Back to Journals » Neuropsychiatric Disease and Treatment » Volume 14
The depressive-like behaviors of chronic unpredictable mild stress-treated mice ameliorated by Tibetan medicine Zuotai: involvement in the hypothalamic–pituitary–adrenal (HPA) axis pathway
Authors Zhao J, Niu C, Wang J, Yang H, Du Y, Wei L, Li C
Received 7 September 2017
Accepted for publication 13 October 2017
Published 3 January 2018 Volume 2018:14 Pages 129—141
DOI https://doi.org/10.2147/NDT.S151107
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Wai Kwong Tang
Jing Zhao,1,2 Cuiying Niu,1–3 Jianv Wang,1,3 Hongxia Yang,1,2 Yuzhi Du,1,2 Lixin Wei,1,2 Cen Li1,2
1Pharmacology and Safety Evaluation Key Laboratory of Tibetan Medicine in Qinghai Province, Northwest Institute of Plateau Biology, 2Key Laboratory of Tibetan Medicine Research, Chinese Academy of Sciences, Xining, Qinghai, 3University of Chinese Academy of Sciences, Beijing, People’s Republic of China
Background: Zuotai, a famous Tibetan medicinal mixture containing metacinnabar, is traditionally used for the purpose of tranquilizing minds and soothing nerves. However, it still lacks substantial experimental data for it to be approved for use.
Aim: This study was designed to assess the effects of Zuotai on depressive-like symptoms in a chronic unpredictable mild stress (CUMS) mouse model, and to explore its potential mechanism, particularly the hypothalamic–pituitary–adrenal (HPA) axis pathway.
Materials and methods: First, Kunming mice were exposed to the CUMS procedure and simultaneously administered Zuotai or imipramine (positive control) by gavage continuously for 6 weeks. Then, depressive-like behaviors of mice in each group were tested with the sucrose preference test, forced swimming test, tail suspension test, and open field test. Meanwhile, the three key neuroendocrine hormones (corticotropin releasing hormone, adrenocorticotropic hormone and corticosterone) in HPA axis pathway, and the level of the emotion-related monoamine neurotransmitters (5-hydroxytryptamine and norepinephrine) were measured using enzyme-linked immunosorbent assay. Furthermore, total mercury in the hypothalamus and hippocampus were determined using an automatic, direct mercury analyzer.
Results: Zuotai or imipramine significantly increased the body weight and the sucrose preference ratio in sucrose preference test, and dramatically improved motor activity in forced swimming test, tail suspension test, and open field test in CUMS mice. Zuotai or imipramine remarkably decreased levels of corticotropin-releasing hormone, adrenocorticotropic hormone, and corticosterone in the HPA axis, and increased levels of 5-hydroxytryptamine and norepinephrine in the serum in CUMS mice. However, a small amount of mercury was deposited in the hypothalamus and hippocampus in Zuotai-treated mice, which may pose a potential risk to the central nervous system.
Conclusion: Zuotai has a strong ability to ameliorate depressive-like behaviors in CUMS-treated mice through inhibition of the HPA axis and upregulation of monoamine neurotransmitters. These findings provide new insight into the pharmacological effect of Zuotai on depression.
Keywords: Tibetan medicine, Zuotai, HgS, depression, chronic unpredictable mild stress, CUMS, HPA axis
Introduction
Depression is a global mental disorder that has high incidence, high recurrence, and high self-mutilation and suicide rates.1,2 Clinically, depression is mainly characterized by persistent depressed mood, loss of interest and enjoyment, anxiety, a significant reduction in volitional activity, cognitive impairment, mental retardation, and other symptoms.3–5 According to the World Health Organization,6 an estimated 350 million people of all ages currently suffer from depression around the world, and more than 800,000 people commit suicide each year. Moreover, depression is expected to be one of the top two leading causes of disability-adjusted life years in 2020. Currently, the effective remission rate of antidepressants ranges from 60% to 80%, and the rate of favorable prognosis is approximately 30%.7–9 Therefore, there is an urgent need to develop new treatment strategies that can significantly improve outcomes. Traditional or natural medicine is one of the most important sources for new antidepressants.10
In many traditional medicine systems, mercuric sulfide (HgS) has been used to treat various diseases across thousands of years.11 Zuotai, a famous Tibetan medicinal mixture containing 54.5% metacinnabar (β-HgS), has been used for tranquilizing the mind, soothing the nerves, invigorating the spleen, strengthening the body, and reducing the toxicity while increasing the efficacy of other drugs.12–15 In the clinic, Zuotai is usually utilized as a core ingredient in many classic compound preparations such as Qishiwei Zhenzhu Wan, Renqing Changjue, Renqing Mangjue, and so on.16,17 Indeed, previous studies have provided a number of evidences about Zuotai to support its traditional medicinal use.18–22 Some studies have preliminarily confirmed the tranquilizing and sedative functions of Zuotai, which could shorten sleep induction time and increase sleep time in response to pentobarbital or chloral hydrate, reduce the convulsion rate due to strychnine, and prolong convulsion due to strychnine and nikethamide in mice.19,21 However, the scientific evidence on this is still scarce.
There is a subset of clinical depression that appears to be related to hypothalamic–pituitary–adrenal (HPA) axis dysfunction.23–25 It has been reported that chronic adverse stress could trigger oversecretion of three core factors (corticotropin-releasing hormone [CRH] by hypothalamus, adrenocorticotropic hormone [ACTH] by pituitary and glucocorticoid [GC] by adrenal cortex) in the HPA-axis pathway, resulting in hyperactivity of HPA-axis function, thereby establishing a vicious cycle;26,27 furthermore, the hyperactivity of the HPA axis could reduce the synthesis of central monoamine neurotransmitters such as 5-hydroxytryptamine (5-HT) and norepinephrine (NE), as well as damage hippocampal neurons, ultimately inducing depression.26,28 Meanwhile, effective antidepressive treatment could restore HPA-axis dysfunction to its normal state.29
The chronic unpredictable mild stress (CUMS) model is a classic, established model for researching depressive disorders and antidepressants.30 The chronic mild stress paradigm resembles the etiology of some human depression disorders.30,31 Moreover, this model can recapitulate some major symptoms of human depression.30,32 Therefore, in the present study, the CUMS model of Kunming albino mice was used to evaluate antidepressive effects of Zuotai by conducting the sucrose preference test (SPT), forced swimming test (FST), tail suspension test (TST), and open field test (OFT). Neuroendocrine factors in the HPA axis and monoamine neurotransmitters in serum were detected to reveal the potential antidepressant mechanism of Zuotai. Total mercury in the hypothalamus and hippocampus was measured to show the potential risk of heavy metal accumulation in the brain.
Materials and methods
Ethics approval
The Animal Experimentation Committee of Northwest Institute of Plateau Biology, Chinese Academy of Sciences approved the protocol for all animal experiments conducted in this study. Procedures involving mice and their care were conducted in conformity with international guidelines, the European Community guidelines (EEC Directive of 1986; 86/609/EEC), and the US guidelines (NIH Publication #85-23, revised in 1985).
Preparation of Zuotai
Zuotai (No 20110705) was prepared by the Company of Tibetan Medicine in the Tibetan Autonomous Region using the following ingredients: 5 kg metallic mercury, 5 kg sulfur, 0.333 kg Nengchi Eight Metal ash (gold, silver, bronze, copper, brass, iron, lead, and tin, all in equal weight proportions), 0.333 kg of Nengchi Eight Minerals ash (gold ore, silver ore, magnet ore, tufa, pyritum, orpiment, realgar, and red mica, all in equal weight proportions), and other auxiliary materials, according to a China patent(88107006.8).12 The producing mixture was designated as ZT20110705 and deposited at the Qinghai Key Laboratory of Tibetan Medicine Pharmacology and Safety Evaluation.
Metallic mercury and sulfur were initially treated by special procedures (eg, washing, refining, detoxifying), then pooled together, and ground into a black powder. The Nengchi Eight Metals and the Nengchi Eight Minerals were also burnt into ashes through complex processes. Finally, the quicksilver powder, Nengchi Eight Metals ashes, and Nengchi Eight Minerals ashes were completely ground into a black fine powder and designated as Zuotai (No 88107006.8, China Patent).12 Synchrotron radiation X-ray absorption fine structure (SR-XAFS), X-ray diffraction (XRD), X-ray fluorescence spectroscopy (XRF), scanning electron microscopy (SEM), and atomic force microscopy (AFM) were employed to verify the quality of the Zuotai. Details of their physicochemical characteristics are published elsewhere.17,33
Reagents and instruments
Imipramine (purity: 98%; 3B Scientific Co., Libertyville, IL, USA) and the enzyme-linked immunosorbent assay (ELISA) kits for Cort and ACTH were purchased from Nanjing Jiancheng Bioengineering Institute (Ningjing, Jiangsu, People’s Republic of China); ELISA kits of CRH, 5-HT, and NE were purchased from Cloud-Clone Corp. Sucrose (AR) was purchased from Sigma-Aldrich Corp. (St Louis, MO, USA). NaCl (PT), NaH2PO4 (AR), KH2PO4 (AR), KCl (AR), absolute ethyl alcohol (AR), and starch (FG) were purchased from Tianjin Fuyu Fine Chemical Co., Ltd. (Tianjing, People’s Republic of China).
The following instruments were used in the present study: Open field experiment system equipment (OFT-100, Chengdu Techman Software Co., Ltd., Sichuan, People’s Republic of China), multi-wavelength microplate reader (Enspire2300, Perkin Elmer Co., Ltd., USA), HD digital video recorder (GZ-MG174AC, Sony Co., Japan), automatic direct mercury analyzer (DMA-80, Milestone Co., Ltd., Italy), UP water purification system (Milli-Q Reference, Millipore Co., Ltd., USA), ultra-low temperature freezer (REVCO Ult-2586-4-v, Thermo-Fisher Co., Ltd., USA), electronic balance (0.0001 g, ME204, Mettler Toledo Co., Ltd., Switzerland), pH meter (PB10, Sartorious Co., Ltd., Germany), high-speed freezing centrifuge (Sigma 3K-15, Sigma Company, Germany), constant temperature magnetic stirrer (85-2A, Jintan Scientific Analytical Instrument Co., Ltd., People’s Republic of China), high-throughput tissue grinder (Scientz-48, NingBo Scientz Biotechnology Co., Ltd., People’s Republic of China), micropipettor (Thermo Electron Co., Ltd., People’s Republic of China), and manual counter (PC3860, Shenzhen Huibo Industry & Trade Co., Ltd., People’s Republic of China).
In addition to this, according to previous reports, the suspended tail test system equipment34 and the forced test system equipment35 were created and used by our laboratory in this study with minor revision.
Experimental animals
Male, random-bred, Kunming albino mice (age 4–5 weeks; mean weight [mean ± SD] 22–25 g) were used in carrying out the studies. These mice (SCXK [Gan] 2015-0002) were purchased from the Gansu University of Traditional Chinese Medicine, Lanzhou, People’s Republic of China.
Housing and husbandry
The Kunming mice were housed in the laboratory animal facility of Northwest Institute of Plateau Biology, Chinese Academy of Sciences, under specific pathogen-free conditions, and maintained with a 12-h light/dark cycle (lights on at 8:00 am) at 22°C–25°C. Mice growth-maintenance feed and sawdust pads were purchased from Beijing Ke’ao Xieli Feed Co., Ltd. (Beijing, People’s Republic of China). The animals had free access to standard diet and water. The Animal Experimentation Committee of Northwest Institute of Plateau Biology, Chinese Academy of Sciences approved the protocol for all animal experiments conducted in this study. The procedures involving mice and their care were conducted in conformity with international guidelines, the European Community guidelines (EEC Directive of 1986; 86/609/EEC), and the US guidelines (NIH Publication #85-23, revised in 1985). All sections of this report comply with ARRIVE guidelines for reporting animal research.
Pharmacological treatments and administration procedures
CUMS procedure
The CUMS procedure was carried out according to the method described by Willner and Katz,30,36 with minor modifications. Briefly, these stress methods include the following: 1) food deprivation for 24 h; 2) drinking water deprivation for 24 h with no drink bottle; 3) cage tilted at a 45° angle for 24 h; 4) placed together as a group for 2 h, then individually separated; 5) application of restraint stress for 2 h; 6) lighting at night for 12 h; 7) clamping of tail for 15 min; 8) forced swimming in cold water (4°C–8°C) for 5 min; 9) exposure to a foreign environment for 24 h; and 10) empty drink bottle. The above stress methods were randomly applied each day for 6 weeks consecutively; the same stress method was not continuously applied to ensure the mice would be unable to anticipate the next type of stress that would be applied.
Drug administration
Zuotai and a positive drug (imipramine, IMI) were suspended or dissolved in 2% starch water solution. Male mice were allowed to acclimate to their surroundings for 7 days prior to initiation of the experiments. Sixty mice were randomly allocated to one of the following six groups (10 mice for each group): control (2% starch solution), CUMS+Veh (CUMS +2% starch solution), CUMS+Zuotai I (CUMS +6.06972 mg·kg−1 Zuotai suspended in 2% starch solution), CUMS+Zuotai II (CUMS +60.6972 mg·kg−1 Zuotai suspended in 2% starch solution), CUMS+Zuotai III (CUMS +606.972 mg·kg−1 Zuotai suspended in 2% starch solution), CUMS+IMI (CUMS +15 mg·kg−1 imipramine dissolved in 2% starch solution). During the period of CUMS paradigm, the experimental animals were correspondingly treated (intragastrical administration [ig],) with Zuotai or a positive drug (imipramine) or 2% starch solution every day for 42 days (ie, 6 weeks). The weight of the mice in each group was measured every week, and the volume of drugs was adjusted according to their weight. Treatment details are shown in Figure 1.
The drug equivalent dose conversion ratio of mouse versus human is 9.1, and was adopted in this study. The clinical dose of Zuotai is 0.667 mg·kg−1; therefore, the equivalent dose in a mouse is 6.06972 mg·kg−1. The experiments were conducted with 1, 10, and 100 times the clinical equivalent doses of Zuotai in mice to explore the dose–effect relationship.
Sucrose preference test
The SPT is an important method that is mainly used to evaluate anhedonia, which is the core symptom of depression. The SPT was conducted in this study as described elsewhere.37 Thus, 72 h before the test, the mice were allowed to adapt to drinking sugar water. Briefly, two water bottles were simultaneously placed in each cage; one bottle was filled with 1% sugar solution, whereas the other contained pure water. The positions of the two bottles were switched every 12 h. Then, the SPT was conducted at 7:00 pm on days 0 and 42 of the present study. The mice were housed in individual cages, and were free to access either of the two bottles containing 1% sucrose solution or water. The volumes of consumed sucrose solution and water were recorded after 2 h. The sucrose preference ratio (SPR) was calculated according to the following equation: SPR = Sucrose intake (g)/[Sucrose intake (g) + Water intake (g)] ×100%.
Forced swimming test
The FST was used to evaluate the learned helplessness state of depressed mice on Day 44 of the experiment. The method was conducted as described by Porsolt et al.35 Briefly, 60 min prior to the FST, mice were moved to a quiet test room to reduce their tension. Then, the mice were individually placed in a glass cylindrical container (total volume: 1,000 mL, 21 cm in height and 12 cm in diameter) that was filled with water (23°C±2°C) to a depth of 10 cm. Each mouse was exposed to a test session for 6 min, and the whole experimental process was recorded by a high-definition digital video camera. The duration of immobility was accurately scored during the last 4 min of the total swimming time by using a timer, which was handled by a blinded observer. A mouse was judged to be in an immobile posture when it remained in a passively floating state in water without struggling or was swimming just to keep its head above the water.
Tail suspension test
The TST is another important method that is used to evaluate the learned helplessness state of the depressed mice, similarly as a forced swimming test. On Day 45 of the experiment, the TST was conducted using a method adapted from Steru et al,34 with a minor modification. Briefly, 60 min prior to the experiment, the mice were moved to a quiet test room to reduce tension. Acoustically and visually isolated mice were suspended by their tail from a ledge with adhesive tape (5 cm in width), 10 cm above the tabletop, for 6 min. The tape was placed approximately 3 cm from the tip of the tail. Immobility was defined as the absence of movement, with the time of immobility recorded during the last 4 min of the total suspended time by using a timer, which was handled by an observer blinded to the drug treatment.
Open field test
The OFT is often used in animal models of anxiety and depression. It is not usually thought of as a model of “depression-like” behavior so much as “anxiety-like” behavior. It is appropriate to use in this case, however, because many antidepressants also have anti-anxiety behaviors. The OFT was conducted according to the method described in a previous report38 on Day 43 of the present experiment. Sixty minutes before the OFT, the mice were moved to the test room and allowed to adapt to the experimental environment. The mice were placed in the center of an OFT box (500×500×415 mm), and the curtain of this experimental apparatus was rapidly pulled. The nine-rectangle-grid mode was selected, and the ratio of the central area versus the quadrilateral area was 0.5. Then, spontaneous locomotor activities (total immobile time, total movement distance, and percentage of time to cross the center) of each mouse were measured during a 5-min period by using this apparatus (OFT-100; Chengdu Techman Software Co., Ltd., People’s Republic of China).
Neuroendocrine hormones in the HPA axis
At 9:00–10:00 am on Day 46 of the experiment, blood was collected from the posterior orbital venous plexus of each mouse without anesthesia (then, the mouse was immediately sacrificed by cervical dislocation) and placed into a coagulation tube, kept for 1 h at room temperature, and the serum was separated by centrifugation at 4,000 rpm for 10 min at 4°C. Then, serum Cort and ACTH levels were determined according to the methods provided in the immunoassay kits. Thereafter, the hypothalamuses were isolated from the brain and placed on ice. Each hypothalamus was placed into 1.5 mL Eppendorf tubes, to which phosphate-buffered solution (pH 1.0–2.0) was added at 9 times the volume of the hypothalamus. Each hypothalamus was homogenized using a tissue homogenizer (frequency: 60 Hz, speed: 1,800 rpm) for 2 min and then centrifuged at 1,000 g for 20 min, and the supernatant was collected. Finally, the CRH level was determined according to the instructions provided in the ELISA kit.
Monoamine neurotransmitters
The levels of the monoamine neurotransmitters (5-HT and NE) in the serum collected from the mouse were determined according to the protocol provided in the ELISA kits (Cloud-Clone Corp.).
Total mercury in hypothalamus and hippocampus
The hypothalamus and hippocampus of the subjected mice were collected on Day 46 of the experiment, and used in the determination of mercury levels using an automatic direct mercury analyzer (DMA-80; Milestone Co., Ltd., Italy).
Statistical analysis
All data were expressed as the mean ± SEM of each group. The multiple comparisons of data were analyzed using One-way ANOVA Post Hoc Multiple Comparisons with the Duncan method (at least three conditions). The independent samples t-test was used for pairwise comparisons. For the 6 weeks data of mice body weight, two-way ANOVA with repeated measures was used (two factors: dose and time). Statistical analysis was done and graphs were constructed using SPSS 20.0 and Office 2013. A P-value of <0.05 was considered statically significant.
Results
Body weight
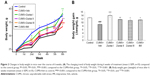
Depression is often accompanied by significant body weight loss or gain. The body weight of mice in each group were monitored weekly during all the studies (Figure 2A). No differences in baseline body weight were observed among each group (at Week 0: P>0.05). In the second week of the CUMS stress procedure, the body weight of mice in the CUMS+Veh group (P<0.001), CUMS+Zuotai I group (P<0.05), CUMS+Zuotai II group (P<0.001), CUMS+Zuotai III group (P<0.001), and CUMS+IMI group (P<0.001) significantly decreased, compared to the age-matched control group. After 6 weeks continuous treatment with Zuotai (6.06972 and 60.6972 mg·kg−1) or imipramine (15 mg·kg−1), weight loss in the CMUS mice was significantly improved compared to that in the CMUS+Veh group (P<0.001 for CUMS+Zuotai I group, P<0.05 for CUMS+Zuotai II and CUMS+IMI groups). The absolute change in mice body weight (Δweight = Final body weight − Initial body weight) is shown in Figure 2B. The body weight decreased significantly in mice after 6 weeks of CUMS exposure compared to that of the control group (P<0.001), which was significantly improved by Zuotai (6.06972, 60.6972, and 606.972 mg·kg−1) or imipramine (15 mg·kg−1).
Sucrose preference test
As a main index for evaluating anhedonia, the SPR of each group of mice was measured at Week 0 and 6 weeks post CUMS and drug procedures (Figure 3). The results showed that there was no significant difference between groups in baseline SPR scores (Week 0: P>0.05, Figure 3A). After 6 weeks of the CUMS procedure, the SPR of the CUMS+Veh group mice was significantly decreased (Week 6: P<0.001, Figure 3B) in comparison to that of the control group. However, compared with the CUMS+Veh group, the SPR of the mice treated with Zuotai or imipramine increased significantly (Week 6: P<0.01, P<0.001, respectively; Figure 3B).
Forced swimming test
After a 6-week CUMS procedure, the CUMS-treated mice exhibited a pronounced increase in the duration of immobility, compared to the non-CUMS mice (P<0.01). Zuotai significantly attenuated the duration of immobility in CUMS mice (P<0.05 for 6.0697 mg·kg−1 group and 60.697 mg·kg−1 group, and P<0.01 for 606.97 mg·kg−1 group) compared to the CUMS+Veh group. In addition, treatment with imipramine (15 mg·kg−1) resulted in a significant reversal in the CUMS-induced increase in the duration of immobility (P<0.05 vs CUMS+Veh group) in CUMS mice (Figure 4).
Tail suspension test
Results from the TST of each group are shown as in Figure 5. Six-week CUMS treatment significantly increased the immobility time of Kunming mice (P<0.01). Zuotai (6.06972, 60.6972, and 606.972 mg·kg−1) or imipramine (15 mg·kg−1) remarkably attenuated CUMS-induced immobility time compared to the mice in the CUMS+Veh group (P<0.001 or P<0.05, Figure 5).
Open field test
To eliminate nonspecific influence, we determined spontaneous locomotor activity (total movement distance, total immobility time, and the percentage of time to cross the center) in all mice using the OFT before the FST and the TST. The OFT results are shown in Figure 6A–D. After 6 weeks of the CUMS procedure, the total movement distance and the percentage of time to reach the central area significantly decreased, whereas total immobility time increased significantly compared to that of control mice. Furthermore, spontaneous locomotor activities of CUMS mice were ameliorated by Zuotai (6.06972, 60.6972, and 606.972 mg·kg−1) or imipramine (15 mg·kg−1).
Neuroendocrine hormones in the HPA axis
Levels of three core neuroendocrine hormones (CRH, ACTH, and Cort) in the HPA axis were determined and are shown in Figure 7A–C. The CRH level in the hypothalamus and levels of serum ACTH and Cort were significantly upregulated after 6 weeks of continuous CUMS treatment, and could be reversed by Zuotai (6.06972 and 60.6972 mg·kg−1, P<0.05 or P<0.01) or imipramine (15 mg·kg−1, P<0.05 or P<0.01) treatment. However, Zuotai failed to show any significant changes in levels of CRH, ACTH and Cort at a dose of 606.972 mg·kg−1, compared to that in the CUMS+Veh group.
Monoamine neurotransmitters (NE and 5-HT) in serum
Given that monoamine neurotransmitters (NE and 5-HT) play critical roles in emotional and cognitive functions and in the development of the nervous system, their serum levels were determined and are shown in Figure 8A and B. After 6 weeks of CUMS administration, serum 5-HT and NE levels were significantly downregulated. Meanwhile, the serum NE level was remarkably rescued by Zuotai (6.06972, 60.6972, and 606.972 mg·kg−1) or imipramine (15 mg·kg−1) after 6 weeks of oral administration (Figure 8A). However, the level of serum 5-HT was only reversed by Zuotai at the doses 6.06972 and 60.6972 mg·kg−1, but not at a dose of 606.972 mg·kg−1 (Figure 8B).
Mercury levels in the hypothalamus and hippocampus
The total mercury concentrations in the hypothalamus and hippocampus of each group mice were detected and are shown in Figure 9. No significant change in mercury level was observed in the hypothalamus and hippocampus in CUMS+Veh and CUMS+IMI mice compared to control mice. After 6 weeks of continuous Zuotai treatment (6.06972, 60.6972, and 606.972 mg·kg−1), the mercury level in the hypothalamus of mice was significantly increased in a dose-dependent manner. Moreover, the mercury level in the hippocampus of mice treated with Zuotai (60.6972 and 606.972 mg·kg−1) was significantly higher than that of the control group, and also changed in a dose-dependent manner.
Discussion
The present study has elucidated the antidepressant activity of Zuotai and its potential mechanism. Zuotai significantly ameliorated depressive behaviors in CUMS-treated mice, characterized by increased SPR in the SPT, increased total movement distance and percentage of time to cross the center in the OFT, and improved body weight as well as motor activities in FST, TST, and OFT. Moreover, Zuotai suppressed the increase in the levels of three important neuroendocrine hormones (CRH, ACTH, and Cort) on HPA axis pathway, and rescued the decrease in the levels of the monoamine neurotransmitters (NE and 5-HT) in CUMS-treated mice. Further, we found that Zuotai could dose-dependently increase the level of mercury in the hypothalamus and hippocampus of mice.
CUMS mouse model was successfully established
The CUMS model is a classic model for testing the efficacy of antidepressants. In the procedure for establishing the CUMS model, rodents are chronically exposed to multiple stress factors that are unpredictable and pose no threat to their survival; this procedure is consistent with the etiology of human depressive disorders.30,31 The CUMS model not only simulates anhedonia – a core symptom of some human depressions – but also recapitulates other major depressive symptoms such as anxiety-like and depressive-like moodiness, decline in sociability and spontaneous locomotor functions, abandonment of violation and attack ability, sex apathy, and weight loss.30,32 In the present study, our CUMS mice displayed conspicuous depressive-like symptoms such as anhedonia, decline in spontaneous locomotor functions, enhanced learned helplessness state, and weight loss, which is consistent with findings in previous reports. This indicates that we successfully established a CUMS mouse model.
Zuotai ameliorates depressive-like behaviors of CUMS mice
Anhedonia, which is a core symptom of depressive disorders, refers to broadly retarded reactions toward rewards or the inability to enjoy happiness.31,39 Previous reports had shown that anhedonia in the form of reduction in SPR in mice is significantly reversed by antidepressants such as imipramine and fluoxetine.40,41 In the current study, the administration of Zuotai remarkably improved the SPR in the CUMS mice, thereby indicating that Zuotai may have potential effects on amelioration of the core symptoms of depression. Moreover, our results showed that Zuotai could reverse weight loss in CUMS mice. Major depressive disorder (MDD) is often associated with appetite changes (loss of appetite or increased appetite) and subsequent weight changes,4,5,42 and treatment with antidepressants is often associated with weight gain in patients with for depression.4,42 Learned helplessness is an important indicator for assessing depressive-like behavior. TST and FST are two classical methods for evaluating the learned helplessness state of rodents, and in screening antidepressants.34,35 These two methods have nearly the same design principle – that is, preventing animals from escaping a harsh environment, thereby leading to immobility behavior.43,44 When mice are suspended by their tail or forced to swim in water, they are subjected to short-term inescapable stress and they adopt an immobile posture.43 However, when antidepressants are administered prior to the test, mice will actively pursue escape-directed behaviors over longer periods of time.43,44 These two methods are sensitive for the screening of antidepressant drugs – for example, imipramine, fluoxetine, and other antidepressants significantly reduced the immobile posture during TST and FST in depressed mice.43,45,46 In the present study, the application of Zuotai (6.0697, 60.697, and 606.97 mg·kg−1) resulted in a significant reduction in the immobile posture during both TST and FST in CUMS mice, and this was similar to the change with the positive drug imipramine.
The OFT is a generally accepted and straightforward test for investigating depressive-like, anxiety-related, and exploratory behavior of rodents.47 Chronic stress can lead to depressive-like and anxiety-like behaviors in mice that are expressed in terms of a reduction in spontaneous activity such as an increase in rest time, a decrease in movement distance and time of crossing into the center, and a reduction in standing times.48 Our results showed that the CUMS procedure significantly reduced the total distance of movement and the time of crossing into the center, and also reduced the resting time of normal mice, whereas these behaviors could prominently be reversed by the administration of Zuotai or imipramine.
Zuotai regulates the HPA-axis pathway and monoamine neurotransmitters in CUMS mice
The occurrence of depression is closely related to the lack/decrease of monoamine neurotransmitters, which is involved in HPA-axis hyperactivity.26,28,49 Under long-term chronic stress and social stress, HPA-axis hyperfunction can be induced, which in turn further leads to a reduction in monoamine neurotransmitters in the central and peripheral nervous systems, thereby resulting in depressive-like symptoms.26 Antidepressant treatment relieves HPA-axis hyperactivity and improves levels of monoamine neurotransmitter.50,51 In the present study, we found that Zuotai could significantly reverse HPA-axis hyperactivity at doses of 6.0697 and 60.697 mg·kg−1 by reducing serum CRH, ACTH, and Cort levels. Furthermore, we observed that Zuotai could remarkably upregulate the level of monoamine neurotransmitters (5-HT and NE). Therefore, the antidepressant effect of Zuotai on CUMS mice may involve inhibition of the HPA axis and cause upregulation of monoamine neurotransmitters levels. However, Zuotai failed to attenuate the hyperactivity of the HPA axis at a higher dose (606.97 mg·kg−1), which implies that the inhibitory effect of Zuotai on HPA-axis hyperactivity involves a certain threshold, and higher doses may be ineffective.
Potential risk of mercury from Zuotai on the nervous system
As a classical processing mixture containing mercury in Tibetan medicine, Zuotai continues to be used in compound preparations for the treatment of various diseases. Mercury is an important toxic heavy metal element that is ubiquitous in the environment, and its toxicity depends on its chemical forms. The mercury chemical species in Zuotai is insoluble cubic crystal mercuric sulfide (β-HgS, Ksp is 1.6×10−52),52 and its toxicity is extremely low.53 Our previous study showed that chronic administration of Zuotai to mice resulted in the accumulation of mercury in the kidneys and liver, whereas only trace amounts were detected in the brain.53 However, the extent of mercury accumulation in different encephalic regions treated by Zuotai remains unclear. In the present study, some level of mercury deposition was observed in the hypothalamus and hippocampus in CUMS mice treated with Zuotai in a dose-dependent manner. Therefore, we suspect that it may pose a potential risk for the use of Zuotai in the clinic. Meanwhile, Zuotai could ameliorate depressive-like behaviors in CUMS mice. Here, we made a bold speculation that the protective effect of Zuotai on depression may be associated with the deposition of mercury from Zuotai in brain.
Probable relationship of mercury deposition and HPA-axis function
Mercury is a well-known endocrine disruptor in the environment. However, mercury levels in vivo and its function on the HPA axis remain elusive, to date. Previous studies have shown that the concentration of Cort, which is one of the key signaling molecules in the HPA-axis pathway, is negatively correlated with mercury levels in the body.54–56 However, some other studies have reported that ACTH and CRH – important signaling molecules in the HPA axis – were positively correlated with mercury levels in vivo.57,58 It has also been reported that the level of Cort in the HPA axis is not associated with mercury levels in vivo.59,60 Therefore, it is difficult to draw a general picture of mercury–HPA-axis relationships at this point.
Conclusion
In summary, the present study has found that Zuotai can ameliorate depression-like behaviors in CUMS mice, and revealed the underlying mechanism that involves the inhibition of the HPA axis neuroendocrine pathway, which can further upregulate the level of monoamine neurotransmitters and finally play a role to ameliorate depressive mood. However, Zuotai can cause significant mercury deposition in the hypothalamus and hippocampus at high doses and with long-term administration, which may pose a potential risk to the central nervous system. Therefore, mechanisms to balance the risk and the efficacy of Zuotai need to be further studied in future research. Taken together, these findings provide new insights into the interventional effect of Zuotai on depression and its potential risk necessitating mercury monitoring.
Abbreviations
HPA, hypothalamic–pituitary–adrenal; CUMS, chronic unpredictable mild stress; IMI, imipramine; SPT, sucrose preference test; SPR, sucrose preference ratio; FST, forced swimming test; TST, tail suspension test; OFT, open field test; CRH, corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone; Cort, corticosterone; 5-HT, 5-hydroxytryptamine or serotonin; NE, norepinephrine; ELISA, enzyme-linked immunosorbent assay.
Acknowledgments
The Science Foundation for Young Scholars of Qinghai Province (2016-ZJ-919Q), “The Dawn of West China” 2014 Talent Training Program of Chinese Academy of Sciences (Y529021211), the National Natural Science Foundation of China (81374063), and Development Program of Key Laboratory in Qinghai Province (2017-ZJ-Y08) supported this study. The authors declare that the sponsors did not play any role in the study design. The authors thank Dr Sheng Song (PhD) and Professor Jie Liu (PhD), National Institute of Environmental Health Sciences (NIEHS), NIH for the English language copyediting of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Luijendijk HJ, van den Berg JF, Dekker MJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394–1401. | ||
Hirokawa S, Kawakami N, Matsumoto T, et al. Mental disorders and suicide in Japan: a nation-wide psychological autopsy case–control study. J Affect Disord. 2012;140(2):168–175. | ||
Angst J, Gamma A, Rössler W, Ajdacic V, Klein DN. Long-term depression versus episodic major depression: results from the prospective Zurich study of a community sample. J Affect Disord. 2009;115(1–2):112–121. | ||
Bentley SM, Pagalilauan GL, Simpson SA. Major depression. Med Clin North Am. 2014;98(5):981–1005. | ||
Otte C, Gold SM, Penninx BW, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. | ||
WHO. Depression. WHO. 2017. Available from: http://www.who.int/mediacentre/factsheets/fs369/en/. Accessed April 19, 2017. | ||
Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:54–63. | ||
Santarsieri D, Schwartz TL. Antidepressant efficacy and side-effect burden: a quick guide for clinicians. Drugs Context. 2015;4:212290. | ||
Wilson E, Lader M. A review of the management of antidepressant discontinuation symptoms. Ther Adv Psychopharmacol. 2015;5(6):357–368. | ||
Butler L, Pilkington K. Chinese herbal medicine and depression: the research evidence. Evid Based Complement Alternat Med. 2013;2013:739716. | ||
Chen CJ, Wu SK, Wang YB, Hou JF, Ma L, Sun XY. [Recent researches of synthetic mercury sulfide in traditional medicine system]. China J Chin Mater Med. 2012;37(19):2968–2970. Chinese. | ||
Gamaqupei, Tudenggesang, Geqiong. The processing method of Mercury medicinal powder (Zuotai). China Patent 88107006.8. 1988. Available from: http://www.soopat.com/Patent/88107006. Accessed November 14, 2017. | ||
Lanke. [Brief review of Tibetan medicine Zuotai]. Chin J Ethnomed Ethnopharm. 1999;(5 Suppl):86. Available from: http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFD9899&filename=ZMYZ1999S1083&uid=WEEvREcwSlJHSldRa1FhcTdWZDhML1N0TTlaTDVQYStZMUFMVHR6cS9GUT0=$9A4hF_YAuvQ5obgVAqNKPCYcEjKensW4ggI8Fm4gTkoUKaID8j8gFw!!&v=MTI4NzVUcldNMUZyQ1VSTDJlWnVkckZ5em1XN3JKUHlEU2RMS3hGOWl2cm85Tlo0UjhlWDFMdXhZUzdEaDFUM3E=. Accessed November 20, 2017. Chinese. | ||
Luosang DJ, Gongga LB. The quality control method of a processing product in Tibetan medicine and its new application. China Patent 200810093949.5. 2008. Available from: http://www.soopat.com/Patent/200810093949. Accessed November 20, 2017. | ||
Suolang. The processing technology of Zuotai. Chin J Ethnomed Ethnopharm. 2007;(5):40. | ||
Wang Z. The reinterpreting of Tibetan medicine essence “Zuota”. Health World (Acad Ed). 2010;4(9):84–85. | ||
Li C, Yang H, Du Y, et al. Chemical species, micromorphology, and XRD fingerprint analysis of Tibetan medicine Zuotai containing mercury. Bioinorg Chem Appl. 2016;2016:7010519. | ||
Chen Z, Xianglan P, Li W, Wu K, Lan G, Cui J. The influence on Drosophila life of Tibetan medicine Zuota. Lishizhen Med Mate Med Res. 2011;22(2):422–423. | ||
Jiang EN, Zhang CG, Wang JH, et al. Study on the pharmacodynamics of Tibetan medicine Zuotai. Lishizhen Med Mater Medic Res. 2009;20(8):3–4. | ||
Zhu T, Shen B, Wang W, Chiren B, Yao G. The proliferation of 239 cell promoted by Tibetan Medicine Zuotai through caspase-3. J Med Pharm Chin Minorities. 2013;5:47–49. | ||
Zeng Y, He S, Liu Y, Wang Z, Zhang Y. Study on the pharmacological effects in central nervous system of Tibetan medicine Zuota. J Sichuan Tradi Chin Med. 2005;23(11):33–34. | ||
Li H, Li WK, Lu YF, Wei LX, Liu J. The Tibetan medicine Zuotai influences clock gene expression in the liver of mice. PeerJ. 2016;4:e1632. | ||
Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry. 2013;18(6):692–699. | ||
Kaestner F, Hettich M, Peters M, et al. Different activation patterns of proinflammatory cytokines in melancholic and non-melancholic major depression are associated with HPA axis activity. J Affect Disord. 2005;87(2–3):305–311. | ||
Harkness KL, Monroe SM. Severe melancholic depression is more vulnerable than non-melancholic depression to minor precipitating life events. J Affect Disord. 2006;91(2–3):257–263. | ||
Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31(9):464–468. | ||
Cowen PJ. Not fade away: the HPA axis and depression. Psychol Med. 2010;40(1):1–4. | ||
Oldehinkel AJ, Bouma EM. Sensitivity to the depressogenic effect of stress and HPA-axis reactivity in adolescence: a review of gender differences. Neurosci Biobehav Rev. 2011;35(8):1757–1770. | ||
De Bellis MD, Gold PW, Geracioti TD Jr, Listwak SJ, Kling MA. Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am J Psychiatry. 1993;150(4):656–657. | ||
Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl). 1997;134(4):319–329. | ||
Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16(4):525–534. | ||
Mao QQ, Ip SP, Ko KM, Tsai SH, Che CT. Peony glycosides produce antidepressant-like action in mice exposed to chronic unpredictable mild stress: effects on hypothalamic-pituitary-adrenal function and brain-derived neurotrophic factor. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(7):1211–1216. | ||
Li C, Zhan-D, Leng-ben-cai-rang, Sang-lao, et al. [Chemical components, mercury coordination structure and micro-morphology of Tibetan Medicine Zuotai]. Guang Pu Xue Yu Guang Pu Fen Xi. 2015;35(4):1072–1078. Available from: http://apps.webofknowledge.com/full_record.do?product=UA&search_mode=GeneralSearch&qid=1&SID=V1eUbVOpnCN9irg8sDn&page=1&doc=2. Accessed November 20, 2017. Japanese. | ||
Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl). 1985;85(3):367–370. | ||
Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229(2):327–336. | ||
Katz RJ. Animal model of depression: pharmacological sensitivity of a hedonic deficit. Pharmacol Biochem Behav. 1982;16(6):965–968. | ||
Dang H, Chen Y, Liu X, et al. Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8): 1417–1424. | ||
Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25(3):235–260. | ||
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. | ||
Duda W, Curzytek K, Kubera M, et al. The effect of chronic mild stress and imipramine on the markers of oxidative stress and antioxidant system in rat liver. Neurotox Res. 2016;30(2):173–184. | ||
Rygula R, Abumaria N, Domenici E, Hiemke C, Fuchs E. Effects of fluoxetine on behavioral deficits evoked by chronic social stress in rats. Behav Brain Res. 2006;174(1):188–192. | ||
Fernstrom MH. Depression, antidepressants, and body weight change. Ann N Y Acad Sci. 1989;575:31–39; discussion 39–40. | ||
Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29(4–5):571–625. | ||
Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl). 2005;177(3):245–255. | ||
Berrocoso E, Ikeda K, Sora I, Uhl GR, Sánchez-Blázquez P, Mico JA. Active behaviours produced by antidepressants and opioids in the mouse tail suspension test. Int J Neuropsychopharmacol. 2013;16(1):151–162. | ||
Wang Z, Gu J, Wang X, et al. Antidepressant-like activity of resveratrol treatment in the forced swim test and tail suspension test in mice: the HPA axis, BDNF expression and phosphorylation of ERK. Pharmacol Biochem Behav. 2013;112:104–110. | ||
Wang W, Liu Z, Wu W, Wan Y, Yan H. The use of open field test in the behavior analysis of mice. Chin J Cell Biol. 2011;33(11):1191–1196. | ||
Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175(1):43–50. | ||
de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–475. | ||
Gupta D, Radhakrishnan M, Kurhe Y. 5HT3 receptor antagonist (ondansetron) reverses depressive behavior evoked by chronic unpredictable stress in mice: modulation of hypothalamic–pituitary–adrenocortical and brain serotonergic system. Pharmacol Biochem Behav. 2014;124:129–136. | ||
Espallergues J, Temsamani J, Laruelle C, Urani A, Maurice T. The antidepressant-like effect of the 3β-hydroxysteroid dehydrogenase inhibitor trilostane involves a regulation of β-type estrogen receptors. Psychopharmacology (Berl). 2011;214(2):455–463. | ||
Cao SF, Liang YQ. Thermodynamics analysis of the mercuric sulfide dissolved in aqua regia. J Southwest China Inst Technol. 1999;14(1):71–73. | ||
Li C, Wang DP, Duo J, et al. [Study on safety of Tibetan medicine zuotai and preliminary study on clinical safety of its compound dangzuo]. China J Chin Mater Med. 2014;39(13):2573–2582. Chinese. | ||
Herring G, Ackerman JT, Herzog MP. Mercury exposure may suppress baseline corticosterone levels in juvenile birds. Environ Sci Technol. 2012;46(11):6339–6346. | ||
Franceschini MD, Lane OP, Evers DC, Reed JM, Hoskins B, Romero LM. The corticosterone stress response and mercury contamination in free-living tree swallows, Tachycineta bicolor. Ecotoxicology. 2009;18(5):514–521. | ||
Wada H, Cristol DA, McNabb FM, Hopkins WA. Suppressed adrenocortical responses and thyroid hormone levels in birds near a mercury-contaminated river. Environ Sci Technol. 2009;43(15):6031–6038. | ||
Schaefer AM, Stavros HC, Bossart GD, Fair PA, Goldstein JD, Reif JS. Associations between mercury and hepatic, renal, endocrine, and hematological parameters in Atlantic bottlenose dolphins (Tursiops truncatus) along the eastern coast of Florida and South Carolina. Arch Environ Contam Toxicol. 2011;61(4):688–695. | ||
Li ZH, Chen L, Wu YH, Li P, Li YF, Ni ZH. Alteration of thyroid hormone levels and related gene expression in Chinese rare minnow larvae exposed to mercury chloride. Environ Toxicol Pharmacol. 2014;38(1):325–331. | ||
Wayland M, Gilchrist HG, Marchant T, Keating J, Smits JE. Immune function, stress response, and body condition in arctic-breeding common eiders in relation to cadmium, mercury, and selenium concentrations. Environ Res. 2002;90(1):47–60. | ||
Tartu S, Angelier F, Wingfield JC, et al. Corticosterone, prolactin and egg neglect behavior in relation to mercury and legacy POPs in a long-lived Antarctic bird. Sci Total Environ. 2015;505:180–188. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.