors of long-term glycemic control in patients with type 2 diabetes. ISRN endocrinol. 2012;2012:526824. doi:10.5402/2012/526824
24. Gross JG, Glassman AR, Jampol LM, et al. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: a Randomized Clinical Trial. JAMA. 2015;314(20):2137–2146. doi:10.1001/jama.2015.15217
25. Rajendram R, Fraser-Bell S, Kaines A, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol. 2012;130(8):972–979. doi:10.1001/archophthalmol.2012.393
26. Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: two-Year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology. 2016;123(6):1351–1359. doi:10.1016/j.ophtha.2016.02.022
27. The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology. 1981;88(7):583–600.
28. The Diabetic Retinopathy Vitrectomy Study Research Group. Early vitrectomy for severe proliferative diabetic retinopathy in eyes with useful vision. Results of a randomized trial--Diabetic Retinopathy Vitrectomy Study Report 3. Ophthalmology. 1988;95(10):1307–1320. doi:10.1016/S0161-6420(88)33015-0
29. Thompson JT, de Bustros S, Michels RG, Rice TA. Results and prognostic factors in vitrectomy for diabetic traction retinal detachment of the macula. Arch Ophthalmol. 1987;105(4):497–502. doi:10.1001/archopht.1987.01060040067035
30. Zhang X, Low S, Kumari N, et al. Direct medical cost associated with diabetic retinopathy severity in type 2 diabetes in Singapore. PLoS One. 2017;12(7):56.
31. Orji A, Rani PK, Narayanan R, Sahoo NK, Das T. The economic burden of diabetic retinopathy care at a tertiary eye care center in South India. Indian j Ophthalmol. 2021;69(3):666–670. doi:10.4103/ijo.IJO_1538_20
32. Parker ED, Lin J, Mahoney T, et al. Economic Costs of Diabetes in the U.S. Diabetes Care. 2023.
33. Kalyani G, Janakiramaiah B, Karuna A, Prasad LVN. Diabetic retinopathy detection and classification using capsule networks. Complex Intelligent Syst. 2023;9(3):2651–2664. doi:10.1007/s40747-021-00318-9
34. Bhandari S, Pathak S, Jain S. A Literature Review of Early-Stage Diabetic Retinopathy Detection Using Deep Learning and Evolutionary Computing Techniques. Arch Comput Methods Eng. 2022;30.
35. Vij R, Arora S. A Systematic Review on Diabetic Retinopathy Detection Using Deep Learning Techniques. Arch Comput Methods Eng. 2022;30.
36. Özbay E. An Active Deep Learning Method for Diabetic Retinopathy Detection in Segmented Fundus Images Using Artificial Bee Colony Algorithm. Artif Intell Rev. 2022;56.
37. Selvachandran G, Quek SG, Paramesran R, Ding W, Son LH. Developments in the detection of diabetic retinopathy: a state-of-The-art review of computer-aided diagnosis and machine learning methods. Artif Intell Rev. 2023;56(2):915–964. doi:10.1007/s10462-022-10185-6
38. Ng CS, Lee JY, Toh MP, Ko Y. Cost-of-illness studies of diabetes mellitus: a systematic review. Diabetes Res Clin Pract. 2014;105(2):151–163. doi:10.1016/j.diabres.2014.03.020
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
