Back to Journals » Patient Preference and Adherence » Volume 17
The Design of a Persuasive Game to Motivate People with Asthma in Adherence to Their Maintenance Medication
Authors Poot CC , de Boer J, Goto L, van de Hei SJ, Chavannes NH , Visch VT, Meijer E
Received 14 July 2023
Accepted for publication 14 October 2023
Published 1 November 2023 Volume 2023:17 Pages 2719—2736
DOI https://doi.org/10.2147/PPA.S423161
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Charlotte C Poot,1,2 Jasmijn de Boer,3 Lyè Goto,3 Susanne J van de Hei,4,5 Niels H Chavannes,1,2 Valentijn T Visch,3 Eline Meijer1,2
1Department of Public Health and Primary Care, Leiden University Medical Centre, Leiden, The Netherlands; 2National eHealth Living Lab (NeLL), Leiden University Medical Centre, Leiden, The Netherlands; 3Department of Human-Centered Design, Faculty of Industrial Design Engineering, Delft University of Technology, Delft, The Netherlands; 4Department of Health Sciences, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; 5General Practitioners Research Institute, Groningen, The Netherlands
Correspondence: Charlotte C Poot, Leiden University Medical Centre, Hippocratespad 21, Leiden, 2333 ZD, The Netherlands, Email [email protected]
Objective: This study aimed to design a persuasive game, using objective adherence data, to motivate people with asthma to adhere to their medication regimen.
Methods: A participatory user-centered design approach was employed, involving end-users and other stakeholders throughout the study. The approach consisted of four phases. Semi-structured interviews and a survey were conducted to understand user needs and reasons for poor adherence (Phase 1: define). Key themes were identified, leading to the formulation of behavior change strategies and design and game requirements. Several design directions were ideated, resulting in a concept for a serious game (Phase 2: ideate). Two rounds of user-tests were performed to evaluate a prototype of the serious game in terms of usability, perceived impact on medication adherence and motivation (Phase 3: prototype and Phase 4: evaluate).
Results: Findings from semi-structured interviews (n = 6) and the online survey (n = 20) revealed that people’s non-adherence was often attributed to the perception of asthma as an episodic condition, the delayed experienced effect of maintenance inhalers, and lack of knowledge regarding difference of effect between maintenance and reliever inhalers. The study used behavior change strategies to translate these insights into design requirements for the development of the narrative-based persuasive game Ademgenoot. This six-week challenge-based game combines various behavior change strategies, including personal goal setting and continuous visual feedback, as well as persuasive game design elements, such as a narrative and rewards, with the aim of enhancing motivation to adhere to their medication regimen. User-testing (n = 8; round 1 and 2) showed that Ademgenoot is feasible in clinical practice and has the potential to support people with mild asthma in adherence to their maintenance medication.
Discussion: Future efforts should be directed towards a larger evaluation to assess the impact on motivation and inhaler use behaviour.
Plain Language Summary: The goal of this study was to create a serious game that encourages people with asthma to take their medication regularly. During the study, we worked closely with individuals who have asthma and other stakeholders throughout the study. We conducted interviews and surveys to understand why people have difficulties using their maintenance inhaler as prescribed by their doctor. Based on the feedback we received, we developed a serious game called “Ademgenoot”. The game uses information on inhaler use automatically collected with a device attached to the inhaler. The game includes features like personal goals and visual feedback on inhaler use to motivate users to take their medication consistently. We tested a prototype of the game with users to see if it was easy to use and if it motivated them to use their maintenance inhaler. The results showed that Ademgenoot is a viable option for helping individuals with mild asthma stay on track with their medication.
Key words: medication adherence, serious game design, behavior change, adherence monitoring, participatory design
Background
Despite the availability of effective inhaler medication, almost half of all asthma patients have poor asthma control.1,2 Poor adherence to maintenance inhalers is a major cause of inadequate asthma control and is associated with 2.5-fold increased risk of uncontrolled asthma. As such, non-adherence is associated with increased risk of severe asthma exacerbations,3 more asthma-related emergency department visits,4 hospitalization and decreased lung function and quality of life.5 Moreover, non-adherence has been shown to increase healthcare costs, as patients with uncontrolled asthma require more frequent medical visits, interventions and step-ups in medication regimen.6,7 In addition, medication non-adherence is often accompanied by over-reliance on short-acting B2 agonists (SABA) for symptom relief.8–11 This is of particular concern as high SABA use is associated with increased exacerbations and 2.2 times higher asthma-related healthcare costs than low SABA use.12 Hence, it is important to address poor medication adherence and promote appropriate SABA usage.
Numerous factors contribute to poor medication adherence, including forgetfulness, limited understanding of the importance of adherence (necessity), and concerns about medication side effects.13,14 Improving medication adherence may be especially challenging among people with mild asthma as they may have a lower feeling of necessity and underestimate the importance of adhering to the maintenance inhaler compared to people with severe asthma.15 Interventions, such as patient education, reminder systems, and simplified medication regimens, have been developed to target the problem of poor adherence.16 More recent technological developments targeting poor adherence include electronic monitoring devices (EMDs) to track and monitor inhaler usage, providing objective and accurate data on medication adherence. EMD data can be used by healthcare providers to identify patients who do not adhere to their medication regimens, intervene with appropriate education, and support or help in decisions on the step-up of treatment. EMDs that connect to patients’ smartphones (also known as smart inhalers) can, in addition, provide patient self-management support by providing medication reminders, personalized feedback and motivational messages. Overall, EMDs have shown promise in improving medication adherence,17–21 however their impact on asthma control and the long-term sustainability in improving adherence and asthma control still require further investigation.17–19
Studies on the effectiveness of smart inhalers have primarily focused on examining the overall effect on medication adherence, rather than identifying which patient groups benefit the most based on determinants of non-adherence. Nevertheless, it is generally believed that smart asthma inhalers, featuring electronic reminders, primarily benefit unintentional nonadherence due to forgetfulness.22 Furthermore, while people with severe or persistent asthma have shown interest in using an app to manage their condition.23 This interest may stem from their heightened necessity for effective asthma control.15,21,23 Moreover, it should be noted that self-management-based interventions are typically used by individuals who are already motivated to self-manage their condition, feel more necessity in doing so and actively monitor their symptoms and behavior. In addition, engagement with such self-monitoring programs can be challenged, as they often require people to enter their symptoms or asthma control manually.21,24–26
Serious games are designed to motivate and engage users for non-entertainment goals, utilizing game elements and game mechanisms. Consequently, they are increasingly used as strategies within behavior change interventions to overcome the challenges of disengagement. Although serious games are often applied to facilitate educational purposes, they have also proven effective in motivating users to adopt specific attitudes and health behaviors such as medication- and therapy adherence or adopting a healthy lifestyle.27 Notably, serious games have demonstrated effectiveness in improving medication adherence among cancer patients,28 promoting physical activity in people with diabetes29 and addressing several mental disorders.27
To understand the underlying mechanisms through which serious games promote beneficial health outcomes, the Persuasive Game Design Model was developed.30,31 According to the model, the skills, attitudes, and beliefs developed within the game world can be transferred to the real world. The Persuasive Game Design Model offers, at the same time, a comprehensive step-by-step framework to standardize and guide the development of serious games. This framework entails (1) defining the transfer effect, including the type of effect and the type of change (eg, reinforcing, altering or forming new behaviors or attitudes) (2) investigating the users’ context, (3) identifying, developing and testing game components (eg, challenges, game levels, and virtual rewards) and game mechanics (eg, collaboration and competition), and (4) evaluating the effectiveness of the game in achieving the intended transfer effect.31 Given the potential of serious game to engage individuals and change behaviors, serious games could prove to be an effective way of motivating people to adhere to their maintenance inhaler.
When designing a persuasive game interconnected to an EDM, it is important to actively involve all stakeholders, to ensure alignment with stakeholder and end-user needs and preferences.22,32 In recent years, a participatory design methodology has emerged as a valuable approach to include end-users and other stakeholders in the development of eHealth. This approach emphasizes several key processes, including at least two iterative cycles of testing and feedback, involving end-users at all stages of the process, and incorporating input from other stakeholders such as clinicians and smart inhaler developers.33
The objective of this study was to apply a participatory design approach to design a persuasive game aimed at improving medication adherence among people with asthma by leveraging the potential of smart inhalers and incorporating behavior change and persuasive game design strategies. This paper describes the development cycle, design requirements and assessment of the final prototype on usability, usefulness, and perceived impact on improving medication adherence.
Method
Study Design
This study employed a user-centered, participatory design approach, during which people with asthma, as the end-users, had an important role in the design process. The approach consisted of four phases: define, ideate, prototype, and evaluate. The define phase (phase 1) was used to gain a deep understanding of the users’ needs, lived experiences and reasons for non-adherence and to identify design requirements. Design requirements were translated into several design directions in the ideation phase (phase 2). In two iterations, a prototype was developed (phase 3) and evaluated with end-users to gather user-feedback and improve the prototype (phase 4). Multiple stakeholder groups were included and consulted throughout the project. Healthcare professionals (ie, general practitioner, practice nurse) provided input on medical content and feasibility for practice. Smart inhaler developers (ie, representatives from pharmaceutical and medical device companies involved in the development and manufacturing of smart inhalers) provided input on technical and commercial feasibility. A visual representation of the study design and phases, including the core study activities and stakeholders involved, is presented in Figure 1.
 |
Figure 1 Schematic overview study design and design phases. |
Design Context
The study was conducted between February 2019 and August 2019 in the area of Zuid-Holland, the Netherlands.
Participants and Recruitment Procedures
Participants
People with asthma were eligible for study participation if they reported the following: 18 years and older, self-reported diagnosis of asthma, use of an ICS inhaler, difficulties being medication adherent, adequate oral fluency in Dutch or English. Participants were sampled purposively to represent diversity in age, gender, frequency of inhaler use and educational level.
Recruitment Procedure
Multifaceted strategies were utilized including advertising on social media and university websites, and posters placed in sporting clubs and on university noticeboards. The recruitment poster included an illustration of three people representing different reasons to be non-adherent, with the question “do you recognize yourself? Participate in our study”. Upon expression of interest in participation a patient information sheet was sent by e-mail. The sheet contained information about the purpose of the study, the description of the study activity, that participation is voluntary, that audio is recorded, that data is treated confidentially and anonymously and that responses are reported pseudonymized. The information sheet was adapted per study activity (ie, interview, survey, user test 1, user test 2, and online survey).
Ethical Considerations
The study was cleared for ethics by the Medical Ethical Review Committee of the Leiden University Medical Centre (No P18.158). The study was conducted in accordance with the Helsinki declaration. Written informed consent was obtained from all participants prior to all study activities.
Study Procedures
The research was conducted in multiple phases using a mixed-methods approach. Throughout the phases, design methods such as personas, creative assignments, and prototyping were used to gain an in-depth understanding of user needs and preferences, identify opportunities for design and facilitate engagement of the participants with the research activities.
Phase 1: Define
To gain an in-depth understanding of people’s inhaler use, reasons for non-adherence and their needs, semi-structured interviews (n = 6) were held. The number of participants were deemed sufficient in light of the specific study activity purpose (ie, to identify design directions). Interviews were guided by a topic list including questions about inhaler usage, beliefs about medication and challenges in everyday life dealing with inhaler use and their asthma. Interviews were – in agreement with the participant – held at the participants’ house or at another place which offered sufficient privacy (eg, university). The duration of the interviews was approximately 30 to 60 minutes. Interviews were audio recorded and transcribed.
A 5-minute online survey was administered among people with asthma (n = 20), containing items on efficacy and attitude from the Knowledge Attitude Self Efficacy Questionnaire (KASE-Q)34 and Beliefs about Medication Questionnaire – Specific (BMQ-specific) used to measure beliefs about asthma medication.35 Both questionnaires were available in Dutch36,37 and used in previous studies.38,39 The online survey was distributed via social media. Descriptive statistics were obtained using SPSS.
Interview transcripts were analyzed and triangulated with results from an online survey. Results were discussed with four primary care healthcare professionals to facilitate contextual understanding. Also, current strategies on how healthcare professionals motivate people to adhere to their maintenance medication were discussed to identify potentially useful behavior change strategies. Themes were identified and mapped onto The Transtheoretical Model (TTM) for Behavior Change to identify behavior change strategies and place these within the five stages of behavior change (ie, precontemplation; contemplation; preparation; action and maintenance). The TTM helped to structure behavioral change strategies, without losing the complex interactions between individual and environmental factors that can impact behavior out of sight.
Phase 2: Ideation
Insights from phase 1 were translated into personas. Personas are used to better understand the needs, goals and behaviors of different user groups and create solutions that tailor to those needs.40 Personas included background information (ie, short biography to give each persona more depth), everyday challenges with asthma self-management, asthma medication beliefs and behaviors, personal goals regarding their asthma-related health and opportunities for design. Personas were used as illustrative scenarios in ideation sessions. Multiple ideation exercises were held with user-centered designers to spark creative thinking, envision a solution, and explore various design directions. Design directions were discussed with an expert on behavior change strategies and a persuasive game design expert (author VV), which led to a number of design and game requirements (ie criteria, constraints, and specifications that a product, system, or service must meet in order to fullfill its intended purpose and meet the needs of its end-users). Design and game requirements were translated into the final design concept.
Phase 3 and 4: Prototype and Formative Evaluation – Iteration 1
The concept was translated to a paper-prototype (ie, a physical representation of the design) to easily gather user feedback and make changes to the design as needed. This helped to improve the overall user experience and ensure that the final product or service is well aligned with user needs and preferences. The paper-prototype consisted of the different screens that the end-users would interact with. User feedback sessions were held with people with mild asthma. We estimated the number of participants based on guidelines for user testing. Five participants generally find 80% of all usability problems in prototype testing.41 During the user-feedback sessions, participants were introduced to the concept, and a step-by-step scenario was used to let the participant walk through all the elements of the concept. Participants were asked to verbalize their thoughts and reasoning. This “think aloud” method allowed the researchers to continuously capture feedback, with participants describing their actions and immediate thoughts for each step.
After the walk-through, participants were asked to rate their level of agreement with self-developed statements developed specifically for this study, regarding usability and usefulness on a 5-point Likert scale (1 = strongly disagree, 5 = strongly agree), for example, “I would find the app useful in managing my inhaler use”. The feedback sessions were audio-recorded. Recordings were transcribed and analyzed using content analysis to determine usability and user preferences and identify further improvements.
Insights from the feedback sessions were discussed with smart inhaler developers. Input was gathered on technical feasibility (ie, does the concept require new technical functionalities to be developed) and commercial feasibility (ie, does the concept add to the market value of existing smart inhalers). Healthcare professionals were asked to comment on accuracy, feasibility, and their overall opinion on the expected impact on medication adherence. Feedback was collected and used in combination with the list of improvements to identify necessary features and improvements.
Phase 3 and 4: Prototype and Evaluation – Iteration 2
Improvements were made to the paper prototype, which was then translated to a WhatsApp prototype. The prototype mimicked the use of the new concept in practice. Using WhatsApp as a communication tool between the investigator and the participant, the participants received the screens they would interact with if they were using the real app. This approach allowed participants to interact with the concept for a longer period of time, within their own setting (eg, at home) and at the same time did not require the costly development of a functional app in this early phase. Participants were asked every day at a fixed time (12 pm) “Did you use your maintenance medication this morning (yes/no)”? and “Did you use your reliever medication yesterday (yes/no)”? Based on their answers, participants received the corresponding screen via WhatsApp. The participants did not receive any instructions on inhaler use. After the five-day testing period, participants were invited for a semi-structured interview. The participants were asked to reflect on the screens they had received in terms of motivation to use their maintenance inhaler, usefulness, inhaler use and usability. During the interview, the other screens (eg, those that were sent with a different combination of answers) were also presented and asked to provide feedback on. In total, the interviews lasted approximately 1.5 hours. The interview was audio recorded and transcribed. Transcripts were analyzed and used to formulate design recommendations.
Results
Phase 1: Define
All participants of the questionnaire (n = 20) expressed that they found it difficult to use their maintenance inhaler on a daily basis. While the initial answer of all participants was that they did not adhere to their inhaler, because they simply forgot, further questioning revealed additional reasons and motives. Based on the interview data and data from the online survey, five key themes were identified regarding reasons for non-adherence to maintenance medication. Each theme is described below. Demographics of the participants of the interviews and the online survey are depicted in Appendix 1. Although not a specific inclusion criterion, participants in the interviews (and subsequent study activities, as chosen design focus) included participants with mild asthma only.
Theme 1: Asthma Feels Episodic and No Need for Medication When Feeling Well
Participants found it difficult to use their inhaler daily because their asthma felt episodic rather than chronic, marked by periods where they hardly experienced any symptoms. During these periods, they did not use their maintenance inhaler until their symptoms increased. Participants initially indicated that they believed there was no necessity to use the maintenance inhaler in the period they hardly felt any symptoms. Not experiencing symptoms or feeling “well enough” were reasons to not use their maintenance medication on a regular basis for the majority of the participants. Comparable results were seen in the online survey. Half of the participants agreed, or completely agreed with the statement “Asthma feels episodic to me”.
Theme 2: Not Wanting to Feel Like a Patient
Participants mentioned that using the inhaler made them feel like “a patient”. Two out of six participants mentioned that even filling in a symptom and asthma control questionnaires reminded them of “being a patient” and made them more conscious of their asthma, which they preferred to not be aware of. They explained that they rather “live in the moment” and not be reminded of having asthma. In line with these qualitative findings, 33.3% of the online survey participants strongly agreed with the statement “using my maintenance inhaler is not necessary when I feel well”. When asked how to deal with flare-up of symptoms, one interviewee answered that he would use the reliever inhaler more often, up to six times a day.
Theme 3: Health and Symptom Burden as an Important Motivator to Use a Maintenance Inhaler
All participants experienced moments of worsening of their asthma. During these moments, all except one participant used their maintenance inhaler daily or occasionally. The increased burden of disease on everyday life activities was a reason to start using the maintenance inhaler after a period of non-adherence. For two participants, important sport performances, requiring optimal physical condition, were reasons to be medication adherent. During these periods of performance (eg, an important hockey tournament) they were more consciously self-managing their asthma which meant using their maintenance inhaler daily (temporarily), stopping smoking and avoiding other triggers.
Theme 4: (Delayed) Experienced Effect of Medication
When discussing reasons why participants rather used the reliever medication, the fact that people did not feel an effect of maintenance medication but did feel the effect (ie, being able to breath and feeling of relieve) of their reliever medication were the main reasons, as one participant explained: “I find it hard to feel the effect of the medication. I do notice it when I feel bad, but I don’t notice it when it goes well.’
Another participant indicated that she found it difficult to distinguish between breathlessness as the result of asthma or ‘bad’ condition.
Theme 5: Need for Knowledge on Inhaler Differences
Other reasons for not using the maintenance inhaler included participants’ limited knowledge regarding the distinction between the maintenance and reliever inhaler, as well as fear of potential side effects (eg, sore throat), they associated with the use of inhaled corticosteroids (ICS). Three participants expressed a desire for more information about the difference in effect between the different inhalers. This need for more information was reinforced by the results of the online survey in which 50% of all respondents agreed or completely agreed with the statement “I would like to have more knowledge about the effect of my medication”.
None of the participants were familiar with EDMs. However, when asked if insight into their inhaler use and symptoms would help them gain a better understanding of how inhaler use can help in controlling their asthma, the majority of the participants affirmed the potential benefits. They clarified that seeing trends between medication use and asthma control would help them understand how proper inhaler use can facilitate asthma control. Two participants also indicated that they also would like to have more insight in their triggers.’
Based on the aforementioned findings, eight behavioral change techniques were identified which were mapped onto the TTM to identify the stage in which the technique should intervene. Behavior change techniques were: i) motivational interviewing, ii) patient education, iii) personal goal setting, and iv) commitment to support contemplation; v) feedback on inhaler usage behavior, vi) self-monitoring of behavior, and vii) self-monitoring of asthma control to support action; and viii) evaluation moments to support maintenance.
Phase 2: Ideation
Five personas were created (see Appendix 2 for an example) and used to identify three design directions each focusing on a separate stage within the TTM (see Appendix 3 for the three design directions and the different ideas). Discussion meeting of the three design directions with an expert on persuasive game design and behaviour change led to the formulation of design requirements (see Table 1). Game requirements were deducted from persuasive game design theory to ensure that the persuasive game is fun, engaging and effective in promoting adherence to maintenance inhalers. These included that the game must be rewarding if the desired behavior is carried out, visualize the effects of medication use, create an overview of medication use over time and be entertaining to engage with (see Table 1).
 |
Table 1 Behavior Change Strategies and Design and Serious Game Requirements |
Final Concept – Ademgenoot
The desired design and game requirements were translated into a serious game concept “Ademgenoot”. The name of the concept was based on a wordplay of the Dutch words “Ademnood” (Breathlessness) and “genoot” (Buddy). Using automatic data logging with an EDM to detect inhaler use and narrative game-elements, Ademgenoot aims to motivate people with mild asthma to adhere to their prescribed maintenance medication treatment. Ademgenoot does so by focusing on the positive effects of taking the daily maintenance medication. Moreover, the application gives the patients the opportunity to try-out the daily intake of their medication as prescribed, by offering them a six-week challenge linked to an asthma-related personal goal (eg, join friends on a skiing weekend). During this six-week period Ademgenoot visualizes inhaler use (maintenance and reliever inhaler) in a playful way to make the effect of the medication visible and to stimulate engagement with the game and thus use of their inhaler. Six weeks was chosen as this is generally the period in which individuals respond to ICS with an improvement in pulmonary function.42 At the end of the challenge, users should have gained insights into the effect of the daily use of their maintenance inhaler and their improved asthma control within their daily lives (see Figure 2 for a visual representation of the game). As such, Ademgenoot supports the intention, action, and evaluation of behavior change.
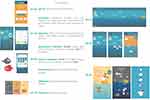 |
Figure 2 Visual representation of the core elements of the persuasive game “Ademgenoot”. |
Intention
Upon onboarding the app, Ademgenoot offers a brief instruction on inhaler technique and the difference between maintenance and reliever inhaler and its effect on the lungs. Motivational Interviewing techniques are included through the use of personal goal setting and creating a commitment. After the onboarding screens, users are offered a six-week challenge, linked to a personal goal. Upon accepting the challenge, users enter the game world.
Action
Use of Narrative and Game Elements
Ademgenoot employs the use of a narrative and metaphors to motivate users to adhere to their maintenance inhaler. The narrative involves a cloud-like character named Brad, whose mission is to reach a treasure island, which represents the user’s personal adherence goal. To do so, Brad must cross over water. Connected to the EDM, Brad is able to “safely” cross the water when inhaler use is detected. However, when inhaler use is not detected he falls into the water. Therefore, it is the patient’s responsibility to create bridges for Brad to safely cross the water by adhering to the inhaler regimen. The user is also confronted with the possibility of Brad falling into the water and drowning when not adhering to the regimen. In the scenario of one missed dose, Brad can hang on to the bridge.
Visualization Effect of Maintenance Inhaler Use
The protective effect of maintenance medication on the lungs is visualized through the environment and Brad’s physical appearance. Whereas one missed dose is not detrimental (ie, Brad is able to hang on to the bridge), the negative impact of multiple non-adherence days is represented through a gradual shift towards a greyer and gloomier environment, with Brad sinking deeper. On the other hand, longer streaks of adherent days, result in a stronger Brad, equipped with a diving goggle to be able to endure longer under water (ie, metaphor for protection of the lungs). The environment also becomes brighter, and users are rewarded with an enriched environment, which for example includes a turtle underwater. Users are provided with a report on the medication usage every three days, represented as intermediate treasure chests.
Visualization Effect of Relieve Inhaler Use
Use of the reliever inhaler is logged through a reliever EMD. When reliever inhaler use is detected, a push-notification is sent to the user, who is then prompted to indicate the reason for use by selecting triggers from a pre-specified list of triggers. Additionally, the user is asked to indicate their level of asthma control using a color scale. To improve engagement with the app and improve the input of self-monitoring data, which can be a challenging aspect of self-monitoring interventions, the to be filled in form is depicted as a piranha, which transforms upon completion of the form into a friendly-looking fish.
Evaluation
Overview Medication Use and Asthma Control
After six weeks, users are provided a report that displays their maintenance inhaler usage, reliever inhaler usage, triggers, and level of asthma control. The report serves as a tool to identify trends and assess the impact of increased adherence to their maintenance inhaler on asthma control.
Phase 3 and 4: Prototype and Evaluation – Iteration 1
Feedback on the paper prototype (see Figure 3) was obtained from four people with mild asthma who had not participated in previous activities. This number was deemed sufficient to identify major issues on usability.43,44 Overall, the participants enjoyed the games and the six-week challenge. They appreciated the ability to set a personal goal. However, two participants mentioned finding it difficult to think of one. The commitment to the challenge itself was motivating enough for them to start using Ademgenoot. One participant suggested to incorporate more daily goals, such as cycling to work without feeling out of breath, rather than linking the goal to a specific event (eg, entering a sporting competition). Some participants preferred a weekly overview instead of updates every 3 days. While two participants considered the cloud character Brad to be childish, the other two felt affection for the character and found him not too childish.
 |
Figure 3 Paper prototype testing of the serious game Ademgenoot with a participant. |
The paper prototype was also reviewed by two general practice nurses who commented on the clinical feasibility and provided additional clinical feedback. They expressed their enthusiasm and found the game to be feasible in practice. However, they pointed out that inhaler use may not always result in better asthma control. Therefore, it is important for healthcare professions and the serious game to manage expectations to maintain user’s motivation to continue with the challenge. Instead of “promising” improvement it is important to reflect on the effect of behavior and asthma control together. The data collected with the app can be useful for health care professionals to evaluate inhaler usage behavior and asthma symptoms. In addition, three patient advocates provided feedback and expressed that the game, despite its playful appearance, could appeal to a broad range of patients, including children and those over the age of 45. They appreciated the breathlessness metaphor but emphasized the importance of providing a proper explanation for the piranha/fish aspect to avoid being perceived as punishment for reliever use. A review and discussion of the prototype with smart inhaler developers showed that the Ademgenoot concept was technically feasible as EDM can be used for several reliever and maintenance inhalers. However, as some patients receive Symbicort as Symbicort Maintenance and Reliever Therapy (SMART), the commercial feasibility (ie, complementing business strategies) may be less clearly defined.
Based on the first prototype evaluation, several improvements were made to the prototype: a weekly report instead of every 3 days, icons instead of text in the trigger self-monitoring form, a more human-like character, a different color pallet and design style to appear less child-like.
Phase 3 and 4: Prototype and Evaluation – Iteration 2
Four novel individuals with mild asthma participated in the WhatsApp prototyping and provided feedback during a semi-structured interview afterwards. Two participants indicated that they never until rarely used their maintenance inhaler, two participants occasionally used their maintenance inhaler. During the test period, one participant had multiple non-adherent days (ie, received the grey and gloomy screens), while the other three were fully adherent during the test period.
Usability and User-Friendliness
All participants understood the purpose of the game and found the game user-friendly and engaging. Participants appreciated the ease of registering trigger moments with the push-notification and the use of icons.
“If I get such a pop-up in my screen, I open it and tap it [the trigger icon]. Easy”.
Use of Metaphors and Narrative
All participants enjoyed the narrative of Brad and indicated that it motivated them to use the app more often. Two out of the four participants mentioned that the character motivated them to use their maintenance inhaler daily. They emphasized with the character and felt responsible for his “wellbeing”, becoming concerned if Brad would fall into the water or drown. This was reinforced when they were presented with the grey and gloomy screens (ie, the result of longer streaks of non-adherence) during the interview. The gloomy screens would motivate them to “get Brad back on track” and use their maintenance inhaler again. The other two participants did not require Brad as a motivator and stated that the challenge in itself was sufficient. The end report was helpful, but they predicted that it without the narrative the reports would only become monotonous over time, and they would lose interest in the game.
Usefulness
All participants indicated that the game helped them to be more aware of their asthma and their inhaler use. Importantly, they also mentioned that due to playful and non-medical appearance it did not make them feel like a patient.
Effect on Adherence Behavior
Three of the four participants indicated that Ademgenoot had a positive effect on their inhaler use during the test period. One participant, who had practically never used her maintenance inhaler before the challenge, reported improvement but still encountered difficulties in using her inhaler in the morning. Nonetheless, the gloomy screens served as motivating factor to exert her best effort:
The confrontation with the screen worked for me. I am very sensitive to color. I like that, the grey sky with the character. I start with sunshine and a beautiful sky. I’m doing a bad job and really have to do something.
Furthermore, participants expressed curiosity to continue using Ademgenoot for the following six-weeks and stated that this first week was a good first motivator. Various reasons to continue after the first week were mentioned. For instance, one participant was curious to see if taking their medication would have a positive effect on their symptoms:
I now have more symptoms, and I want to try to see if it [using maintenance inhaler daily] helps. I have not used it for such a long period now and I am learning to live with that. I am however short of breath. If I use it for a longer period, do I notice it? I am very curious about that.
Another participant mentioned that especially getting insight into her trigger moments would be a reason to continue with Ademgenoot.
Effect on Sense of Control Over the Asthma
Three participants expressed their expectation that, if Ademgenoot helped them to adhere to their maintenance inhaler and experience fewer symptoms, they would gain a greater sense of control over their asthma. They mentioned that continuing to track symptom triggers over time would help them get better insight into their triggers, recognize trigger events, and respond better to the triggers. In addition, the weekly overviews provided them with a sense of control. Participants trusted that the Ademgenoot could also help in controlling their asthma symptoms. However, two participants mentioned that relying on reliever use as a benchmark was not appropriate, as they rarely used their reliever medication despite feeling breathless and experiencing symptom flare-ups. They suggested the possibility of reporting on their level of asthma control regardless of reliever use, and the user could be rewarded with a fish in the water for doing so.
Evaluation of the concept with two patient advocates showed similar findings. The patient advocates found Ademgenoot easy to use and particularly valuable for individuals recently diagnosed with asthma and children, owing to its playful and fun approach. The combination of game elements and medication usage statistics was especially appreciated, as they expected that this would motivate people to be adherent. However, the patient advocates emphasized the importance of evaluating the inhaler behavior and asthma control with a healthcare professional during regular consultations as improvement may be less when medication is not properly adjusted. They considered the weekly reports to be helpful in reflecting together on medication use, asthma control and trigger events. Furthermore, they emphasized that the report on symptoms or control should reflect how one feels, as one might be medication adherent. Yet stich experience significant discomfort. Finally, both patient advocates suggested that Ademgenoot could also be beneficial for individuals with limited health literacy due to its prominent visual component.
Discussion
This paper demonstrates that a participatory user-centered design approach, combined with involvement from healthcare professionals, behavior change experts and persuasive game design experts, can result in an engaging, fun persuasive game that has the potential to motivate people with mild asthma to be adherent to their maintenance medication. We identified reasons for non-adherence and user needs, identified behavior change strategies (phase 1) and translated these into design and serious game requirements (phase 2). Several iterations were performed in which prototypes were evaluated with end-users, which led to the final persuasive game Ademgenoot (phase 3 and 4). Final evaluation showed that Ademgenoot has the potential to motivate people to adhere to their maintenance inhaler, promote long-term behavior change and is feasible in clinical practice.
A key finding during the define phase (phase 1) was that people’s non-adherence was often due to their perception of asthma as an episodic rather than chronic condition. This perception stems from the intermittent periods of symptom worsening and has been previously reported in qualitative research studies on reasons for non-adherence.45 Furthermore, non-adherence was influenced by the delayed perceived effect of maintenance medication, and a lack of comprehension regarding the distinction between maintenance and reliever inhaler. Consistent with our findings, multiple studies have reported perceived lack of efficacy and suboptimal knowledge of reliever and maintenance medications as drivers for non-adherence.45–48
Our study is unique as it targets multiple drivers for non-adherence, serving as a foundation for the design of Ademgenoot, a six-week challenge-based game designed to increase medication adherence. Ademgenoot aims to motivate users, employ goal-oriented strategies, and provide visual feedback on adherence behavior to raise awareness of the effect of adherence on asthma control. Ademgenoot incorporates various behavioral change strategies, such as personal goal setting and continuous direct feedback, along with persuasive game design elements, including a narrative and rewards. These combined elements are intended to increase patients’ motivation to adhere to their maintenance inhaler regimen.
Self-Determination Theory and Motivation
Increasing motivation to adhere to the maintenance inhaler and sustain the behavior is an important design strategy and in accordance with the Self-Determination Theory’s motivational underpinnings. According to this theory, there are three essential psychological needs – autonomy, relatedness, and competence – that are critical in forming motivation.49,50 In the context of asthma medication adherence, Self-Determination Theory suggests that patients are more likely to adhere to their medication regimen when they have a sense of autonomy in decision-making, feel competent in managing their asthma, and have a supportive relationship with their healthcare provider.
To promote autonomy, the six-week challenge of Ademgenoot, along with personal goal setting, contributes to patients’ sense of control. Competence is targeted through patient education on inhaler differences and fostering a sense of accomplishment through being adherent. Generally, patients experience better well-being and a greater sense of being in control over their asthma when they comply with their medication regimen. Relatedness, another vital aspect, can be achieved by integrating Ademgenoot into a blended-care setting, as suggested by the patient advocates, and by incorporating evaluation moments that focus on successes, thereby providing support for competence. Ademgenoot could facilitate conversations between patients and healthcare providers on adherence behavior, asthma control and decisions regarding therapy step-up.
Ademgenoot employs a combination of internal motivation (eg, personal goal setting), external motivation (such as motivating screens featuring Brad's progression), additional rewards (eg, the environment becoming richer) and intrinsic motivation by fostering engagement and enjoyment with the app itself (instead of using the inhaler itself having to be enjoyable). While short-term motivation is achieved through these fun and rewarding elements, long-term adherence requires the internalization of extrinsic motivation according to the Self-Determination Theory.51 The six-week challenge stimulates long-term engagement, allowing users to experience the benefits of using a maintenance inhaler and facilitating the process of internalizing extrinsic motivation, thereby increasing the chances of maintaining desired behavior in the long run.
Use of Narrative to Complement Behavior Change
The internalization of motivation is further facilitated by a narrative with the protagonist (Brad). Narratives have been successfully employed in serious games to complement behavior change theories and have shown to effectively support behavior change.52 Through immersion and the creation of affection for the narrative protagonist, people can mentally envision the health benefits related to the desired behavior and apply them in the real life. Furthermore, narratives can provide intriguing incentives for people who, having affection for the protagonist, feel obliged to finish the story and act on feeling of relatedness to the protagonist.53 As such, our protagonist Brad may foster internalization of external motivation to complete the game and adopt the behavior promoted in the game. Furthermore, a narrative can serve as an analogy of a real-world setting, enhancing a possibly boring and unstimulating context, and inspiring players by adding a narrative overlay.54
Ongoing Feedback on Behavior to Change Behavior
Ademgenoot utilizes EDM logging to offer direct and continuous feedback on inhaler usage. Feedback delivered through digital technologies, in order to facilitate behavior change’, has been promising to disrupt and change undesired habits or automatic behavior.55 Effective feedback depends on factors such as timing, delivery, modality, and content.55 Generally, continuous, real-time feedback delivered visually, enabling reflection-in-action (ie, in the moment), is the most effective. Ademgenoot offers continuous real-time visual feedback on inhaler usage and perceived asthma control, which is an improvement over the current reflection-on-action-based (ie, afterwards) practice during regular consultations. Future developments should explore how best to provide feedback on perceived asthma control, for example, by incorporating asthma questionnaires such as the Asthma Control Questionnaire (ACQ). Moreover, integration of automatic monitoring of environmental triggers (eg, pollen data) or peak flow data could provide a more comprehensive self-management tool.56
Strengths and Limitations
Our paper takes a distinctive approach by utilizing participatory user-centered design with service design techniques, behavior change principles, and persuasive game theory. This approach involves creating personas and multiple prototype iterations. By actively involving end-users, key design requirements were identified, increasing the chances of the intervention being accepted and adopted. Furthermore, by integrating theoretical frameworks, we promote understanding of mechanisms explaining motivational effects.57 As such, our study contributes to the knowledge base of persuasive game design as an effective method for changing health-related behavior. Another strength of our study is that besides the end-users, multiple other stakeholders were involved, including healthcare professionals, patient advocates and developers of smart inhalers. Their involvement ensured the accuracy, feasibility, and commercial and technical viability of Ademgenoot, making it a strong candidate for future implementation.
Our study is subject to several limitations. First, the relatively young participants could have limited the representativeness of our findings and the applicability of the Ademgenoot concept. However, by involving various stakeholders, we were able to achieve a wider perspective. Second, the small number of participants per design phase may have an influence on the study findings. Nonetheless, considering that we were able to involve participants throughout all phases of design and development, involving individuals that had not been involved during the previous phase and iterate on the previous feedback, we believe we were able to capture their needs in a valid way. Third, the formative evaluation of Ademgenoot may also present some limitations as not all components of the Ademgenoot game were tested. In addition, interference by the researcher may have led to changes in behavior (ie, improved medication adherence) due to the individual being observed. Finally, the limited duration of the testing period (ie, five days) calls for caution in drawing conclusions regarding long-term usage. Furthermore, the focus of our study on individuals with mild asthma may limit the generalizability of our findings to other patient groups.
Implications for Practice
Our paper demonstrated how data logging through EDMs can be utilized to provide a fun persuasive game to motivate, in the first place, adults with mild asthma to adhere to their medication regimen. Despite its potential, EMDs have not yet been implemented on a large scale in practice due to, amongst others, the need for evidence on long-term effectiveness and disengagement challenges with existing apps.56 Adoption of EDMs may be facilitated by offering different types of adherence support programs that cater to the needs, reasons for non-adherence, capabilities and preferences of individual patients, in a shared-decision making way.22,58 This requires EMD programs to be compatible with existing inhalers preferably integrated within existing electronic health records. Inquiring into reasons for poor-adherence should be standard practice to provide appropriate adherence support. We observed, in our study, behind the initial answer of “simply forgetting” were several underlying reasons for nonadherence, which should be addressed to support medication adherence effectively. Moreover, to reach its full potential, developments with EMD should focus on providing feedback on inhalation techniques to ensure proper usage. Ademgenoot offers an engaging way to incorporate inhalation techniques into self-management interventions for asthma. Finally, the concept of providing feedback on medication use via automatic logging, such as through electronic pill bottles, may also be applicable to other chronic diseases with medication non-adherence as a common challenge and where there is no direct tangible benefit of being adherent.
Implications for Research and Future Directions
The evaluation of Ademgenoot through WhatsApp prototype testing provided a low-cost and efficient way to assess people’s perceived impact on medication adherence and motivation. However, further research should focus on a real-world evaluation of Ademgenoot as a fully functional prototype to assess its long-term usage, impact on inhaler usage, and the expected shift from external to internal motivation, and include people with limited (health)literacy.59 Long-term evaluation should in addition provide more understanding of the degree of adherence to the app to acquire the desired behavior.60 Eventually, effectiveness on clinical outcomes (eg, asthma control) and medication adherence should be evaluated by including considering study design appropriate for summative evaluation of eHealth interventions (eg, step-wedged-design). While the involvement of healthcare professionals within this study ensured that Ademgenoot fits with care processes, feasibility in practice should also be assessed through pilot studies, taking into account the perspectives of patients, healthcare professionals in both first (ie, GP and practice nurses) and secondary care (ie, respiratory physicians), and decision makers. Finally, considering that smart inhalers are a relatively new field of research, further research is needed to determine which patients would benefit the most from these programs.39 Hence, we encourage that studies into the effectiveness of smart asthma inhalers include patient characteristics (eg, beliefs about medication and eHealth literacy) to gain insight into which smart inhaler programs benefit which patients based on these characteristics and reasons for non-adherence.57
Conclusion
Combining a participatory user-centered design with behavior change principles and persuasive game theory, we developed an innovative six-week challenge-based persuasive game that was evaluated as user-friendly and useful and has the potential to increase motivation to be medication adherent. Active involvement of all stakeholders throughout the design process ensured that our solution does not only meet the end-users needs but is also technically, commercially, and practically feasible and has the potential to improve medication adherence among people with mild asthma and other chronic conditions in which non-adherence is a common challenge.
Data Sharing Statement
Individual de-identified participant data supporting the findings are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank all participating patients, healthcare professionals, policymakers and developers for their participation in this study. We thank Sander Hermsen for his valuable input on behavior change strategies and the members of our patient advisory panel (J Donkers, B Frankemölle, J Groenendijk and S Sturkenboom) for their valuable input in the setup and execution of the study. AstraZeneca was provided the opportunity to review the manuscript; full editorial control remained with the authors.
Author Contributions
JdB and CCP conceptualized and designed the study. JdB was responsible for data collection, data analysis and interpretation. CCP, LG, VTV and EM made substantial contributions to the design and collection of data. CCP, EM, SJvH and NHC made substantial contributions to interpretation of data. CCP wrote the manuscript and EM provided feedback at each version of the manuscript. All authors reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage. All authors gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study is funded by AstraZeneca following a research collaboration agreement. The funding body was not involved in designing the study, data collection, study management nor the analysis and interpretation of data and writing the manuscript. AstraZeneca was provided the opportunity to review the manuscript and full editorial control remained with the authors.
Disclosure
All authors declare no competing interests in this work.
References
1. Price D, Fletcher M, van der Molen T. Asthma control and management in 8000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009. doi:10.1038/npjpcrm.2014.9
2. Van Boven J, Lavorini F, Richard Dekhuijzen N. Urging Europe to put non-adherence to inhaled respiratory medication higher on the policy agenda: a report from the First European Congress on Adherence to Therapy. Eur Respir J. 2017;49:1700076.
3. Engelkes M, Janssens HM, de Jongste JC, Sturkenboom MC, Verhamme KM. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J. 2015;45(2):396–407. doi:10.1183/09031936.00075614
4. Williams LK, Peterson EL, Wells K, et al. Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid nonadherence. J Allergy Clin Immunol. 2011;128(6):1185–1191.e2. doi:10.1016/j.jaci.2011.09.011
5. Ebmeier S, Thayabaran D, Braithwaite I, Bénamara C, Weatherall M, Beasley R. Trends in international asthma mortality: analysis of data from the WHO Mortality Database from 46 countries (1993–2012). Lancet. 2017;390(10098):935–945.
6. Dekhuijzen R, Lavorini F, Usmani S, Van Boven O. Addressing the impact and unmet needs of nonadherence in asthma and chronic obstructive pulmonary disease: where do we go from here? Eur Respir J. 2018;6.
7. Guilbert TW, Garris C, Jhingran P, et al. Asthma that is not well-controlled is associated with increased healthcare utilization and decreased quality of life. J Asthma. 2011;48(2):126–132. doi:10.3109/02770903.2010.535879
8. Lasmar L, Camargos P, Champs NS, et al. Adherence rate to inhaled corticosteroids and their impact on asthma control. Allergy. 2009;64(5):784–789. doi:10.1111/j.1398-9995.2008.01877.x
9. Looijmans-van den Akker I, Werkhoven A, Verheij T. Over-prescription of short-acting beta agonists in the treatment of asthma. Fam Pract. 2021;38(5):612–616. doi:10.1093/fampra/cmab013
10. Bright IN, Magnus E, Pål H, Fredrik W, Gunilla T, Christer J. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55(4):1901872. doi:10.1183/13993003.01872-2019
11. Molina J, Plaza V, Nuevo J, Gutiérrez M, Sicras-Mainar A, Valero A. Clinical consequences of the overuse of short-acting β2-adrenergic agonists (SABA) in thetreatment of asthma in Spain: the SABINA study. Open Respiratory Archives. 2023;5(2). doi:10.1016/j.opresp.2023.100232
12. Doz M, Chouaid C, Com-Ruelle L, et al. The association between asthma control, health care costs, and quality of life in France and Spain. BMC Pulm Med. 2013;13:15. doi:10.1186/1471-2466-13-15
13. Dima AL, Hernandez G, Cunillera O, Ferrer M, de Bruin M. Asthma inhaler adherence determinants in adults: systematic review of observational data. Eur Respir J. 2015;45(4):994–1018. doi:10.1183/09031936.00172114
14. Horne R. Compliance, adherence, and concordance: implications for asthma treatment. Chest. 2006;130(1 Suppl):65s–72s. doi:10.1378/chest.130.1_suppl.65S
15. Chapman S, Dale P, Svedsater H, et al. Modelling the effect of beliefs about asthma medication and treatment intrusiveness on adherence and preference for once-daily vs. twice-daily medication. NPJ Prim Care Respir Med. 2017;27(1):61. doi:10.1038/s41533-017-0061-7
16. Normansell R, Kew KM, Stovold E. Interventions to improve adherence to inhaled steroids for asthma. Cochrane Database Sys Rev. 2017;4:Cd012226. doi:10.1002/14651858.CD012226.pub2
17. Sulaiman I, Mac Hale E, Holmes M, et al. A protocol for a randomised clinical trial of the effect of providing feedback on inhaler technique and adherence from an electronic device in patients with poorly controlled severe asthma. BMJ open. 2016;6(1):e009350. doi:10.1136/bmjopen-2015-009350
18. Foster JM, Usherwood T, Smith L, et al. Inhaler reminders improve adherence with controller treatment in primary care patients with asthma. J Allergy Clin Immunol. 2014;134(6):1260–1268.e3. doi:10.1016/j.jaci.2014.05.041
19. Merchant RK, Inamdar R, Quade RC. Effectiveness of population health management using the propeller health asthma platform: a randomized clinical trial. J Allergy Clin Immunol Pract. 2016;4(3):455–463. doi:10.1016/j.jaip.2015.11.022
20. Jochmann A, Artusio L, Usemann J, et al. A 3-month period of electronic monitoring can provide important information to the healthcare team to assess adherence and improve asthma control. ERJ Open Res. 2021;7(3):56.
21. Chan A, De Simoni A, Wileman V, et al. Digital interventions to improve adherence to maintenance medication in asthma. Cochrane Database Sys Rev. 2022;6(6):Cd013030. doi:10.1002/14651858.CD013030.pub2
22. Blakey JD, Bender BG, Dima AL, Weinman J, Safioti G, Costello RW. Digital technologies and adherence in respiratory diseases: the road ahead. Eur Respir J. 2018;52(5).
23. Jácome C, Almeida R, Pereira AM, et al. Asthma app use and interest among patients with asthma: a multicenter study. J Investig Allergol Clin Immunol. 2020;30(2):137–140. doi:10.18176/jiaci.0456
24. Howard S. Electronic monitoring devices: necessary steps for their successful integration with current asthma care. University of Nottingham; 2017.
25. Howard S, Lang A, Patel M, Sharples S, Shaw D. Electronic monitoring of adherence to inhaled medication in asthma. Curr Respir Med Rev. 2014;10:50.
26. Morton RW, Elphick HE, Rigby AS, et al. STAAR: a randomised controlled trial of electronic adherence monitoring with reminder alarms and feedback to improve clinical outcomes for children with asthma. Thorax. 2017;72(4):347–354. doi:10.1136/thoraxjnl-2015-208171
27. Damaševičius R, Maskeliūnas R, Blažauskas T. Serious games and gamification in healthcare: a meta-review. Information. 2023;14(2). doi:10.3390/info14020105
28. Bruggers CS, Baranowski S, Beseris M, et al. A prototype exercise–empowerment mobile video game for children with cancer, and its usability assessment: developing digital empowerment interventions for pediatric diseases. Original Research. Front Pediatrics. 2018;6.
29. Yates T, Edwardson CL, Henson J, et al. Walking away from type 2 diabetes: a cluster randomized controlled trial. Diabetic Medicine. 2017;34(5):698–707. doi:10.1111/dme.13254
30. Visch V, Vegt N, Anderiesen H, van der Kooij K. Persuasive Game Design: a model and its definitions. 2013.
31. Siriaraya P, Visch V, Vermeeren A, Bas M. A cookbook method for persuasive game design. Int J Serious Games. 2018;5(1):57.
32. van de Hei SJ, Stoker N, Flokstra-de BMJ, et al. Anticipated barriers and facilitators for implementing smart inhalers in asthma medication adherence management. NPJ Prim Care Respir Med. 2023;33(1):22. doi:10.1038/s41533-023-00343-w
33. van Gemert-Pijnen JE, Nijland N, van Limburg M, et al. A holistic framework to improve the uptake and impact of eHealth technologies. J Med Internet Res. 2011;13(4):e111. doi:10.2196/jmir.1672
34. Wigal JK, Stout C, Brandon M, et al. The knowledge, attitude, and self-efficacy asthma questionnaire. Chest. 1993;104(4):1144–1148. doi:10.1378/chest.104.4.1144
35. Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14(1):1–24. doi:10.1080/08870449908407311
36. Ridder D, Theunissen N. De rol van ziektepercepties in therapietrouw bij hypertensie. / The role of illness perceptions in adherence to hypertension regimens. Gedrag Gezondheid. 2003;237–249.
37. van der Meer V, van Stel HF, Detmar SB, Otten W, Sterk PJ, Sont JK. Internet-based self-management offers an opportunity to achieve better asthma control in adolescents. Chest. 2007;132(1):112–119. doi:10.1378/chest.06-2787
38. Ryan D, Price D, Musgrave SD, et al. Clinical and cost effectiveness of mobile phone supported self monitoring of asthma: multicentre randomised controlled trial. 10.1136/bmj.e1756. BMJ. 2012;344.
39. Susanne J, Charlotte CP, NvdB L, et al. Effectiveness, usability and acceptability of a smart inhaler programme in patients with asthma: protocol of the multicentre, pragmatic, open-label, cluster randomised controlled ACCEPTANCE trial. BMJ Open Respir Res. 2022;9(1):e001400. doi:10.1136/bmjresp-2022-001400
40. Grudin J. 12 - Why personas work: the psychological evidence. In: Pruitt JS, Adlin T, editors. The Persona Lifecycle. Morgan Kaufmann; 2006:642–663.
41. Andre TS. Determining the effectiveness of the usability problem inspector: a theory-based model and tool for finding usability problems. Human Factors. 2003;45:455–482.
42. Martin RJ, Szefler SJ, King TS, et al. The Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trial. J Allergy Clin Immunol. 2007;119(1):73–80. doi:10.1016/j.jaci.2006.10.035
43. Nielsen J, Landauer TK A mathematical model of the finding of usability problems.
44. Kirakowski J, Bevan N RESPECT: Handbook of User-Centred Design, Deliverable D6.2. 2007:117. Available from: https://uxp.ie/INUSE_Handbook_of_UCD.pdf.
45. Amin S, Soliman M, McIvor A, Cave A, Cabrera C. Understanding patient perspectives on medication adherence in asthma: a targeted review of qualitative studies. Patient Prefer Adherence. 2020;14:541–551. doi:10.2147/ppa.S234651
46. Peláez S, Bacon SL, Aulls MW, Lacoste G, Lavoie KL. Similarities and differences between asthma health care professional and patient views regarding medication adherence. Can Respir J. 2014;21(4):221–226. doi:10.1155/2014/738654
47. Mowrer JL, Tapp H, Ludden T, et al. Patients’ and providers’ perceptions of asthma and asthma care: a qualitative study. J Asthma. 2015;52(9):949–956. doi:10.3109/02770903.2015.1010731
48. George M, Keddem S, Barg FK, Green S, Glanz K. Urban adults’ perceptions of factors influencing asthma control. J Asthma. 2015;52(1):98–104. doi:10.3109/02770903.2014.947651
49. Deci EL, Ryan RM. Motivation and Self-Determination in Human Behavior. NY: Plenum Publishing Co; 1985.
50. Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self-determination of behavior. Psychol Inq. 2000;11(4):227–268.
51. Patrick H, Williams GC. Self-determination theory: its application to health behavior and complementarity with motivational interviewing. Int J Behav Nutrition Phys Activity. 2012;9(1):18. doi:10.1186/1479-5868-9-18
52. Lu AS, Baranowski T, Thompson D, Buday R. Story immersion of videogames for youth health promotion: a review of literature. Games Health J. 2012;1(3):199–204. doi:10.1089/g4h.2011.0012
53. Sailer M, Hense JU, Mayr SK, Mandl H. How gamification motivates: an experimental study of the effects of specific game design elements on psychological need satisfaction. Comput Human Behav. 2017;69:371–380. doi:10.1016/j.chb.2016.12.033
54. Nicholson S. A RECIPE for meaningful gamification. In: Reiners T, Wood LC, editors. Gamification in Education and Business. Springer International Publishing; 2015:1–20.
55. Hermsen S, Frost J, Renes RJ, Kerkhof P. Using feedback through digital technology to disrupt and change habitual behavior: a critical review of current literature. Comput Human Behav. 2016;57:61–74. doi:10.1016/j.chb.2015.12.023
56. Himes BE, Leszinsky L, Walsh R, Hepner H, Wu AC. Mobile health and inhaler-based monitoring devices for asthma management. J Allergy Clin Immunol Pract. 2019;7(8):2535–2543. doi:10.1016/j.jaip.2019.08.034
57. Stewart S-JF, Moon Z, Horne R. Medication nonadherence: health impact, prevalence, correlates and interventions. Psychol Health. 2023;38(6):726–765. doi:10.1080/08870446.2022.2144923
58. van Boven JF, Trappenburg JC, van der Molen T, Chavannes NH. Towards tailored and targeted adherence assessment to optimise asthma management. NPJ Prim Care Respir Med. 2015;25:15046. doi:10.1038/npjpcrm.2015.46
59. Perez MF, Coutinho MT. An overview of health disparities in asthma. Yale J Biol Med. 2021;94(3):497–507.
60. Sieverink F, Kelders SM, van Gemert-Pijnen JE. Clarifying the concept of adherence to eHealth technology: systematic review on when usage becomes adherence. J Med Internet Res. 2017;19(12):e402. doi:10.2196/jmir.8578
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
