Back to Journals » Infection and Drug Resistance » Volume 14
The Development of mRNA Vaccines for Infectious Diseases: Recent Updates
Received 4 October 2021
Accepted for publication 25 November 2021
Published 9 December 2021 Volume 2021:14 Pages 5271—5285
DOI https://doi.org/10.2147/IDR.S341694
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Nitika,1,2 Jiao Wei,1,2 Ai-Min Hui1,2
1Fosun Pharma USA Inc., Boston, MA, USA; 2Shanghai Fosun Pharmaceutical Industrial Development, Co., Ltd., Shanghai, People’s Republic of China
Correspondence: Ai-Min Hui
Shanghai Fosun Pharmaceutical Industrial Development, Co., Ltd., 1289 Yishan Road, Shanghai, 200233, People’s Republic of China
Tel +86-21-33987000
Fax +86-21-33987020
Email [email protected]
Abstract: mRNA-based technologies have been of interest for the past few years to be used for therapeutics. Several mRNA vaccines for various diseases have been in preclinical and clinical stages. With the outbreak of the COVID-19 pandemic, the emergence of mRNA vaccines has transformed modern science. Recently, two major mRNA vaccines have been developed and approved by global health authorities for administration on the general population for protection against SARS-CoV-2. They have been proven to be successful in conferring protection against the ongoing SARS-CoV-2 and its emerging variants. This will draw attention to various mRNA vaccines against infectious diseases that are in the early stages of clinical trials. mRNA vaccines offer several advantages ranging from rapid design, generation, manufacturing, and administration and have strong potential to be used against various diseases in the future. Here, we summarize the mRNA-based vaccines in development against various infectious diseases.
Keywords: vaccines, mRNA, infectious diseases, lipid nanoparticles, immune response
Introduction
Vaccines are important for protection against infectious agents and confer protection against various diseases to humans1. Vaccines have been prominent in eradicating various deadly diseases such as smallpox, polio, measles, mumps, rubella, and other such infections.2–4 Conventionally, vaccines were developed using chemical or heat treatment and live attenuated pathogens in cell lines or animals. While live attenuated vaccines (LAV) have been successful in the eradication of numerous deadly diseases, their safety and efficacy remain questionable in some diseases.2 Some disadvantages of live attenuated vaccines include their failure if there are mutations in the surface antigens of the pathogens, causing the disease in immune-compromised individuals and chances of reversion to a virulent form due to reverse mutations in the pathogen.5,6 These vaccines are less efficient in mounting CD8+ immune response, which is important for protection against bacteria and viruses.7
Recently, nucleic acid therapeutics have emerged as an alternative to conventional vaccines. One of the nucleic acid vaccine approaches involves the development of messenger ribonucleic acid (mRNA) vaccines.8 The first mRNA vaccine was developed in rats to reverse diabetes insipidus using intrahypothalamic injection of vasopressin mRNA.9 mRNA vaccines are thought to be beneficial in comparison to the conventional vaccines due to the non-infectious and non-integrative nature of mRNA in the human body.10 Additionally, mRNA half-life can be modulated in a cell by adding modifications onto the mRNA molecule.10 Nucleic acid-based vaccines mimic infection with live pathogens and stimulate follicular T helper and germinal B cell immune response.11
Here we review different types of mRNA vaccines, delivery, and immune responses generated by mRNA vaccines and various mRNA-based vaccines against infectious diseases in clinical trials.
Types of mRNA Vaccines
Currently, there are two forms of mRNA vaccines: non replicating mRNA vaccines (modified and unmodified mRNA (NRM)) and self-amplifying mRNA (SAM) vaccines, which are derived from positive-strand RNA virus.12–17 mRNA vaccines are produced by artificially synthesizing mRNA via a cell-free in vitro enzymatic transcription reaction. This in vitro reaction consists of linearized plasmid DNA, RNA polymerase and nucleoside triphosphates. NRM vaccines consist of an open reading frame (ORF) for a target antigen, untranslated regions (UTRs) at both ends and a terminal poly A tail.18 Once inside the body, they drive antigen expression. Naked unmodified mRNA is prone to be degraded as soon as it is delivered inside the body. However, it was discovered that incorporation of naturally occurring chemically modified nucleosides such as pseudouridine and 1-methylpseusouridine results in the efficient translation of RNA.19,20
Another mRNA vaccine platform is based on alphavirus, a positive-strand virus.21 In these vaccines, the structural proteins are replaced with the gene of interest. These self-amplifying mRNA can direct self-replication RNA-dependent RNA polymerase complex and generate multiple copies of the antigen-encoding mRNA.22 They express high levels of the heterologous gene when introduced into the cytoplasm of host cells. They mimic the production of antigens in vivo by viral pathogens and trigger both humoral and cellular immune responses.22 SAMs produce higher levels of antigens as compared to NRM vaccines. Both NRM and SAM vaccines do not integrate into the host genome. mRNA vaccines are much safer than other vaccine platforms and hold promise to be effective against infectious pathogens.13,15,20,23,24
Delivery of mRNA Vaccines
The delivery of mRNA vaccines is crucial due to their unstable nature. Intravenous administration of unmodified mRNA leads to its digestion by ribonucleases and stimulates innate immune response.25,26 The immune system cells including antigen-presenting cells (APCs), B cells and T cells should be stimulated by mRNA delivery.27–29 Delivery of mRNA molecules is challenging as mRNA molecule is 4 times larger than other molecules. In addition, mRNA is negatively charged and repulsed by the cell membrane.30 This limitation can be overcome by using modified RNA and better delivery systems. Various mRNA platforms have been designed including protamine, dendrimers, polyethyleneimine and lipid nanoparticles.31 Lipid nanoparticles (LNP) loaded with nucleoside modified NRM is used often for administering these vaccines generally in clinical trials.31 LNPs are composed of phospholipids, cholesterol, ionizable lipids and lipid-anchored polyethylene glycol (PEG).32 LNPs help cellular uptake, aid endosomal uptake, and enhance cytoplasmic delivery.33 LNPs also protect mRNA from being recognized by endosomes and toll-like receptors (TLRs) and prevent overactivation of the innate immune system.34 Some mRNA vaccines have adjuvant properties, which can be beneficial.35 mRNAs can be modified to enhance immune activation without blocking mRNA expression. This includes modification of a nucleoside with a TLR-4 agonist monophosphoryl lipid A (MPLA) which aids in the induction of T cell activation.36,37 Another strategy employed is using a short double-stranded region in poly-A tail or 3ʹUTR of mRNA. The addition of a short poly U or poly-A tail induces IFN-B and IL-6 and enhances dendritic cell activation and migration. The ds poly U or poly-A tail is recognized by TLR3 and RIG-I.10,38 Recently, two SARS-CoV-2 RNA lipid nanoparticle vaccines based on nucleoside modified mRNA (modRNA) were designed.39,40 BNT162b1 is a modRNA with blunted immune sensor activating capacity and enhanced RBD expression.41 BNT162b2 is a modRNA vaccine that expresses P2 mutant, prefusion spike glycoprotein (P2 S) (version 9).42,43
Immune Response to mRNA Vaccines
The mechanism of immune response to mRNA vaccine is still being studied. In humans, there are two kinds of RNA sensors the RIG-I-like receptor family and TLRs.38 There are four TLRs: TLR3, TLR7, TLR8 and TLR9 which are present in dendritic cells, macrophages, and monocytes. TLR3 recognizes double-stranded RNA (dsRNA) and single-stranded RNA (ssRNA). TLR7 binds to both dsRNA and ssRNA and TLR8 recognizes ssRNA only.44,45 The RIG-I family includes RIG-I, MDA-5 and LGP2. RIG-I recognizes ssRNA and dsRNA and stimulates interferon production.46,47 MDA5 is a cytosolic RNA sensor that detects long ds RNA generated during viral RNA replication. This recognition of ds RNA activates IRF-3 and NF-KB and increased the production of IFN-I.48,49 The induction of interferons (IFNs) by mRNA vaccines depends on the in vitro transcribed mRNA, administration route and the delivery vehicle.50 Post-mRNA vaccination, pattern recognition receptors (PRRs) are activated, and there is enhanced type I IFN production.51 The IFN production can be positive via activation of the immune response or negative by blocking mRNA translation.52
Vaccine-Induced Immunity
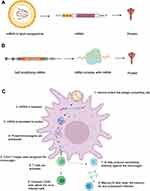
A cellular immune response such as cytotoxic T cells (CTLs) directly targets the viral-infected cells in contrast to neutralizing the virus, which is achieved by humoral immunity.53,54 The level of antibody generation depends on the size of LNPs which are transport to the draining lymph nodes, thus activating B cells. B cells can uptake the LNPs with mRNA and facilitate the production of protein from the mRNA.55 In addition, B cells interact with the B cell receptors expressing the foreign antigens. B cells located in the draining lymph nodes (with the LNP-mRNA) secrete specific low-affinity antibodies and some may enter a germinal center (GC).56 B cells that enter GC undergo somatic hypermutation (SHM) and affinity maturation. The affinity matured B cells later become plasmablasts and secrete high-affinity antibodies. However, they can also re-enter GCs that undergo SHM and become memory B cells57 (Figure 1).
For efficient CTL induction, antigen should enter the route of antigen processing. The pathogenic antigens are then delivered to the cytosol for proteasomal processing. Later, the peptides generated from the proteasomal pathway are delivered to the endoplasmic reticulum (ER) by the transporter associated with antigen processing (TAP).58 In the ER, these peptides bind to the major histocompatibility complex class I (MHC). The MHC I-antigen peptide complex is then recognized by the CD8 T lymphocytes at the cell surface.59 mRNA-based vaccines are particularly suitable for the generation of potent CTL responses because they can express the antigen in the cytosol of the antigen-presenting cells.60,61
Antigen Selection
While designing mRNA-based vaccines, various factors such as antigen selection, vaccine platforms, vaccine routes and regimen must be taken into consideration. Antigen selection is a crucial step for vaccine design.62 In the case of SARS-CoV-2, the structural proteins are S protein, N protein, matrix protein (M) and envelope protein (E). N protein is responsible for coating the large positive-strand RNA genome encased in a lipid envelope derived from the host cell membrane. S, M and E proteins are inserted into this membrane.63 It has been found that in SARS-CoV-2 only the antibodies against the S protein can neutralize the virus and prevent infection. Therefore, most of the vaccines for SARS-CoV-2 are directed against the S1 domain of the receptor-binding domain.64
RNA Vaccines in Infectious Diseases
SARS-CoV-2
COVID-19 is caused by SARS-CoV-2 (severe acute respiratory syndrome–coronavirus 2) that causes respiratory illness, which in severe cases can lead to death.65 Bats are the main host of coronavirus and when transmitted to humans66; it is primarily spread via direct transmission. Coronavirus is composed of spike protein (S), envelope protein (E), membrane protein (M), nucleocapsid protein (N) and hemagglutinin esterase dimer protein (H) which are bound to negative sense RNA.67 COVID-19 is an infectious disease and emerged as a global pandemic in December 2019. Therefore, there is an urgent need to develop a vaccine against the SARS-CoV-2 virus. Various COVID-19 vaccines have been in development since the beginning of the pandemic.68 BNT162b2 is a lipid nanoparticle-formulated nucleoside-modified RNA vaccine. It encodes a prefusion stabilized, membrane-anchored SARS-CoV-2 full-length spike protein. This vaccine was approved by the FDA in December for administration on the public after it showed 95% efficacy in Phase 3 clinical trials.69,70 A few weeks later, mRNA1273, which is also a lipid nanoparticle-encapsulated mRNA-based vaccine encoding the prefusion stabilized full-length spike, was approved by the FDA after its efficacy was 94.1% in phase 3 clinical trials (Table 1).71,72 Currently, CV2nCoV containing sequence-optimized unmodified mRNA coding for a stabilized form of spike protein encapsulated in lipid nanoparticles (LNP) remains in preclinical development. CV2nCoV elicits high titers of cross-neutralizing antibodies against the B.1.1.7, B.1.1.298 and B.1.351 variants in rat.73 CV2nCoV is optimized to have enhanced translation, immunogenicity, and 1.8-fold higher protein expression than the previous candidate vaccine CVnCoV.73,74 CVnCoV showed 47% efficacy against SARS-CoV-2 of any severity in Phase II/III clinical trials and failed to meet prespecified statistical success criteria75 (NCT04652102). It has been now withdrawn from the regulatory review due to a potential overlap with approval timelines for CV2nCoV (CureVac).
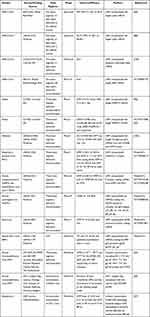 |
Table 1 mRNA Vaccines Against Infectious Diseases |
ARCoV is another such lipid nanoparticle-encapsulated mRNA (mRNA-LNP) encoding the receptor-binding domain (RBD) of SARS-CoV-2 is in Phase III clinical trials (NCT04847102). ARCoV elicited high SARS-CoV-2 specific IgG antibodies and strong virus neutralization titers in mice and cynomolgus monkeys.76
mRNA vaccines can be generally designed faster than any other platform. This is evident from the SARS-CoV-2 nucleoside modified mRNA-LNP vaccine for human use in 42 days post obtaining the SARS-CoV-2 sequence by Pfizer-BioNTech and Moderna.77,78 mRNA-based vaccines may prove to be better than other platforms and efficacious against the mutant virus strains as the mRNA can be designed for a mix of multiple sequences for a broad coverage during a pandemic.79 It is clear from the immediate availability of mRNA vaccine amidst a pandemic that this technology holds promise and can be scaled up from a small batch required for the clinical trials as well as massive production in case of a global pandemic.80
Various mutants of SARS-CoV-2 have emerged so far. The first one to emerge was spike mutation D614G, which enhanced the spike binding to ACE2 receptor.81 The next one to emerge was N501Y found in multiple locations on the globe including the UK (B1.1.7), Brazil (P.1) and South Africa (B.1.351). This mutant strain increases the binding of the spike to the ACE2 receptor and enhances the viral transmission.82,83 Another mutation E484K has emerged in many variants that emerged in New York (B.1.526), Nigeria (B.1.525) and the Philippines (P3).84,85 It has been observed that the BNT162b2 mRNA vaccine confers neutralizing activity against B.1.1.7, B.1.351, B.1.429, B.1.526, P.1 and B.1.1.7/E484K mutant viral strains.86 Recently, new strains B.1.617.2, B.1.617.2 and B.1.618 have spread to various countries. All these variants are currently circulating in the US. New strains included WT, B.1.525-spike, B.1.617.1-spike, B.1.617.2-spike, B.1.617.2-v2-spike, and B.1.618-spike viruses. BNT162b2 demonstrated neutralization of all the variants except for B.1.617.1, which was least neutralized, probably due to the presence of both L452R and E484Q substitutions at the receptor-binding site.84
Moderna has designed a new booster candidate (mRNA-1273.351) against the B.1.351 variant. mRNA-1273.351 has advanced to Phase III clinical trials in the US to assess the immunological benefit of boosting with strain-specific spike proteins.87,88 mRNA-1273 has now been approved for a third dose booster against SARS-CoV-2 in the US (NCT04927065). Pfizer and BioNTech have also evaluated the safety and immunogenicity of a third booster dose of the BNT162b2 vaccine. This study aimed to understand the efficacy of booster dose against the South African variant. This vaccine has now been approved for the third dose booster against SARS-CoV-289 (NCT04955626). Initially, there were some concerns regarding the safety of mRNA-based vaccines for vulnerable groups such as the elderly, children, pregnant women, and immunocompromised patients. However, emerging data from phase II/III clinical trials show that mRNA vaccines are safe for administration in children and pregnant women90,91 (NCT04796896, NCT04816643, NCT04958304, NCT04754594). With the success of mRNA vaccines for SARS-CoV-2, it is evident that in the future mRNA vaccine platform can be expanded to other infectious diseases.
Rabies
Rabies is a disease of the central nervous system caused by the rabies virus. Rabies virus is a member of the rhabdovirus family and bullet or rod-shaped single-stranded, negative-sense enveloped RNA virus.92,93 The virus is transmitted from the bite of an infected mammal (cat or dog) to humans. After infection, humans develop flu-like symptoms followed by severe neurotropic symptoms caused by progressive encephalomyelitis.16 Various vaccines have been approved against the rabies virus but still, there is high mortality due to rabies infection.94 Thus, there is a need for better and efficacious vaccine candidates. Various mRNA-based rabies vaccines are being tested in clinical trials. CV7201 and CV7202 are two such lyophilized, temperature-stable mRNA candidate vaccines consisting of mRNA encoding the rabies virus glycoprotein (RABV-G) in free form and complexed with the cationic protein protamine.17 CV7201 was approved for Phase 1 clinical trials in Germany. A total of 101 participants were enrolled and a total of 306 doses of mRNA were administered. Vaccination by needle-free induced virus-neutralizing antibody titers of 0.5 IU/mL in 32 (71%) of 45 participants given 80 μg or 160 μg CV7201 doses intradermally and six (46%) of 13 participants given 200 μg or 400 μg CV7201 doses intramuscularly. After a year, eight (57%) of 14 participants were boosted with an 80 μg needle-free intradermal dose of CV7201 and achieved titers of 0.5 IU/mL or more. The vaccine was ineffective in only one participant (320 μg intradermal vaccination) with a detectable immune response. CV7202 is a new vaccine for rabies virus which is still under phase 1 clinical trials.17,95,96 So far, 53 participants have been recruited and the study is expected to be completed by 2023.
Influenza
Influenza is caused by influenza viruses, which are members of the Orthomyxoviridae family.97 They are negative-sense single-stranded RNA viruses. Influenza virus consists of RNA polymerase subunits, viral glycoproteins (haemagglutinin (HA), neuraminidase (NA)), nucleoprotein (NP), matrix protein (M1), membrane protein (M2), nonstructural protein (NS1) and nuclear export protein (NEP).98 In humans, influenza viruses A and B cause infectious respiratory illness.99 Like SARS-CoV-2, severe influenza pandemic led to >40 million deaths worldwide in 1918.100 Henceforth, there is a need for an effective influenza vaccine. Currently, three influenza vaccines are administered in the clinic: inactivated, live attenuated and recombinant HA vaccines. These vaccines target the HA protein responsible for viral entry in the host. However, the rapid mutation in the virus causes antigenic drift, which leads to yearly modification of the influenza vaccine.101 Therefore, there is a real need for alternative antigen targets and rapid vaccine production as soon as the new influenza strain emerges. Several mRNA vaccines have been developed for influenza virus.102 VAL-506440 vaccine consists of modified mRNAs encoding the full-length, membrane-bound form of the hemagglutinin (HA) glycoprotein from the H10N8 influenza strain or the H7N9 influenza strain in lipid nanoparticles (LNP). For both vaccines, the immunogenicity outcomes measured were HAI (humoral immunization inhibition) and MN (microneutralization assays).31,103 A total of 201 participants were recruited for the H10N8 study. The 100-µg intramuscular dose induced HAI titers ≥ 1:40 in 100% and MN titers ≥1:20 in 87.0% of the participants. The 25-µg intradermal dose induced HAI titers >1:40 in 64.7% of the participants compared to 34.5% of the participants receiving the IM dose.104
For H7N9, 156 participants were chosen, intramuscular doses of 10, 25, and 50 µg achieved HAI titers ≥1:40 in 36.0%, 96.3%, and 89.7% of the participants, respectively. MN titers ≥1:20 were achieved by 100% in the 10- and 25-µg groups and 96.6% in the 50-µg group. Seroconversion rates identified were 78.3% (HAI) and 87.0% (MN) for H10N8 (100 µg IM) and 96.3% (HAI) and 100% (MN) in H7N9 (50 µg).104 These high neutralization antibody titer data from clinical trials suggest that mRNA vaccines hold a great promise against influenza infection.
Respiratory Syncytial Virus (RSV)
RSV is caused by a negative-sense, single-stranded RNA virus that belongs to the Paramyxoviridiae family.105 RSV consists of M2-1 (transcription processivity factor), M2-2 (switches transcription to DNA replication. It also consists of lipid bilayer displaying the fusion (F), attachment (G), and small hydrophobic proteins (SH).106 RSV infection leads to acute bronchiolitis in children and is associated with high morbidity and mortality. Current RSV vaccine candidates target the highly conserved F protein, which prevents viral fusion; however, most of them failed the clinical trials because of insufficient neutralizing antibody titers. Recently, mRNA-1345 has been developed as the potential mRNA-based vaccine for RSV.107 The sequence of mRNA-1345 has been engineered and codon-optimized to enhance translation and immunogenicity relative to the previous vaccine candidate mRNA-1777.108 The mRNA encodes for a stabilized prefusion F glycoprotein of RSV by engineering the coding sequence. The Phase I clinical trials for mRNA-1345 are still ongoing. A total of 160 participants have been recruited so far, and tolerability and reactogenicity of several doses of mRNA lipid nanoparticle injections on adults and children will be evaluated by the completion of the study in 2023.108
Human Metapneumovirus (HMPV) and Parainfluenza Virus Type 3 (PIV3)
Human metapneumovirus and parainfluenza virus are respiratory pathogens that belong to the Paramyxoviridiae family. HMPV is a negative-strand RNA virus that encodes three viral glycoproteins: fusion (F), attachment (G) and short hydrophobic proteins (SH). HMPV causes respiratory tract infections in infants and young children.109 PIV3 is a single-stranded negative-sense RNA virus. It consists of three nucleocapsid associated proteins, the nucleoprotein (N), phosphoprotein (P), large polymerase protein (L), an accessory protein (C) encoded by the second open reading frame (ORF) in the P mRNA; an internal matrix protein (M) and two envelope-associated proteins, the fusion (F) and the hemagglutinin-neuraminidase (HN) glycoproteins, which are the major neutralization and protective antigens.110 Currently, there is no licensed vaccine for HMPV and PIV3; however, several vaccine candidates have been developed which are being tested in preclinical studies. An mRNA vaccine, mRNA-1653 targeting fusion proteins (F protein) of both HMPV and PIV3 has been evaluated in phase I clinical trials. A total of 160 patients were recruited to evaluate the safety, reactogenicity and immunogenicity of the vaccine in children and adults. The interim results indicate that 1 month after a single vaccination, geometric mean titers were 6.04 for HMPV-A, 6.33 for HMPV-B and 3.24 for PIV3. Second interim data demonstrated that the antibody titers remained above the baseline after 7 months post vaccination111 (NCT04144348). Therefore, mRNA vaccines have the potential to prevent significant virus-associated morbidities and mortalities worldwide due to HMPV and PIV3.
Human Cytomegalovirus (HCMV)
Human cytomegalovirus belongs to the human herpes type 5 family. These viruses have a double-stranded linear DNA in an icosahedral nucleocapsid with a protein matrix.112 HCMV can be transmitted via direct transmission from human to human.113 There are numerous candidate vaccines against HCMV infection and disease in development. However, no vaccine has been approved for administration.114,115 mRNA vaccine candidates, mRNA-1647 and mRNA-1443 use envelope glycoprotein B precursor (gB) to generate neutralizing antibodies against HCMV. These mRNA vaccines were tested for their safety, reactogenicity and tolerability in phase I clinical trials. For mRNA-1647, CMV-seropositive groups who received 30 µg, 90 µg and 180 µg groups had geometric mean ratios (GMRs) of the neutralizing antibody ranging from 14-fold to 31-fold across the three doses against epithelial cell infection whereas the GMRs against fibroblast infections were 6-fold to 8-fold. For Phase II, CMV-seropositive groups who received 50 µg, 100 µg and 150 µg groups had geometric mean ratios (GMRs) of the neutralizing antibody ranging from 12-fold to 14-fold across the three doses against epithelial cell infection, whereas the GMRs against fibroblast infections were 0.8-fold to 1.2-fold (NCT03382405). These high neutralizing antibody titers suggest that mRNA vaccines against HCMV infections may offer protection against this lethal disease.
Zika Virus
Zika is caused by the Zika virus (ZIKV) which is an arthropod-borne virus that belongs to the Flaviviridae family. Zika virus is transmitted to humans by the bite of Aedes mosquitoes and then direct transmission from human to human via saliva or sexual contact from an infected person. Zika is a positive-sense RNA virus consisting of three structural proteins: capsid (C), pre-membrane (prM), envelope (E) and seven structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5). ZIKV infection leads to mild influenza-like illness and multi-organ failure, meningitis, and encephalitis in severe cases.116 The common antigen choice for mRNA vaccines against Zika virus is the membrane and envelope protein (prM-E) as the neutralizing antibodies against prM-E can prevent viral fusion. One study showed that a single 30 μg or 50 μg dose of an LNP-prM-E mRNA-protected mice and rhesus macaques, respectively, from a Zika infection. The neutralizing antibody titers generated by the mRNA vaccine were 50–100 times higher than other vaccines.117,118 mRNA-1893 is another mRNA-based vaccine that targets pre-membrane and envelope (prM-E) glycoproteins of the Zika virus. prM-E is an antigen choice for mRNA vaccines against Zika virus because the neutralizing antibodies against it prevent viral fusion to the host cell. Phase I clinical trials of this vaccine to evaluate the safety, tolerability and immunogenicity are underway and expected to be completed by 2021 (NCT04917861). mRNA1325 is another mRNA-based vaccine that completed the phase I clinical trials in 2019 but the data has not been published yet (NCT03014089). So far there is no approved vaccine against the Zika virus, hence these mRNA-based vaccines are of therapeutic value against Zika virus infection.119
Epstein–Barr Virus (EBV)
Epstein–Barr virus, the causative agent for mononucleosis belongs to the Herpes family. EBV consists of a torsoid-shaped protein core, envelope protein, double-stranded DNA, icosahedral capsid of 162 capsomeres, a viral tegument and nucleocapsid. EBV is transmitted via direct transmission from saliva of infected individuals. Efforts have been made to develop a prophylactic vaccine against EBV to prevent primary infection and subsequent chronic disease but there is still no vaccine for clinical use.120 mRNA-1189 is an RNA vaccine against antigen glycoprotein 350 (gp350).121 Only preclinical studies have been conducted so far. Naïve Balb/c mice were administered with two doses of mRNA-1189 and the antibody titers against viral proteins involved in epithelial cell entry (gH/gL and gB) or B cell entry (gp350, gH/gL and gB) were measured in peripheral blood after 57 days (ModernaTx). With the development of these two mRNA vaccine candidates and improvements in antigen selection, there is hope for sustainable immunity and protection against EBV-associated malignancies.
Human Immunodeficiency Virus (HIV)
HIV causes acquired immunodeficiency syndrome (AIDS) and belongs to the Retroviridiae family.122 Structurally, HIV is composed of a 5ʹ long terminal repeat region (LTR) which codes for promoter for the transcription of viral genes, the reading frame of gag gene encoding outer core membrane (MA), capsid protein (CA), the nucleocapsid (NC) and a nucleic acid stabilizing protein. After gag, pol gene encodes for protease (PR), reverse transcriptase (RT), RNase H and integrase (IN). Adjacent to pol gene, env gene encodes two envelope glycoproteins gp120 and gp41.123 HIV emerged as a pandemic and globally 17.5 million people were infected by the virus. HIV is transmitted via direct human to human transmission.123 Despite numerous years of research, there is still no effective vaccine against HIV due to the antigenic diversity of the envelope protein and the dense “glycan shield” that conceals the envelope protein epitopes. There have been efforts to develop mRNA vaccines against HIV in recent years.124 Self-amplifying mRNA vaccine encoding a clade C envelope glycoprotein and viral replicon particle (VRP) was evaluated in rhesus macaques. Animals receiving the HIV SAM and HIV-VRP vaccines reached anti-Env levels of 103–104.5, with GMTs of 103.94 and 103.41, respectively.24,124 Anti-Env antibody titers increased significantly after boosting with Env/MF59, reaching levels 10–100-fold higher than those found after priming with peak GMTs of 106.14, 106.25, and 104.79 for Env/MF59, HIV SAM, and HIV-VRP respectively.125,126 Another preclinical study investigated the mRNA encoding gag gene of HIV which induced antigen-specific, functional T cells resulting in potent cytotoxic T lymphocytes.28 One study investigated the effect of intravenous injection of an LNP-encapsulated, nucleoside-modified mRNA expressing VRC01, to produce neutralizing antibodies in mice. This approach using a single dose protected mice from intravenous challenge with HIV-1.127 Hence mRNA vaccines have the potential to become efficacious vaccine candidates against HIV infection.
Streptococcus
Streptococci are coccoid gram-positive bacteria. They are non-motile and form spores. They cause numerous diseases and invasive infections. There are three groups of Streptococcus: Group A, B and C. Invasive Group A Streptococcus (GAS) causes infection of the skin, soft tissue, and respiratory tract. Most of the GAS infections are severe and, in some cases, fatal. Group B Streptococcus (GBS) leads to invasive infections in infants, pregnant women, and older adults. GBS infection leads to pneumonia, meningitis, and sepsis. Group C Streptococcus infection is zoonotic and transmitted from exposure to infected farm animals. Group C infection leads to infections in the skin and soft tissues. Currently, there are no vaccines approved against GAS/GBS and due to severity of the infections, there is an urgent need for a vaccine.128 Recently, self-amplifying mRNA (SAM) has been used to develop vaccines against GAS and GBS. Bacterial antigens (the double-mutated GAS Streptolysin-O (SLOdm) and the GBS pilus 2a backbone protein (BP-2a)) were expressed by SAM vectors. These SAM vectors bound to cationic nano-emulsion (CNE) were tested for immunogenicity in CD-1 mice. For immunogenicity, GMT of BP-2a after 2 and 3 weeks were 4.7×102 and 4.7×103, respectively. GMTs of SLOdm after 2 and 3 weeks were 1.25×104 and 2.5×104, respectively.37
Ebola Virus
Ebola virus belongs to the Filoviridae family. Ebola virus is composed of negative-sense RNA, surface glycoprotein, lipid membrane envelope and tubular helical nucleocapsid. Ebola virus is a zoonotic virus transmitted from vertebrate animals to humans and other mammalian species.129 The secondary transmission occurs from human to human by contact with infected blood and body fluids. The virus affects the gastrointestinal system and the central nervous system. In severe cases, it leads to multi-organ failure, coma, and death. Ebola emerged as an endemic in Central Africa in 2014.130 Although the FDA approved a recombinant vesicular stomatitis virus (VSV)-based Ebola vaccine (rVSV-EBOV) in 2019, there are some safety concerns including acute arthritis and skin rash at high doses.131 mRNA vaccines against the Ebola virus may prove to be safer than this virus-based vaccine as they do not replicate inside the body. Two mRNA vaccines based on the EBOV envelope glycoprotein (EBOV GP) were developed. The mRNAs were encapsulated with lipid nanoparticles to facilitate delivery. The vaccine was tested for neutralizing antibodies in guinea pigs. Two groups (vaccine A, B) of guinea pigs were immunized with the two vaccines. EBOV GP serum IgG was detected in both groups after 21 days. After 42 days, EBOV GP titers were increased in the Vaccine B group. After the second dose, the vaccine A group has 1:140, while vaccine B group increased to 1:1800.132
Advantages and Challenges Associated with mRNA Vaccines
mRNA vaccines are advantageous because mRNA is non-infectious and does not integrate into the genome. In addition, the incorporation of modified nucleosides in the mRNA sequence reduces its inflammatory capacity. Hence, mRNA-based vaccines are safer than other virus-based vaccines.10 mRNA-based vaccines have several benefits over conventional vaccines, in that they are precise and only express a specific antigen and induce a directed immune response. These vaccines promote both humoral and cellular immune responses and induce the innate immune system. mRNA expression in the body does not require nuclear entry, which diminishes the possibility of genomic integration.36 mRNA is quickly degraded by cellular processes after the induction of immune response. The mRNA vaccine manufacturing is easily standardized because a change in the antigen does not affect the mRNA backbone’s physical-chemical characteristics. Additionally, the safety concerns for the viral contaminants are minimized since production is based on an in vitro cell-free transcription reaction.133 Although there are several benefits of mRNA vaccines, certain challenges also exist. Currently, a well-established manufacturing platform for the generation of mRNA is lacking and several combinations of manufacturing steps are required. These can be grouped into upstream processing, which comprises the enzymatic generation of mRNA, and downstream processing, which includes the unit operations required to purify the mRNA product.8 In addition, the need for cold chain storage for mRNA vaccines poses logical difficulties. Therefore, there is a need for the development of thermostable vaccines for warm countries or those without reliable cold chain storage.
Concluding Remarks
Although mRNA vaccines have been in existence for almost three decades, these vaccines were approved for emergency patient use for the SARS-CoV-2 pandemic by the FDA. There is a need for understanding the molecular mechanism of action of mRNA vaccines.134 Continual optimization of UTRs and coding mRNA will provide greater stability and potency of the vaccine in terms of the production of antigens in the body. In addition, improved stability of mRNA vaccines can also be achieved using base or sugar modifications that can lead to higher translational properties. The optimization of carrier systems such as the development of ionizable lipids and formulations, enhanced cellular uptake, endosomal release, and enhanced potency is desired.135 Bioinformatics tools can be utilized for sequence optimization of coding mRNA sequences. These tools can also assist in predicting the correlation between preclinical and clinical studies on mRNA vaccines.136 The focus of current mRNA vaccines is to optimize immunogenicity and efficacy while monitoring the safety of the vaccine in humans. Activation of immune response can be advantageous but can also lead to some complications. Hence, there is a need to optimize the titers of the vaccine dose, therefore there is a need for the design of clinical trials in a way that captures the immune response effectively.8,52,137 Several technologies such as transcriptomics and proteomics-based approaches can be used to identify these immune responses.138 The humoral response generated by the mRNA vaccines so far has not been at par as compared to the live attenuated vaccines. Therefore, a detailed investigation is required to identify formulations that will enable achieving the desired immune responses.8,139 Currently, the regulatory guidelines on the clinical development of mRNA vaccines have not been fully outlined. There is a need to develop guidelines for the study of novel carriers of mRNA vaccines and monitor their safety and efficacy.70
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors affiliated with Fosun Pharma USA Inc., and Shanghai Fosun Pharmaceutical Industrial Development, Co., Ltd. The authors report no other conflicts of interest in this work.
References
1. Greenwood B. The contribution of vaccination to global health: past, present and future. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130433. doi:10.1098/rstb.2013.0433
2. Minor PD. Live attenuated vaccines: historical successes and current challenges. Virology. 2015;479–480:379–392. doi:10.1016/j.virol.2015.03.032
3. Plett PC. [Peter Plett and other discoverers of cowpox vaccination before Edward Jenner]. Sudhoffs Arch. 2006;90:219–232. German.
4. Jenner E. Dr. Jenner, on the Vaccine Inoculation. Med Phys J. 1800;3:502–503.
5. Pandey A, Cabello A, Akoolo L, et al. The case for live attenuated vaccines against the neglected zoonotic diseases brucellosis and bovine tuberculosis. PLoS Negl Trop Dis. 2016;10:e0004572. doi:10.1371/journal.pntd.0004572
6. Jang H, Elaish M, Kc M, et al. Efficacy and synergy of live-attenuated and inactivated influenza vaccines in young chickens. PLoS One. 2018;13:e0195285. doi:10.1371/journal.pone.0195285
7. Li J, Arevalo MT, Chen Y, Chen S, Zeng M. T-cell-mediated cross-strain protective immunity elicited by prime-boost vaccination with a live attenuated influenza vaccine. Int J Infect Dis. 2014;27:37–43. doi:10.1016/j.ijid.2014.05.016
8. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279.
9. Jirikowski GF, Sanna PP, Maciejewski-Lenoir D, Bloom FE. Reversal of diabetes insipidus in Brattleboro rats: intrahypothalamic injection of vasopressin mRNA. Science. 1992;255:996–998. doi:10.1126/science.1546298
10. Wang Y, Zhang Z, Luo J, et al. mRNA vaccine: a potential therapeutic strategy. Mol Cancer. 2021;20:33. doi:10.1186/s12943-021-01311-z
11. Pardi N, Hogan MJ, Naradikian MS, et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med. 2018;215:1571–1588. doi:10.1084/jem.20171450
12. Magini D, Giovani C, Mangiavacchi S, et al. Self-amplifying mRNA vaccines expressing multiple conserved influenza antigens confer protection against homologous and heterosubtypic viral challenge. PLoS One. 2016;11:e0161193. doi:10.1371/journal.pone.0161193
13. Brazzoli M, Magini D, Bonci A, et al. Induction of broad-based immunity and protective efficacy by self-amplifying mRNA vaccines encoding influenza virus hemagglutinin. J Virol. 2016;90:332–344. doi:10.1128/JVI.01786-15
14. McCullough KC, Bassi I, Milona P, et al. Self-replicating replicon-RNA delivery to dendritic cells by Chitosan-nanoparticles for translation in vitro and in vivo. Mol Ther Nucleic Acids. 2014;3:e173. doi:10.1038/mtna.2014.24
15. Hekele A, Bertholet S, Archer J, et al. Rapidly produced SAM ® vaccine against H7N9 influenza is immunogenic in mice. Emerg Microbes Infect. 2013;2:e52. doi:10.1038/emi.2013.54
16. Schnee M, Vogel AB, Voss D, et al. An mRNA vaccine encoding rabies virus glycoprotein induces protection against lethal infection in mice and correlates of protection in adult and newborn pigs. PLoS Negl Trop Dis. 2016;10:e0004746. doi:10.1371/journal.pntd.0004746
17. Alberer M, Gnad-Vogt U, Hong HS, et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390:1511–1520. doi:10.1016/S0140-6736(17)31665-3
18. Schlake T, Thess A, Fotin-Mleczek M, Kallen KJ. Developing mRNA-vaccine technologies. RNA Biol. 2012;9:1319–1330. doi:10.4161/rna.22269
19. Andries O, Mc Cafferty S, De Smedt SC, et al. N(1)-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J Control Release. 2015;217:337–344. doi:10.1016/j.jconrel.2015.08.051
20. Kim J, Eygeris Y, Gupta M, Sahay G. Self-assembled mRNA vaccines. Adv Drug Deliv Rev. 2021;170:83–112. doi:10.1016/j.addr.2020.12.014
21. Perri S, Greer CE, Thudium K, et al. An alphavirus replicon particle chimera derived from Venezuelan equine encephalitis and sindbis viruses is a potent gene-based vaccine delivery vector. J Virol. 2003;77:10394–10403. doi:10.1128/JVI.77.19.10394-10403.2003
22. Fleeton MN, Chen M, Berglund P, et al. Self-replicative RNA vaccines elicit protection against influenza A virus, respiratory syncytial virus, and a tickborne encephalitis virus. J Infect Dis. 2001;183:1395–1398. doi:10.1086/319857
23. Geall AJ, Verma A, Otten GR, et al. Nonviral delivery of self-amplifying RNA vaccines. Proc Natl Acad Sci U S A. 2012;109:14604–14609. doi:10.1073/pnas.1209367109
24. Bogers WM, Oostermeijer H, Mooij P, et al. Potent immune responses in rhesus macaques induced by nonviral delivery of a self-amplifying RNA vaccine expressing HIV type 1 envelope with a cationic nanoemulsion. J Infect Dis. 2015;211:947–955. doi:10.1093/infdis/jiu522
25. Martinon F, Krishnan S, Lenzen G, et al. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur J Immunol. 1993;23:1719–1722. doi:10.1002/eji.1830230749
26. Petsch B, Schnee M, Vogel AB, et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat Biotechnol. 2012;30:1210–1216. doi:10.1038/nbt.2436
27. Zhao M, Li M, Zhang Z, Gong T, Sun X. Induction of HIV-1 gag specific immune responses by cationic micelles mediated delivery of gag mRNA. Drug Deliv. 2016;23:2596–2607. doi:10.3109/10717544.2015.1038856
28. Li M, Zhao M, Fu Y, et al. Enhanced intranasal delivery of mRNA vaccine by overcoming the nasal epithelial barrier via intra- and paracellular pathways. J Control Release. 2016;228:9–19. doi:10.1016/j.jconrel.2016.02.043
29. Kranz LM, Diken M, Haas H, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi:10.1038/nature18300
30. Linares-Fernandez S, Lacroix C, Exposito JY, Verrier B. Tailoring mRNA vaccine to balance innate/adaptive immune response. Trends Mol Med. 2020;26:311–323. doi:10.1016/j.molmed.2019.10.002
31. Bahl K, Senn JJ, Yuzhakov O, et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol Ther. 2017;25:1316–1327. doi:10.1016/j.ymthe.2017.03.035
32. Guevara ML, Persano F, Persano S. Advances in lipid nanoparticles for mRNA-based cancer immunotherapy. Front Chem. 2020;8:589959. doi:10.3389/fchem.2020.589959
33. Eygeris Y, Patel S, Jozic A, Sahay G. Deconvoluting lipid nanoparticle structure for messenger RNA delivery. Nano Lett. 2020;20:4543–4549. doi:10.1021/acs.nanolett.0c01386
34. Reichmuth AM, Oberli MA, Jaklenec A, Langer R, Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther Deliv. 2016;7:319–334. doi:10.4155/tde-2016-0006
35. Edwards DK, Jasny E, Yoon H, et al. Adjuvant effects of a sequence-engineered mRNA vaccine: translational profiling demonstrates similar human and murine innate response. J Transl Med. 2017;15:1. doi:10.1186/s12967-016-1111-6
36. Maruggi G, Zhang C, Li J, Ulmer JB, Yu D. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol Ther. 2019;27:757–772. doi:10.1016/j.ymthe.2019.01.020
37. Maruggi G, Chiarot E, Giovani C, et al. Immunogenicity and protective efficacy induced by self-amplifying mRNA vaccines encoding bacterial antigens. Vaccine. 2017;35:361–368. doi:10.1016/j.vaccine.2016.11.040
38. Heine A, Juranek S, Brossart P. Clinical and immunological effects of mRNA vaccines in malignant diseases. Mol Cancer. 2021;20:52. doi:10.1186/s12943-021-01339-1
39. Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi:10.1038/s41586-020-2639-4
40. Pu J, Yu Q, Yin Z, et al. The safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in Chinese adults aged 18–59 years: a phase I randomized, double-blinded, controlled trial. Vaccine. 2021;39:2746–2754. doi:10.1016/j.vaccine.2021.04.006
41. Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi:10.1056/NEJMoa2027906
42. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi:10.1056/NEJMoa2034577
43. Ji RR, Qu Y, Zhu H, et al. BNT162b2 vaccine encoding the SARS-CoV-2 P2 S protects transgenic hACE2 mice against COVID-19. Vaccines (Basel). 2021;9:324.
44. Chen N, Xia P, Li S, et al. RNA sensors of the innate immune system and their detection of pathogens. IUBMB Life. 2017;69:297–304. doi:10.1002/iub.1625
45. Kowalczyk A, Doener F, Zanzinger K, et al. Self-adjuvanted mRNA vaccines induce local innate immune responses that lead to a potent and boostable adaptive immunity. Vaccine. 2016;34:3882–3893. doi:10.1016/j.vaccine.2016.05.046
46. Kell AM, Gale M. RIG-I in RNA virus recognition. Virology. 2015;479–480:110–121. doi:10.1016/j.virol.2015.02.017
47. Saito T, Gale M. Differential recognition of double-stranded RNA by RIG-I-like receptors in antiviral immunity. J Exp Med. 2008;205:1523–1527. doi:10.1084/jem.20081210
48. Loo YM, Gale M. Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi:10.1016/j.immuni.2011.05.003
49. Yin X, Riva L, Pu Y, et al. MDA5 governs the innate immune response to SARS-CoV-2 in lung epithelial cells. Cell Rep. 2021;34:108628. doi:10.1016/j.celrep.2020.108628
50. Pollard C, Rejman J, De Haes W, et al. Type I IFN counteracts the induction of antigen-specific immune responses by lipid-based delivery of mRNA vaccines. Mol Ther. 2013;21:251–259. doi:10.1038/mt.2012.202
51. Lei X, Dong X, Ma R, et al. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat Commun. 2020;11:3810. doi:10.1038/s41467-020-17665-9
52. Park JW, Lagniton PNP, Liu Y, Xu RH. mRNA vaccines for COVID-19: what, why and how. Int J Biol Sci. 2021;17:1446–1460. doi:10.7150/ijbs.59233
53. Chatzikleanthous D, O’Hagan DT, Adamo R. Lipid-based nanoparticles for delivery of vaccine adjuvants and antigens: toward multicomponent vaccines. Mol Pharm. 2021;18:2867–2888. doi:10.1021/acs.molpharmaceut.1c00447
54. Cubas R, Zhang S, Kwon S, et al. Virus-like particle (VLP) lymphatic trafficking and immune response generation after immunization by different routes. J Immunother. 2009;32:118–128. doi:10.1097/CJI.0b013e31818f13c4
55. Lindgren G, Ols S, Liang F, et al. Induction of robust B cell responses after influenza mRNA vaccination is accompanied by circulating hemagglutinin-specific ICOS+ PD-1+ CXCR3+ T follicular helper cells. Front Immunol. 2017;8:1539. doi:10.3389/fimmu.2017.01539
56. Cagigi A, Lore K. Immune responses induced by mRNA vaccination in mice, monkeys and humans. Vaccines (Basel). 2021;9. doi:10.3390/vaccines9010061
57. De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nat Rev Immunol. 2015;15:137–148. doi:10.1038/nri3804
58. Hewitt EW. The MHC class I antigen presentation pathway: strategies for viral immune evasion. Immunology. 2003;110:163–169. doi:10.1046/j.1365-2567.2003.01738.x
59. Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–473. doi:10.1146/annurev-immunol-032712-095910
60. Buschmann MD, Carrasco MJ, Alishetty S, Paige M, Alameh MG, Weissman D. Nanomaterial delivery systems for mRNA vaccines. Vaccines (Basel). 2021;9:65.
61. Rahman MM, Zhou N, Huang J. An overview on the development of mRNA-based vaccines and their formulation strategies for improved antigen expression in vivo. Vaccines (Basel). 2021;9:244.
62. Jackson NAC, Kester KE, Casimiro D, Gurunathan S, DeRosa F. The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines. 2020;5:11. doi:10.1038/s41541-020-0159-8
63. Satarker S, Nampoothiri M. Structural proteins in severe acute respiratory syndrome Coronavirus-2. Arch Med Res. 2020;51:482–491. doi:10.1016/j.arcmed.2020.05.012
64. Naqvi AAT, Fatima K, Mohammad T, et al. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165878. doi:10.1016/j.bbadis.2020.165878
65. Pal M, Berhanu G, Desalegn C, Kandi V. Severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2): an update. Cureus. 2020;12:e7423. doi:10.7759/cureus.7423
66. Dhama K, Patel SK, Sharun K, et al. SARS-CoV-2 jumping the species barrier: zoonotic lessons from SARS, MERS and recent advances to combat this pandemic virus. Travel Med Infect Dis. 2020;37:101830. doi:10.1016/j.tmaid.2020.101830
67. Haque SM, Ashwaq O, Sarief A, Azad John Mohamed AK. A comprehensive review about SARS-CoV-2. Future Virol. 2020;15:625–648. doi:10.2217/fvl-2020-0124
68. Wei J, Hui A-M. Journey of the COVID-19 vaccine from 10 years to 1 year - reassured with real world evidence. Am J Transl Med. 2021;5:1–12.
69. Lamb YN. BNT162b2 mRNA COVID-19 vaccine: first approval. Drugs. 2021;81:495–501. doi:10.1007/s40265-021-01480-7
70. Anand P, Stahel VP. Review the safety of Covid-19 mRNA vaccines: a review. Patient Saf Surg. 2021;15:20. doi:10.1186/s13037-021-00291-9
71. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi:10.1056/NEJMoa2028436
72. Kyriakidis NC, Lopez-Cortes A, Gonzalez EV, Grimaldos AB, Prado EO. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6:28. doi:10.1038/s41541-021-00292-w
73. Roth N, Schön J, Hoffmann D, et al. CV 2 CoV, an enhanced mRNA based SARS-CoV-2 vaccine candidate, supports higher protein expression and improved immunogenicity in rats. 2021. Available from https://www.biorxiv.org/content/10.1101/2021.05.13.443734v1. Accessed December 02, 2021.
74. Rauch S, Roth N, Schwendt K, et al. mRNA-based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus-neutralising antibodies and mediates protection in rodents. NPJ Vaccines. 2021;6:57. doi:10.1038/s41541-021-00311-w
75. Kremsner PG, Guerrero RA, Arana E, et al.Efficacy and Safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate: results from Herald, a phase 2b/3, randomised, observer-blinded, placebo-controlled clinical trial in ten countries in Europe and Latin America. Lancet. 2021.
76. Zhang NN, Li X-F, Deng Y-Q, et al. A thermostable mRNA vaccine against COVID-19. Cell. 2020;182:1271–1283 e1216. doi:10.1016/j.cell.2020.07.024
77. Granados-Riveron JT, Aquino-Jarquin G. Engineering of the current nucleoside-modified mRNA-LNP vaccines against SARS-CoV-2. Biomed Pharmacother. 2021;142:111953. doi:10.1016/j.biopha.2021.111953
78. Laczko D, Hogan MJ, Toulmin SA, et al. A single immunization with nucleoside-modified mRNA vaccines elicits strong cellular and humoral immune responses against SARS-CoV-2 in mice. Immunity. 2020;53:724–732 e727. doi:10.1016/j.immuni.2020.07.019
79. Corbett KS, Edwards DK, Leist SR, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi:10.1038/s41586-020-2622-0
80. Funk CD, Laferriere C, Ardakani A. A snapshot of the global race for vaccines targeting SARS-CoV-2 and the COVID-19 pandemic. Front Pharmacol. 2020;11:937. doi:10.3389/fphar.2020.00937
81. Harvey WT, Carabelli AM, Jackson B, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409–424. doi:10.1038/s41579-021-00573-0
82. Sanyaolu A, Okorie C, Marinkovic A, et al. The emerging SARS-CoV-2 variants of concern. Ther Adv Infect Dis. 2021;8:20499361211024372. doi:10.1177/20499361211024372
83. Slavov SN, Patané JS, Bezerra RD, et al. Genomic monitoring unveil the early detection of the SARS-CoV-2 B.1.351 (beta) variant (20H/501Y.V2) in Brazil. J Med Virol. 2021;93:6782–6787. doi:10.1002/jmv.27190
84. Liu J, Liu Y, Xia H, et al. BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants. Nature. 2021;596(7871):273–275.
85. Annavajhala MK, Mohri H, Wang P, et al. A novel and expanding SARS-CoV-2 variant, B.1.526, identified in New York. medRxiv. 2021. doi:10.1101/2021.02.23.21252259
86. Gomez CE, Perdiguero B, Esteban M. Emerging SARS-CoV-2 variants and impact in global vaccination programs against SARS-CoV-2/COVID-19. Vaccines (Basel). 2021;9:243.
87. Wu K, Choi A, Koch M, et al. Variant SARS-CoV-2 mRNA vaccines confer broad neutralization as primary or booster series in mice. bioRxiv. 2021. doi:10.1016/j.vaccine.2021.11.001
88. Mahase E. Covid-19: moderna applies for US and EU approval as vaccine trial reports 94.1% efficacy. BMJ. 2020;371:m4709. doi:10.1136/bmj.m4709
89. Tegally H, Wilkinson E, Giovanetti M, et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592:438–443. doi:10.1038/s41586-021-03402-9
90. Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273–2282. doi:10.1056/NEJMoa2104983
91. Riley LE. mRNA Covid-19 vaccines in pregnant women. N Engl J Med. 2021;384:2342–2343. doi:10.1056/NEJMe2107070
92. Fooks AR, Cliquet F, Finke S, et al. Rabies. Nat Rev Dis Primers. 2017;3:17091. doi:10.1038/nrdp.2017.91
93. Davis BM, Rall GF, Schnell MJ. Everything you always wanted to know about rabies virus (but were afraid to ask). Annu Rev Virol. 2015;2:451–471. doi:10.1146/annurev-virology-100114-055157
94. Hampson K, Coudeville L, Lembo T, et al. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis. 2015;9:e0003709. doi:10.1371/journal.pntd.0003709
95. Aldrich C, Leroux–Roels I, Huang KB, et al. Proof-of-concept of a low-dose unmodified mRNA-based rabies vaccine formulated with lipid nanoparticles in human volunteers: a phase 1 trial. Vaccine. 2021;39:1310–1318. doi:10.1016/j.vaccine.2020.12.070
96. Armbruster N, Jasny E, Petsch B. Advances in RNA vaccines for preventive indications: a case study of a vaccine against rabies. Vaccines (Basel). 2019;7:996–998. doi:10.3390/vaccines7040132
97. Te Velthuis AJ, Fodor E. Influenza virus RNA polymerase: insights into the mechanisms of viral RNA synthesis. Nat Rev Microbiol. 2016;14:479–493. doi:10.1038/nrmicro.2016.87
98. Bouvier NM, Palese P. The biology of influenza viruses. Vaccine. 2008;26(Suppl 4):D49–53. doi:10.1016/j.vaccine.2008.07.039
99. Krammer F, Smith GJD, Fouchier RAM, et al. Influenza. Nat Rev Dis Primers. 2018;4:3. doi:10.1038/s41572-018-0002-y
100. Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12:15–22. doi:10.3201/eid1209.05-0979
101. Zost SJ, Parkhouse K, Gumina ME, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A. 2017;114:12578–12583. doi:10.1073/pnas.1712377114
102. Rockman S, Laurie KL, Parkes S, Wheatley A, Barr IG. New technologies for influenza vaccines. Microorganisms. 2020;8:1745. doi:10.3390/microorganisms8111745
103. Zhang JJ, Dong X, Cao -Y-Y, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi:10.1111/all.14238
104. Feldman RA, Fuhr R, Smolenov I, et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019;37:3326–3334. doi:10.1016/j.vaccine.2019.04.074
105. Collins PL, Fearns R, Graham BS. Respiratory syncytial virus: virology, reverse genetics, and pathogenesis of disease. Curr Top Microbiol Immunol. 2013;372:3–38. doi:10.1007/978-3-642-38919-1_1
106. Battles MB, McLellan JS. Respiratory syncytial virus entry and how to block it. Nat Rev Microbiol. 2019;17:233–245. doi:10.1038/s41579-019-0149-x
107. Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev. 2014;35:519–530. doi:10.1542/pir.35-12-519
108. Aliprantis AO, Shaw CA, Griffin P, et al. A phase 1, randomized, placebo-controlled study to evaluate the safety and immunogenicity of an mRNA-based RSV prefusion F protein vaccine in healthy younger and older adults. Hum Vaccin Immunother. 2021;17:1248–1261. doi:10.1080/21645515.2020.1829899
109. Crowe JE, Williams JV. Paramyxoviruses: respiratory syncytial virus and human metapneumovirus. In Viral Infections of Humans. Springer; 2014;601–627.
110. Bailly JE, McAuliffe JM, Durbin AP, et al. A recombinant human parainfluenza virus type 3 (PIV3) in which the nucleocapsid N protein has been replaced by that of bovine PIV3 is attenuated in primates. J Virol. 2000;74:3188–3195. doi:10.1128/JVI.74.7.3188-3195.2000
111. Shaw C, Lee H, Knightly C, et al. 2754. Phase 1 trial of an mRNA-based combination vaccine against hMPV and PIV3. Open Forum Infect Dis. 2019;6:S970–S970. doi:10.1093/ofid/ofz360.2431
112. Jeffries AM, Nitika T, Marriott I. The intracellular DNA sensors cGAS and IFI16 do not mediate effective antiviral immune responses to HSV-1 in human microglial cells. J Neurovirol. 2020;26:544–555. doi:10.1007/s13365-020-00852-1
113. Schottstedt V, Blümel J, Burger R, et al. Human cytomegalovirus (HCMV) - revised. Transfus Med Hemother. 2010;37:365–375.
114. Plotkin SA, Wang D, Oualim A, et al. The status of vaccine development against the human cytomegalovirus. J Infect Dis. 2020;221:S113–S122. doi:10.1093/infdis/jiz447
115. Anderholm KM, Bierle CJ, Schleiss MR. Cytomegalovirus vaccines: current status and future prospects. Drugs. 2016;76:1625–1645. doi:10.1007/s40265-016-0653-5
116. Noorbakhsh F, Abdolmohammadi K, Fatahi Y, et al. Zika virus infection, basic and clinical aspects: a review article. Iran J Public Health. 2019;48:20–31.
117. Richner JM, Himansu S, Dowd KA, et al. Modified mRNA vaccines protect against zika virus infection. Cell. 2017;168:1114–1125 e1110. doi:10.1016/j.cell.2017.02.017
118. Pardi N, Hogan MJ, Pelc RS, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543:248–251. doi:10.1038/nature21428
119. Poland GA, Kennedy RB, Ovsyannikova IG, et al. Development of vaccines against Zika virus. Lancet Infect Dis. 2018;18:e211–e219. doi:10.1016/S1473-3099(18)30063-X
120. Cohen JI, Fauci AS, Varmus H, Nabel GJ. Epstein-Barr virus: an important vaccine target for cancer prevention. Sci Transl Med. 2011;3:107fs107. doi:10.1126/scitranslmed.3002878
121. Fugl A, Andersen CL. Epstein-Barr virus and its association with disease - a review of relevance to general practice. BMC Fam Pract. 2019;20:62. doi:10.1186/s12875-019-0954-3
122. Chand HS, Vazquez-Guillamet R, Royer C, et al. Cigarette smoke and HIV synergistically affect lung pathology in cynomolgus macaques. J Clin Invest. 2018;128:5428–5433. doi:10.1172/JCI121935
123. German Advisory Committee Blood. Human Immunodeficiency Virus (HIV). Transfus Med Hemother. 2016;43:203–222. doi:10.1159/000445852
124. Esteban I, Pastor-Quiñones C, Usero L, et al. In the era of mRNA vaccines, is there any hope for HIV functional cure? Viruses. 2021;13:501. doi:10.3390/v13030501
125. Emmer KL, Wieczorek L, Tuyishime S, et al. Antibody responses to prime-boost vaccination with an HIV-1 gp145 envelope protein and chimpanzee adenovirus vectors expressing HIV-1 gp140. AIDS. 2016;30:2405–2414. doi:10.1097/QAD.0000000000001224
126. Spearman P, Lally MA, Elizaga M, et al. A trimeric, V2-deleted HIV-1 envelope glycoprotein vaccine elicits potent neutralizing antibodies but limited breadth of neutralization in human volunteers. J Infect Dis. 2011;203:1165–1173. doi:10.1093/infdis/jiq175
127. Pardi N, Secreto AJ, Shan X, et al. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat Commun. 2017;8:14630. doi:10.1038/ncomms14630
128. Parks T, Barrett L, Jones N. Invasive streptococcal disease: a review for clinicians. Br Med Bull. 2015;115:77–89. doi:10.1093/bmb/ldv027
129. Kourtis AP, Appelgren K, Chevalier MS, McElroy A. Ebola virus disease: focus on children. Pediatr Infect Dis J. 2015;34:893–897. doi:10.1097/INF.0000000000000707
130. Matua GA, Van der Wal DM, Locsin RC. Ebolavirus and haemorrhagic syndrome. Sultan Qaboos Univ Med J. 2015;15:e171–176.
131. Agnandji ST, Huttner A, Zinser ME, et al. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med. 2016;374:1647–1660. doi:10.1056/NEJMoa1502924
132. Meyer M, Huang E, Yuzhakov O, et al. Modified mRNA-based vaccines elicit robust immune responses and protect guinea pigs from Ebola virus disease. J Infect Dis. 2018;217:451–455. doi:10.1093/infdis/jix592
133. Rosa SS, Prazeres DMF, Azevedo AM, Marques MPC. mRNA vaccines manufacturing: challenges and bottlenecks. Vaccine. 2021;39:2190–2200. doi:10.1016/j.vaccine.2021.03.038
134. Abbasi J. COVID-19 and mRNA vaccines-first large test for a new approach. JAMA. 2020;324:1125–1127. doi:10.1001/jama.2020.16866
135. Wadhwa A, Aljabbari A, Lokras A, Foged C, Thakur A. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics. 2020;12:102. doi:10.3390/pharmaceutics12020102
136. Xu S, Yang K, Li R, Zhang L. mRNA vaccine era-mechanisms, drug platform and clinical prospection. Int J Mol Sci. 2020;21:6582. doi:10.3390/ijms21186582
137. Bettini E, Locci M. SARS-CoV-2 mRNA vaccines: immunological mechanism and beyond. Vaccines (Basel). 2021;9. doi:10.3390/vaccines9020147
138. Manzoni C, Kia DA, Vandrovcova J, et al. Genome, transcriptome and proteome: the rise of omics data and their integration in biomedical sciences. Brief Bioinform. 2018;19:286–302. doi:10.1093/bib/bbw114
139. Clem AS. Fundamentals of vaccine immunology. J Glob Infect Dis. 2011;3:73–78. doi:10.4103/0974-777X.77299
140. Gebre MS, Rauch S, Roth N, et al. Optimization of non-coding regions for a non-modified mRNA COVID-19 vaccine. Nature. 2021. doi:10.1038/s41586-021-04231-6
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.