Back to Journals » Risk Management and Healthcare Policy » Volume 13
The Diagnostic Performance of Chest-X-Ray and Erythrocyte Sedimentation Rate in Comparison with GeneXpert® for Tuberculosis Case Notification Among Patients Living with Human Immunodeficiency Virus in a Resource-Limited Setting: A Cross-Sectional Study
Authors Sorsa A
Received 25 May 2020
Accepted for publication 10 August 2020
Published 21 September 2020 Volume 2020:13 Pages 1639—1646
DOI https://doi.org/10.2147/RMHP.S264447
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Marco Carotenuto
Abebe Sorsa
Arsi University College of Health Science, Assela, Ethiopia
Correspondence: Abebe Sorsa Email [email protected]
Introduction: For many years, chest-X-ray (CXR) has been used as a diagnostic tool to complement clinical diagnosis of bacteriologically negative tuberculosis (TB) cases. Increased erythrocyte sedimentation rate (ESR) was also used as laboratory surrogates to augment the diagnosis of bacteriological test negative TB cases. The objective of this study was to evaluate the diagnostic performance of CXR and ESR in comparison with geneXpert® for TB case notification among PLHIV in a resource-limited setting.
Methods: An institution-based cross-sectional study was carried out from February 1, 2018–January 31, 2019. During regular HIV-clinic visits, PLHIVs were assessed for TB using the National and WHO screening tool, and those with positive results were further evaluated using ESR, CXR and sputum for AFB and GeneXpert®. Patients were interviewed for demographic and clinical data using a standardized tool. Collected data were analyzed using SPSS 21. Frequency, percentage, cross tabulation and proportion were used to describe variables of importance. Logistic regression was used to assess factors affecting CXR and ESR findings. Kappa was used to assess reliability, and a p-value < 0.05 was considered significant.
Results: A total of 384 patients with presumptive TB-HIV co-infection were included of which 165 (43.0%) were diagnosed to have TB, and 79 (53.3%) of these were confirmed by GeneXpert®, and the remaining 77 (46.7%) TB cases were diagnosed using clinical judgment, CXR and ESR. The sensitivity, specificity, positive predictive value and negative predictive value of CXR were 67.9%, 77.3%, 43.8%, and 90.3%, and that of ESR were 49.4%, 55.1%, 22.1%, and 83.0%, respectively. The overall agreement between CXR and GeneXpert® was good with a kappa value of 0.38 while that of ESR and GeneXpert® was poor with a kappa value of 0.028.
Conclusion: CXR in diagnostic work of TB among PLHIV plays an unprecedented role while ESR has little clinical significance in the evaluation of TB.
Keywords: CXR, ESR, GeneXpert, HIV, TB, PLHIV
Introduction
The World Health Organization (WHO) recommends regular symptom screening for TB for HIV-positive individuals in TB endemic settings.1,2 There has been no single diagnostic test implemented till now which addresses all ideal diagnostic tools to satisfy all the demands of “rapid,” “affordable,” and “easy” in TB case findings.3,4 Diagnosis of TB is challenging in HIV-positive individuals, especially when the stage of the disease is advanced. Standard TB diagnostic approaches and clinical algorithms should be followed to guide the diagnosis of TB in PLHIV. Sputum smear acid fast bacilli (AFB)-microscopy has been in clinical use for decades for the diagnosis of TB. For many years, WHO has recommended chest-X-ray (CXR) as a diagnostic tool to be used to substantiate the diagnosis of microbiologically negative TB cases.3 CXR shows important radiological features regarding disease extent and progress, but there is no valid and reliable grading system to assess the severity of TB on CXR. Similarly, chest radiography can help the diagnosis of tuberculosis in patients with a smear negative result that mainly averts diagnosis delay.6–11 There are radiologic features compatible with TB which includes hilar lymph node enlargement, pleurisy with fluid collection and military infiltrates and the reactivated form of TB which mainly involves the upper lobe of the lung with consolidation, calcification, and cavitary lesions.5–8 The sensitivity of CXR is good for the diagnosis of intra thoracic TB, hence, it is a valuable tool to identify TB as a likely diagnosis for patients; however, it is limited by modest specificity with a high interobserver variability in radiological reports.3,10-13 A study from Kenya evaluated the performance of CXR through classifying the lung radiologic lesions into “highly consistent with TB, consistent with TB, pathology but not TB and no pathology.” The CXR reading with “highly consistent with TB” and “consistent with TB” had a sensitivity of 72% and 42%. However, from the same report, the specificity of CXR reading with “no pathology” was 87%, taking Mycobacterium culture as the “gold standard.”9,14 Similarly a study reported by Riccardo et al, showed a high of 91.5% negative predictive value with slightly lower positive predictive value of 50%. Some centers use CXR as an augmenting tool after a microbiological study turned out to be negative to substantiate the diagnosis of microbiological negative TB among people living with HIV.15,16 Patients with a chronic inflammatory condition like TB were more likely to have hematologic changes like anemia and elevated ESR.16
Objective
The objective of this study was to evaluate the diagnostic performance of CXR and erythrocyte sedimentation rate (ESR) in comparison with geneXpert® for TB case notification among PLHIV in a resource-limited setting:
Methods and Materials
An institution-based cross-sectional study was conducted for 1 year from February 1, 2018- January 31, 2019 among patients living with HIV/AIDS (PLHIV) who were under care and treatment at Asella teaching and referral hospital (ATRH). The hospital is the only teaching and referral hospital for the south-central part of Ethiopia serving close to 4 million people. There are about 3,560 PLHIV having follow up and getting HIV care and treatment at the hospital.
Inclusion criteria: all patients fulfilling the screening criteria for presumptive TB cases per the revised WHO/National Comprehensive HIV Prevention, Care and Treatment guideline1 recommendation for intensified TB case finding among PLHIV were included. Accordingly, any patient during the regular follow up visit who has one or more of the following was included:
- Current cough,
- Fever,
- Weight loss,
- Night Sweats.
Data were collected using patient interview, antiretroviral therapy (ART) log book review, laboratory log books for important demographics (age, sex, address), clinical symptoms (cough, fever, weight loss) and laboratory parameters (Hct, CD4, ESR, viral load).
Exclusion criteria: Patients who were on TB treatment during the time of study.
Sputum, Blood Sample Collection and Laboratory Procedure
Included patients were requested to give a morning spot sputum specimen (about two mL) for GeneXpert. Each patient gave the expectorate of sputum samples which was not induced. It was collected under direct observation of a laboratory technologist. The sputum was processed using a fully self-automated GeneXpert® MTB/RIF v 4.3 (Cepheid, Sunnyvale, CA, USA) machine at the reference laboratory of the hospital by a laboratory technologist. Similarly, complete blood cell (CBC) count and ESR determination were performed at the reference laboratory of the hospital. The laboratory technologists were blinded to the results of each other.
Erythrocyte sedimentation rate (ESR): was measured using Westergren method. Two mL of venous blood was added into a tube containing 0 0.5 mL of sodium citrate. The tube is placed in a rack in a strictly vertical position for 1 hour at room temperature, at which time the distance from the lowest point of the surface meniscus to the upper limit of the red cell sediment is measured. The distance of fall of erythrocytes, expressed as millimeters in 1 hour, is the ESR.
Chest-X-Ray
Posterior anterior CXR was taken in an upright position using a digital X-ray machine. Then the CXR film was examined by two radiologists assigned blindly for the purpose of the research and not aware of the reports of each other. CXR was considered as a compatible finding with pulmonary TB or not if they fulfill the following as defined below. Discordant results of CXR readings were taken to discussion at the presence of two radiologists for agreement. Results that still remained discordant were taken as inconclusive and discarded.
Data Analysis
Data were initially coded and entered to EpiInfo 7.2 for clean-up and exported to SPSS 21 for analysis. Frequency, proportion and percentage were calculated for categorical variables and mean with standard deviation (SD) was used for continuous variables. Cross tabulation and ROC curve were used to assess the sensitivity, specificity, positive (PPV) and negative (NPV) in reference to GeneXpert as “reference.” Univariate and multivariate logistic regressions were used to assess factors associated with outcome variables. Factors associated with ESR and CXR findings on univariate analysis at a p-value <0.2 were further analyzed using multivariate logistic regression. Significance levels of p<0.05 with confidence intervals of 95% CI were used.
Operational Definition
- Presumptive TB cases for positive HIV patients: Current cough with/without any of the following clinical symptoms: fever, night sweats or weight loss.
- Compatible CXRwith pulmonary TB: defined with any one of the following: miliary mottling, pleurisy with effusion, cavitary lesion, calcification or enlarged hilar lymph nodes.
- TB case in this report implies: All forms of TB which include
- Pulmonary TB: A form of TB which is contained only in the lung,
- Disseminated TB: A clinical spectrum of TB involving lung and one or more of other organsystems.
- Bacteriologically confirmed TB: TB case confirmed by bacteriological tests which include smear AFB, GeneXpert, culture.
- Probable TB case among PLHIV is diagnosed if: Patients whose bacteriological test is negative and if they fulfilled two or more of the following criteria: clinical feature suggestive of TB, reactive tuberculin, skin test >10 mm, or radiographic findings compatible with TB, no response to broad spectrum antibiotic trial.
- Anemia: defined as hematocrit value of <34% for blood drawn from venous blood which is the case in our study.
Ethical Considerations
The research proposal was initially submitted to Arsi University College of health science institutional review board (IRB) and ethical approval was secured to pursue the study. After briefing about the purpose and benefit of the study, a written informed consent form was signed by the patient before commencing data collection. For patients under the age of 18 years, a parent/legal guardian signed a written informed consent on behalf of them.
The study was conducted in accordance with the Declaration of Helsinki. Confidentiality of information about the patients’ medical problem was secured with maximum effort and was not exposed to any other third party.
Results
A total of 3,560 patients who were living with HIV/AIDS were attending our antiretroviral therapy (ART) clinic at ATRH of which 384 patients who fulfilled the definition of an intensified TB case were included in our study. Males accounted for 199 (51.9%), the median age was 37 years and most of the patients were in the age range of 18–60 years. Majority of patients were WHO HIV/AIDS clinical stage T1 and stage T3, each accounting for 119 (30.9%) and 130 (33.9%) patients respectively and about 104 (38.2%) had a CD4 cell count of less than 200/mL (Table 1).
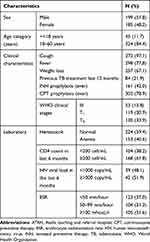 |
Table 1 Demographic and Some Clinical Data of Presumptive TB-HIV Co-Infection Patients Among Presumptive TB Cases, ATRH, 2019 |
All patients have been on ART with a duration of ART treatment ranging from 0 to 132 months with a median duration of treatment of 52 months. The mean CD4 count with SD was 272 ± 240.2 cell/mL among GeneXpert TB diagnosed-patients and 367.2+289.8 cell/mL among GeneXpert-negative patients (Table 2).
 |
Table 2 Hematologic, Immunologic and HIV Viral Load Outline of Patients GeneXpert Positive and Negative, ATRH, 2019 |
A total of 165 TB cases were diagnosed during the study period from which 79 (49.0%) were bacteriologically confirmed TB cases by GeneXpert. The remaining cases were “probable” TB cases.
Pulmonary TB accounted for 98 (59.4%) of all TB cases while the remaining were disseminated TB cases. All presumptive TB-HIV co-infection had undergone CXR imaging of which 122 (35.5%) had a compatible report.
Sensitivity, Specificity, PPV and NPV of CXR and ESR
The sensitivity and specificity of ESR at the cut point level of ≥50 mm/hour were 32.2% and 38.1% with a positive predictive value (PPV) and a negative predictive value (NPV) of 22.1% and 83.0% respectively, taking GeneXpert as reference (Table 3 and Figure 1). There was no statistically significant difference between the higher cut point level of ESR (≥100 mm/hour) and >50mm/hour, p=0.213. The reliability test was also evaluated and the kappa value was found to be 0.041 and 0.028 at ESR values of >50 mm/hour and >100 mm/hour indicating poor reliability.
 |
Table 3 Sensitivity, Specificity, PPV, NPV of ESR Taking GeneXpert as Gold Standard, ATRH, 2019 |
Using CXR as the screening tool while taking GeneXpert as the “reference,” the sensitivity, specificity, PPV, and NPV of CXR were 67.9%, 77.3%, 43.8%, and 90.3%, respectively (Table 3). The overall agreement of GeneXpert and CXR were good with a kappa value of 0.38.
Factors Affecting the CXR Finding
Using the univariate analysis test, shorter duration of cough, previous TB treatment, absence of INH prophylaxis provision, presence of anemia, lower BMI, non-advanced WHO clinical stage of HIV/AIDS were significantly associated with compatible CXR finding. However, on the multivariate logistic regression only absence of provision of INH and previous TB treatment were significantly associated with a compatible CXR finding (Table 4).
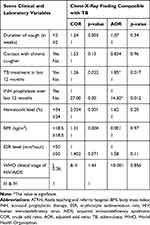 |
Table 4 Factors Associated with Compatible Chest-X-Ray Finding with TB on Univariate and Multivariate Logistic Regression, ATRH, 2019 |
Factors Associated with Increased ESR (>50 mm/hour)
Using univariate analysis, low hematocrit, lower BMI, advanced stage of HIV/AIDS disease, disseminated form of TB and lower CD4 cell count were associated with increased ESR. When these factors were further analyzed using multivariate logistic regression only disseminated TB and low hematocrit remained significantly associated with increased with ESR (Table 5).
 |
Table 5 Factors Associated with Increased ESR Level on Univariate and Multivariate Logistic Regression, ATRH, 2019 |
Discussion
In our study, we tried to assess the performance of CXR and ESR for the diagnosis of TB. A total of 165 TB cases were diagnosed of which 79 (47.8%) were bacteriologically confirmed TB cases while the remaining 86 (52.2%) were bacteriologically negative “probable” TB cases. In the case of “probable” TB cases the diagnosis was made using clinical evaluation, CXR and other laboratory tests like ESR. Compatible CXR findings were reported among 105 (63.6%) of all TB cases and these compatible CXR findings became even higher among bacteriologically confirmed TB cases. Taking GeneXpert as gold standard, the sensitivity of CXR was found to be 68% implying that among patients with compatible CXR findings, about two-thirds of them were bacteriologically confirmed TB cases. The specificity was a bit higher (77.4%) indicating that those patients with non-compatible CXR with TB patients were unlikely to have actual TB. The sensitivity reported in our study was comparable with reports from Demoor et al13 in which the reported sensitivity range was 66–72% and a specificity of 87–91.5% in a high-risk population but lower sensitivity than the reports by WHO and Pande et al,6 who reported the sensitivity of CXR was 86% taking sputum Mycobacterium culture as the gold standard. The difference could be related to how the CXR was described, and the difference with what tests were taken asthe “gold standard.”3–8,16
Similarly, the PPV of compatible CXR findings was 43.8% implying that less than half the cases with CXR readings compatible with TB actually had TB. This finding is in congruence with the study from Kenya which reported PPV of CXR to be 63% while its negative predictive value (90.3%) implied that if the CXR is read as not compatible with TB, one can be 90% confident that there is no TB. Therefore, CXR can be used for triaging patients to those in actual need of further GeneXpert evaluation without missing significant TB cases and can be used for effective and efficient utilization of the GeneXpert service in resource-limited countries.9−12
The Radboud group and their collaborators at the University of Cape Town, South Africa and a systematic review by Lancelot et al assessed the algorithmic approach of CXR as a test for sorting out patients with suspected TB in reference to definitive testing with GeneXpert. They concluded that early screening using CXR would have allowed for reductions in the number of suspected TB cases undertaking definitive testing, but at the cost of significantly underestimating the number of TB cases (eg, 11% of PTB cases might have been omitted). The screening approach failed to uncover fewer cases in patients without HIV infection, partly because GeneXpert is more sensitive in patients with an intact immune system.3,8–12 The radiologic feature of TB among HIV patients could vary from normal to atypical findings unlike non-HIV patients. Factors associated with CXR findings include previous TB treatment and lack of IPT prophylaxis. The justification for the association between previous TB treatment and current compatible CXR could be related to the fact that some of TB radiologic findings could remain for years as a scar after TB treatment.15,17–19
In this study, we tried to evaluate the significance of ESR which has been in routine clinical use for decades in the work-up procedure of diagnosing TB. We tried to evaluate at two cut point values: >50 mm/hour and >100 mm/hour, its sensitivity, specificity, PPV and NPV were mostly less than 50%, hence they do not play such a significant clinical role for routine laboratory evaluation of TB patients. ESR is a non-specific measure of inflammation. Non-inflammatory conditions that can cause raised ESR include anemia, kidney failure, obesity, ageing, and female sex. ESR is also higher in women during menstruation and pregnancy. Also, ESR is increased in autoimmune disorders (such as rheumatoid arthritis and lupus), infections, some kidney diseases and some cancers (such as lymphoma and multiple myeloma). This is in line with the research findings of Amilo et al16 who reported non-specific clinical significance of ESR in routine TB evaluation.16
Conclusion
Our study concluded that CXR was a good augmenting tool for TB case notification among PLHIV with TB-HIV co-infection in resource-constraint countries. However, ESR has an insignificant role in the evaluation of TB-HIV co-infection and routine determination of ESR adds little value for patient care other than incurring additional cost on the patients and work load burden on health professionals.
Abbreviations
AFB, Acid fast bacilli; ATRH, Asella teaching and referral hospital; BMI, body mass index; CXR, Chest-X-ray; INH, isoniazid prophylactic therapy; ESR, erythrocyte sedimentation rate; HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; COR, crude odd ratio; AOR, adjusted odd ratio; TB, tuberculosis; WHO, World Health Organization.
Acknowledgments
I am very grateful to the health professionals working at Asella teaching and referral hospital infectious disease clinic (ID) for their unstinting cooperation and commitments during data collection. Next, I am equally grateful to Mr Mohammed Kaso for his constructive and continuous technical support during the research. Lastly, I would like to extend heartfelt gratitude to Arsi university for allowing me to carry out the research.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Ethiopia federal minister of health. National Comprehensive HIV Prevention, Care and Treatment Guideline; 2018.
2. WHO. Global Tuberculosis Report; 2019.
3. WHO. Chest Radiography in Tuberculosis Detection; 2017.
4. Pinto LM, Pai M, Dheda K, Schwartzman K, Menzies D, Steingart KR. Scoring systems using chest radiographic features for the diagnosis of pulmonary tuberculosis in adults: a systematic review. Eur Respir J. 2013;42:480–494.
5. Piccazzo R, Paparo F, Garlaschi G, Piccazzo R, Paparo F, Garlaschi G. Diagnostic accuracy of chest radiography for the diagnosis of tuberculosis (TB) and its role in the detection of latent TB infection: systematic review. J Rheumatol Suppl. 2014;91:32–40.
6. Pande T, Cohen C, Pai M, Khan FA. Computer aided detection of pulmonary tuberculosis on digital chest radiographs: a systematic review . Int J Tuberc Lung Dis. 2016;20(9):1226–1230.
7. Agizew TB, Services H, Bachhuber MA, Tedla Z, Tallaksen RJ. Association of chest radiographic abnormalities with tuberculosis disease in asymptomatic HIV-infected adults. Int J Tuberc Lung Dis. 2014;14(3):324.
8. Kaguthi G, Nduba V, Nyokabi J, Onchiri F, Gie R, Borgdorff M. Chest radiographs for pediatric TB diagnosis: interrater agreement and utility. Interdiscip Perspect Infect Dis. 2014;2014.
9. Van Cleeff MRA, Kivihya-ndugga LE, Meme H, Odhiambo JA, Klatser PR. The role and performance of chest X-ray for the diagnosis of tuberculosis: a cost-effectiveness analysis in Nairobi, Kenya. BMC Infect Dis. 2005;9:1–9.
10. Ebrahimzadeh A, Mohammadifard M, Naseh G. Comparison of chest x-ray findings of smear positive and smear negative patients with pulmonary tuberculosis. Iran J Radiol. 2014;11(4):10–13.
11. Nishikiori N, Van Weezenbeek C. Target prioritization and strategy selection for active case-finding of pulmonary tuberculosis: a tool to support country-level project planning. BMC Public Health. 2013;13(1). doi:10.1186/1471-2458-13-97
12. Ralph AP, Ardian M, Wiguna A, et al. A simple, valid, numerical score for grading chest x-ray severity in adult smear-positive pulmonary tuberculosis. Thorax. 2010;65:863–870.
13. Demoor T, Bracke KR, Joos GF, Brusselle GG, Pintelaan D. Diagnostic accuracy of digital chest radiography for pulmonary tuberculosis in a UK urban population. Eur Respir J. 2008;35(3):689–692.
14. Shi X, Guy ES, Barbosa EJM, et al. Pulmonary tuberculosis: role of radiology in diagnosis and management. Radiographics. 2017;1:52–72.
15. Samandari T, Bishai D, Luteijn M, Mosimaneotsile B, Motsamai O, Postma M. Costs and consequences of additional Chest X-Ray in a tuberculosis prevention program in Botswana. Adv Life Sci Technol. 2010;11.
16. Amilo GI, Meludu SC, Ele PU, Ezechukwu C, Onyenekwe C, Chukwu MI. Haematologic indices in pulmonary tuberculosis with or without HIV co-infection in South Eastern Nigeria. Adv Life Sci Technol. 2013;11(47):1–8.
17. Lombardi G, Di Gregori V, Girometti N, Tadolini M, Bisognin F, Monte PD. Diagnosis of smear-negative tuberculosis is greatly improved by Xpert MTB/RIF. PLoS One. 2017;12:e0176186.
18. Hanrahan CF, Haguma P, Ochom E, et al. Implementation of Xpert MTB/RIF in Uganda: missed opportunities to improve diagnosis of tuberculosis. Open Forum Infect Dis. 2014;3:1–6.
19. Elsevier. Author Information Pack. J Clin Tuberc Other Mycobact Dis. 2020;1–10
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

