Back to Journals » International Journal of Nanomedicine » Volume 18
The Current Progress of Tetrahedral DNA Nanostructure for Antibacterial Application and Bone Tissue Regeneration
Authors Hong S , Jiang W, Ding Q, Lin K, Zhao C, Wang X
Received 9 March 2023
Accepted for publication 19 June 2023
Published 10 July 2023 Volume 2023:18 Pages 3761—3780
DOI https://doi.org/10.2147/IJN.S403882
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Yan Shen
Shebin Hong,1– 3,* Weidong Jiang,1– 3,* Qinfeng Ding,1– 3,* Kaili Lin,1– 3 Cancan Zhao,1– 3 Xudong Wang1– 3
1Department of Oral & Cranio-Maxillofacial Surgery, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2College of Stomatology, Shanghai Jiao Tong University, Shanghai, People’s Republic of China; 3National Center for Stomatology, National Clinical Research Center for Oral Diseases, Shanghai Key Laboratory of Stomatology, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xudong Wang; Cancan Zhao, Department of Oral and Cranio-Maxillofacial Surgery, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University, No. 639, Zhizaoju Road, Shanghai, 200011, People’s Republic of China, Tel +86-21-23271699, Fax +86-21-63136856, Email [email protected]; [email protected]
Abstract: Recently, programmable assembly technologies have enabled the application of DNA in the creation of new nanomaterials with unprecedented functionality. One of the most common DNA nanostructures is the tetrahedral DNA nanostructure (TDN), which has attracted great interest worldwide due to its high stability, simple assembly procedure, high predictability, perfect programmability, and excellent biocompatibility. The unique spatial structure of TDN allows it to penetrate cell membranes in abundance and regulate cellular biological properties as a natural genetic material. Previous studies have demonstrated that TDNs can regulate various cellular biological properties, including promoting cells proliferation, migration and differentiation, inhibiting cells apoptosis, as well as possessing anti-inflammation and immunomodulatory capabilities. Furthermore, functional molecules can be easily modified at the vertices of DNA tetrahedron, DNA double helix structure, DNA tetrahedral arms or DNA tetrahedral cage structure, enabling TDN to be used as a nanocarrier for a variety of biological applications, including targeted therapies, molecular diagnosis, biosensing, antibacterial treatment, antitumor strategies, and tissue regeneration. In this review, we mainly focus on the current progress of TDN-based nanomaterials for antimicrobial applications, bone and cartilage tissue repair and regeneration. The synthesis and characterization of TDN, as well as the biological merits are introduced. In addition, the challenges and prospects of TDN-based nanomaterials are also discussed.
Keywords: tetrahedral DNA nanostructure, carriers, antibacterial treatment, bone regeneration, cartilage regeneration
Graphical Abstract:
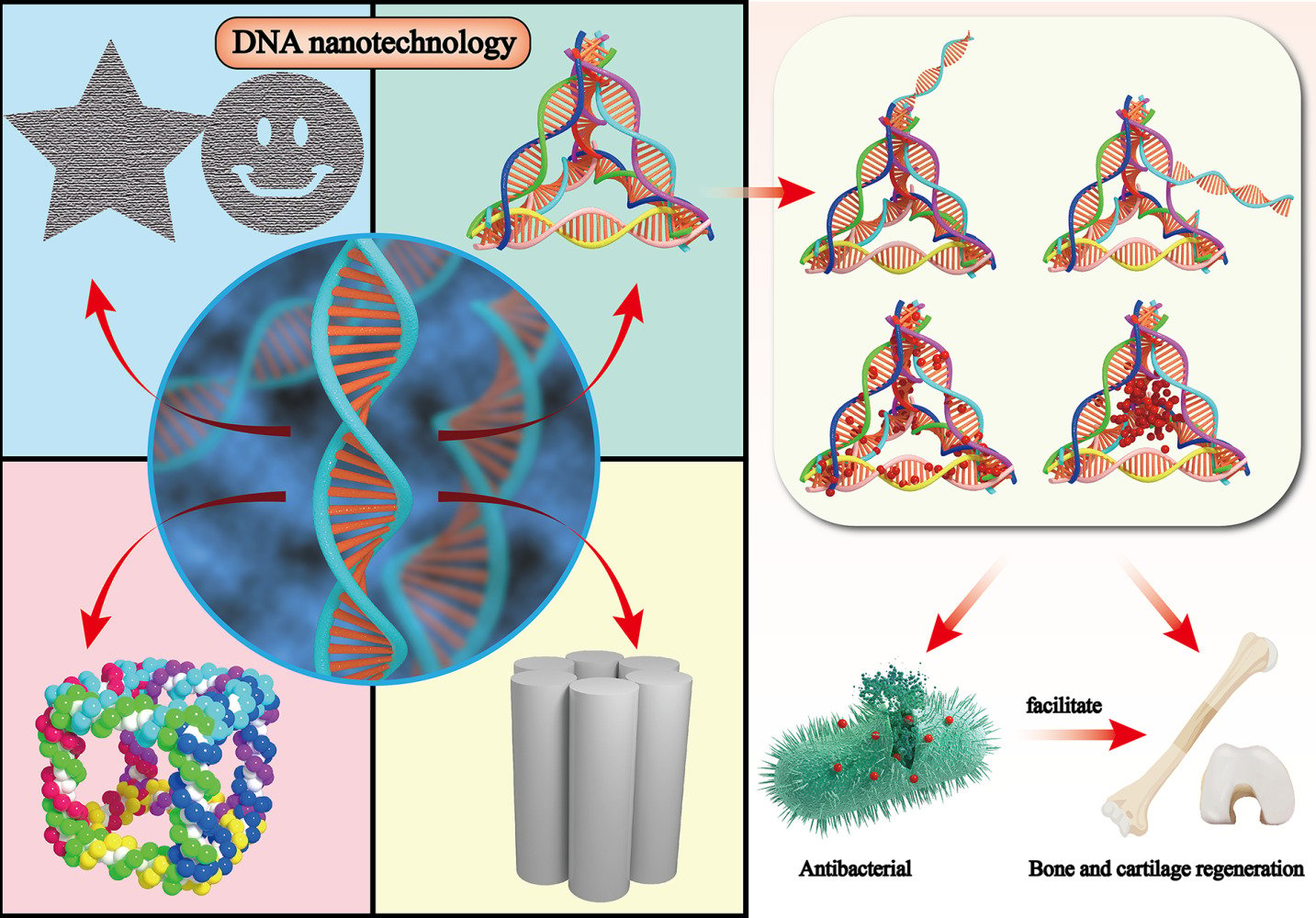
Introduction
DNA nanotechnology, which applies the specific self-assembly property of base pairs, holds great promise for the development of novel materials and medical treatments.1 However, precise manipulation and accurate control of the sizes and shapes of DNA at a nanometer scale are still very challenging. The immobile DNA junction as the building blocks was firstly presented by Nadrian C Seeman in the early 1980s.2 Breakthroughs in these fields prompt the prosperity of three-dimensional molecular nanofabrication with highly spatial programmability, followed by DNA building blocks assembling, tiled-based assembly, and the evolution of DNA origami technique.3–6 Until now, a large range of DNA nanostructures were fabricated in three-dimensional modalities including cube,7 tetrahedron,8 octahedron,9 icosahedron,10 tube,11 and DNA origami structure.12,13 Of these, the tetrahedral DNA nanostructure (TDN) stood out because of a series of unique performance, including admirable cellular membrane and tissue permeability, high yield, and multiple cell biological functions.14
TDN is synthesized by four appropriately designed single DNA strands (ssDNA) based on the complementary rules of base pairing.8 It was more easily synthesized with yields as high as 95% in one-step process within a few seconds in contrast to other challenging assembling of DNA cages like DNA cubes7 and octahedra,15 which were reported to assemble in complex process with yield as low as 1%. Moreover, the most prominent characteristic of TDN compared with other DNA nanostructures is that TDN can enter cells autonomously in large quantities without the assistance of any functional molecules. Previous studies reported that the cell entry of TDN was size-independent in the range of several tens of nanometers and dependent on the morphology of DNA nanostructures.16 The pyramid structure of TDNs can minimize electrostatic repulsion and induce uneven charge redistribution in the membrane, which play important roles in the internalization of TDNs.16 In addition to these unique advantages compared with other 2D or 3D DNA nanostructures, TDNs also offer a variety of other benefits. First, as a natural genetic material, TDNs exhibit excellent biocompatibility and positive effects to various living cells like mouse L929 fibroblasts, human corneal epithelial cells and chondrocytes, etc.17–19 Second, TDN features in rigidity and structural stability that resist nucleases degradation and increase circulation time in vivo.20,21 A latest founding indicated that the DNA crosshair had a rapid degradation within 20 min, while TDN remained sustaining existence after 1 hour in a single living cell.22 Last but not least, TDN possesses abundant functional modification sites due to the programmability. Oligonucleotides can be monovalently or multivalently modified to the vertex or side of TDN by extending ssDNA during synthesis or chemical crosslinking via complementary sequence.23,24 Besides, some functional molecules can be intercalated into duplex DNA via electrostatic interaction or be capsuled into the DNA cage.25,26 These merits contribute TDN to be promising biomedical application for biosensing, antitumor, antibacterial therapy and tissue regeneration.
Currently, TDNs-based biosensors have gained widespread application in anchoring small molecules, nucleic acids, proteins, and cells to specific sites due to their high specificity, rapid analysis, accuracy, and cost-effectiveness.27–30 For instance, Miao et al developed an ultrasensitive electrochemical system using TDN-modified AuNPs tags that can distinguish targeted miRNA from interfering miRNAs with slightly different sequences.31 TDNs-based nanoplatforms have also shown potential in clinical diagnosis, as exemplified by the use of DNA walking nanomachine on a tetrahedral DNA scaffold interface for ultrasensitive electrochemical analysis of miRNA (miR-141), a stable serum-based biomarker for prostate cancer diagnosis.27 Additionally, TDNs have been utilized to target tumor cells and enhance the stability and cell entry of chemical drugs. Lin et al developed an intelligent DNA nanorobot by anchoring an anti-HER2 DNA aptamer on a TDN, which demonstrated improved stability of the aptamer and specific induction of lysosomal degradation of the membrane protein HER2 in HER2-positive breast cancer cells.32 Despite the promising outlook for regenerative medicine applications, such as bone, cartilage, nerve, and dental regeneration,33–35 it is worth noting that TDN also exhibit potential in addressing infectious bone defects.
The clinical treatment of infected bone defects is a complex and intractable problem in orthopedics, where infection can significantly impair the repair capacity of mesenchymal stem cells, making it difficult to heal.36,37 In this regard, infection control and bone regeneration are the primary objectives for repairing infectious bone defects.36 Effective infection control measures involve debridement of the infected area, systemic administration of antibiotics, and the use of antibiotic-impregnated spacers. However, the systemic administration of antibiotics poses a significant challenge in achieving adequate local concentrations due to vascular destruction and osteonecrosis caused by the infection.38 The emergence of drug-resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) and multidrug-resistant Acinetobacter baumannii (MRAB), have made the treatment of wound infections increasingly challenging, requiring the development of novel antimicrobial agents and therapeutic approaches to effectively combat these multidrug-resistant pathogens.39,40 In addition, biofilm formation at the wound site further enhances antibiotic resistance and prevents effective treatment, while biofilm can cause excessive and persistent inflammation that inhibits bone healing, which is one of the main reasons for chronic non-healing wounds.41,42 Therefore, the development of bone substitutes that exhibit dual functionality in terms of anti-bacterial efficacy and the promotion of osteogenesis represents a highly significant treatment strategy for managing infectious bone defects. TDN has proven to be an exceptional delivery vehicle with tremendous potential for multifunctional antibacterial application and bone regeneration. In this comprehensive review, we have provided an insightful summary of the current antibacterial strategy based on TDN and their potential mechanisms for combating infections. Moreover, we have discussed the latest research in TDNs-based bone and cartilage repair and regeneration, shedding light on novel ideas for the therapy of infectious bone defects. Finally, we have also addressed the significant obstacles facing the development of TDN-based nanomaterials and highlighted the future prospects for this promising field.
Synthesis and Identification of TDN
Turberfield et al firstly introduced a single-step self-assemble procedure of TDN in 2004.8 The TDNs, comprised of four vertexes and six edges, were accomplished via the use of four specifically designed oligonucleotides. Each oligonucleotide was constructed with three distinct sequences, which were complementary to the other three oligonucleotides in accordance with Watson-Crick base-pairing rules. To ensure the preservation of the 60° angle at each vertex, unpaired “hinges” were incorporated within the structure. Subsequently, Goodman was able to synthesize a family of TDN molecules with varying sizes in seconds, resulting in a remarkable yield as high as 95%.43 Recently, TDN synthesized from four 63-mer oligonucleotides separated by a single base-pair hinges at every vertex to ensure sufficient flexibility, are commonly used by Lin group.14 These TDNs exhibited higher synthesis efficiency, fewer instances of secondary structure, and demonstrated more positive effects on various types of cells.44 To ensure their good programmability and high predictability, four appropriately designed ssDNA at equivalent mole concentration were mixed in TM buffer (10 mM Tris, 20 mM MgCl2, pH 8.0), then a thermal annealing process (95°C for 10 minutes, then rapidly reduced to 4°C for over 20 minutes) were performed (Figure 1A).21,45,46 To confirm the successful preparation of TDN, several analytical techniques are typically employed. The molecular weight of TDN is typically analyzed using 8% polyacrylamide gel electrophoresis (PAGE) as shown in Figure 1B. Furthermore, the structure of TDN can be characterized using atomic force microscopy (AFM) and transmission electron microscopy (TEM) as shown in Figures 1C and D respectively. Additionally, the dynamic light scattering (DLS) technique is commonly utilized to analyze TDN size and zeta-potential as depicted in Figures 1E and F. Collectively, these techniques provide valuable insight into the structure and general properties of TDN.
 |
Figure 1 Synthesis and identification of TDN. (A) Four paired ssDNA molecules were assembled into a tetrahedral DNA nanostructure (TDN). (B) The molecular weights of ssDNA and TDN were determined using 8% PAGE, with the size of TDN estimated to be around 200 base pairs. The morphology of TDN was characterized using AFM (C) and TEM (D). The red circle indicated a pyramid-shaped structure of TDN, with a size of approximately 10–20 nm. Scale bar: 50 nm for the main figure and 20nm for detailed figure. (E) The size of TDN was analyzed using dynamic light scattering (DLS), which yielded an average size of 18 nm. (F) The zeta potential of the TDN exhibited a negative electric charge. (A–F) Reprinted with permission from Zhou M, Zhang T, Zhang B, et al. A DNA nanostructure-based neuroprotectant against neuronal apoptosis via inhibiting toll-like receptor 2 signaling pathway in acute ischemic stroke. ACS Nano. 2022,16,1,1456–1470. Copyright 2022 American Chemical Society.46 |
Biological Merits of TDN
Positive Regulation of Cellular Function
As a natural biological genetic material, TDN possess splendid biocompatibility and are easier to realize diverse biological application. In 2016, Lin group explored the biological effects of pure TDNs on mammalian cells, taking advantage of TDN’s natural ability to enter cells without the aid of transfection agents.17 The effects of various concentrations of TDNs, ranging from 62.5 nM to 500 nM, on cellular proliferation was investigated in this study. The results demonstrated that TDNs, specifically at a concentration of 250 nM, significantly enhanced cell proliferation by modulating the Wnt/β-catenin signaling pathway. Subsequently, numerous studies have demonstrated that TDN can enhance the proliferation of various cell types, including mesenchymal stem cells,33,47 neural stem cells,48 and umbilical endothelial cells.24 Moreover, TDNs also promote cell migration in various cell types, including endothelial cells,24 human corneal epithelial cells,18 neural stem cells (NSCs)49 and Schwann cells (SCs),50 all of which are involved in the intricate tissue healing process. TDNs have been shown to facilitate NSC differentiation into neurons and oligodendrocytes in the nervous system, promoting neural tissue formation in injured spinal cords.34 In the immune system, TDNs exhibit immunomodulatory effects, influencing cytokine secretion and signal transducer and activator of transcription (STAT) signaling to regulate B and T cell differentiation.51
In addition to regulating cell proliferation, migration, and differentiation, TDNs also exhibit anti-apoptotic properties. Apoptosis is a crucial programmed cell death process involving significant changes in morphology and metabolic activity, leading to cell death and playing a critical role in the development and normal function of an organism.52 Studies have shown that TDNs can effectively suppress cell apoptosis induced by excessive reactive oxygen species (ROS) production and inflammatory responses. In retinal ganglion cell injury model, TDNs regulated the expression of oxidation-related enzymes to reduced ROS production and affect the expression of apoptosis-related proteins, inhibiting cell apoptosis.53 Additionally, TDNs upregulated the expression of the antioxidant enzyme heme oxygenase-1 (HO-1) to attenuate ROS generation and oxidative stress, inhibiting cell apoptosis in RAW264.7 cells stimulated with lipopolysaccharide (LPS).47
Enhanced Cellular and Tissue Permeability
As is well-known, mammalian cells exhibit low efficiency in taking up exogenous DNA due to its polyanionic nature. However, in the absence of viral vectors or transfection agents, TDN can be readily internalized into cells. In a study conducted by Walsh et al,54 the ability of TDN to enter live cells was investigated, revealing that TDN can effectively enter human embryonic kidney cells in the absence of transfection reagents, and can remain substantially intact in the cytoplasm for up to 72 hours (Figure 2A). Previous studies have shown that TDN can penetrate cells through the caveolin-dependent endocytosis pathway.55 Subsequently, Liang et al56 utilized single-particle tracking to monitor the entry and transport pathways of TDN in Hela cells, and discovered that TDN could fuse with the plasma membrane over a period of approximately 87 seconds before entering the cell. This fusing process was facilitated by the unique tetrahedral structure of TDN, which allowed its corners to approach the cytomembrane, thereby reducing electrostatic repulsion. Additionally, the short-range attraction that occurs after charge redistribution at the membrane interface was assisted by caveolin.16
 |
Figure 2 Enhanced cellular and tissue permeability. (A) Confocal images of intracellular internalization of Cy5-labeled naked TDN without the aid of transfection reagent. Blue: nuclear stain; red: Cy5; green: lysosomes; gray: phase contrast. Scale bars: 20 μm. Adapted with permission from from Walsh AS, Yin H, Erben CM, Wood MJA, Turberfield AJ. DNA Cage Delivery to Mammalian Cells. ACS Nano. 2011;5(7):5427–5432. Copyright 2021, with permission from American Chemical Society.54 (B) Schematic illustration of 8 kinds of DNA nanostructures including Th21, Th37, Th337, 6H714, 6H14498, R13730, B14498, T14498. (C) Representative fluorescence images of mouse skin histology post various DNA nanostructures penetration (red: Cy5.5-labeled DNA nanostructures, blue: Hoechst 33342-stained skin). (D) Cy5.5 fluorescence signal from various DNA nanostructures along skin depth. (B–D) Reproduced from Wiraja C, Zhu Y, Lio DCS, et al. Framework nucleic acids as programmable carrier for transdermal drug delivery. Nat Commun. 2019;10(1):1147. Copyright 2019, with permission from Springer Nature.26 Abbreviations: TH21, tetrahedron with 21 bps; TH31, tetrahedron with 37 bps; TH337, tetrahedron with 337 bps; 6H714, 6-helical rods with 714 bps; 6H14498, 6-helical rods with 14,498 bps; R13730, rectangular plane with 13,730 bps; B14498, rectangular box with 14,498 bps; T14498, triangular plane with 14,498 bps. |
In addition to their improved endocytosis capabilities, recent studies have reported on the enhanced tissue penetration behavior of TDN structures. Wiraja. C investigated the skin penetration ability of TDNs with sizes ranging from 20 to 200 nm.26 The results showed that TDN with sizes ≤75 nm could effectively reach the dermis layer, with TDN of 20 nm exhibiting the strongest penetration ability, indicating a size-dependent penetration phenomenon (Figure 2B–D).26 This characteristic is an important factor to consider in drug development, as it can significantly affect the efficacy of drug delivery. Unlike other DNA nanostructures that are hindered by their dimensions, TDN structures can overcome these limitations with their improved tissue penetration, which could further broaden their potential applications as drug delivery vehicles or sensor platforms.
Multiple Drug Delivery Options
DNA possesses several drug binding mechanisms including intercalation, electrostatic interaction, and chemical cross-linking, due to its unique structure.57 Previous studies have explored various methods for loading drugs onto TDNs, such as embedding nucleic acids, intercalating or encapsulating small-molecule drugs.58–60 Despite the fact that TDNs offer a backbone for incorporating various drugs, there exist differences in both the loading mechanism and efficiency, as well as the subsequent release profile.
Through sequence extension or hybridization techniques, nucleic acids such as DNA, RNA, and PNA can bind to the vertex or side edge of TDNs.14 The sequence extension method is a molecular biology technique that utilizes homopolymeric oligonucleotide end ligation via terminal transferase to attach diverse nucleic acid molecules, including DNA, RNA, and other nucleotides, to the 5′- or 3′-ends of ssDNA.61 Through this approach, a multifunctional TDN with targeted or therapeutic efficacy can be synthesized from four normal and functional ssDNAs utilizing the same synthesis procedure of TDNs. Compared with the directly oligonucleotide extension, the sticky-ended hybridization may be a more practical approach.62 Functional molecules or groups could hybridize with ssDNA or ssDNA extensions located at either the termini or internal regions of their sequences. This approach has facilitated the development of diverse nucleic acids with distinct functionalities, which have shown promising results in tumor suppression, immune regulation, and stem cell regulation.63,64 Despite their potential, prior investigations have failed to provide a satisfactory explanation regarding the detachment of nucleic acid drugs from TDNs, which is expected to be transported to the lysosome. Small-molecule drugs exhibit distinct binding mechanisms for different targets, and the unique helical structure of DNA offers a multitude of binding options. For example, peptides and proteins with positive charges, such as antimicrobial peptide histatin 5, can be loaded onto TDNs with negative charges by leveraging electrostatic force.65 In addition, a variety of functional molecules, including anticancer drugs, traditional Chinese medicine monomers, metal complexes, and fluorescent molecules, can be intercalated into the TDNs’ double helix through groove docking and hydrogen bonding.55,66–68 Furthermore, functionalized molecules could be wrapped into the cage structure of TDNs.60 Turberfield et al estimated that a sphere with a radius of approximately 2.6 nm could fit within the central cavity of a tetrahedron.69
Utilizing TDNs for drug delivery offers numerous benefits, such as enhancing drug solubility, mitigating off-target effects, and increasing intracellular accumulation. However, the binding of small molecules with TDN can significantly alter its structure. Recent research has demonstrated that DOX intercalation with DNA can lead to partial B-to-A-DNA conformational transitions.70 It should be noted that the intercalation mechanism is susceptible to environmental factors such as pH value and Mg2+ concentration.71
TDN-Based Nanostructures for Antibacterial Application
Bacterial infections are a significant global health concern, causing a range of diseases and health complications that affect millions of people each year. While the immediate impact of bacterial infection is often attributed to the physical damage it causes to tissues, it can also have significant and long-lasting effects on the body’s ability to regenerate damaged tissue. Bacterial toxins and inflammation can interfere with the complex cellular processes involved in tissue repair and regeneration, leading to delayed or incomplete healing. Furthermore, the emergence of antibiotic-resistant strains of bacteria has made it increasingly difficult to treat infections and promote tissue regeneration. Therefore, there is a critical need to develop new therapeutic approaches to combat bacterial infections and support tissue regeneration.
TDN has emerged as a promising multifunctional antibacterial assistant due to its excellent delivery potential, abundant modification sites, and high bacterial absorption rate.72 Current research has shown that the TDN-based nanoplatform is capable of inhibiting multiple bacterial strains, including Methicillin-resistant Staphylococcus aureus (MRSA),73 Porphyromonas gingivalis (P. gingivalis),74 and Escherichia coli (E. coli),75 by enhancing the uptake rate of antibiotics, preventing efflux of antibiotics, suppressing bacterial growth through transcription regulation, or inhibiting biofilm formation. Currently, TDN has been developed into a novel delivery system to load antibiotics,76 peptide nucleic acids (PNAs),73 antimicrobial peptides (AMPs),74 and antisense oligonucleotides (ASOs)77 into multiple bacteria with rich abundance. In this section, we provide a comprehensive summary of the novel antibacterial strategies based on TDN in recent years, along with their potential mechanisms of action as illustrated in Figure 3.
The Combined Use of TDN with Antibiotics
MRSA has become a significant pathogen of nosocomial and community infections, accounting for 13% to 74% of all S. aureus infections worldwide, posing an enormous threat to public health.78 The broad-spectrum β-lactam antibiotic, ampicillin, often shows little influence on MRSA when used alone at prescribed doses due to its multiple drug resistance mechanisms.79 To enhance its antibacterial efficacy against MRSA, Sun et al76 incubated TDN with an ampicillin solution, which exhibited a high encapsulation rate and favorable stability. The uptake rate of TDN-ampicillin by MRSA18908 was significantly higher than that of ampicillin alone, as demonstrated by flow cytometry and confocal microscopy. Moreover, the minimum inhibitory concentrations (MIC) of TDN-ampicillin to MRSA were half that of ampicillin alone, significantly lower than the recommended resistance dose by the European Committee on Antimicrobial Susceptibility Testing.80 Morphological observation revealed that TDN-ampicillin had a stronger cell wall-damaging effect on MRSA than free ampicillin, possibly because TDN improved the efficiency of ampicillin binding to penicillin-binding protein 2 (PBP2), a protein involved in the synthesis of peptidoglycan, the primary component of bacterial cell walls.81 Similarly, Sun et al75 loaded erythromycin into TDN and evaluated its antibacterial activity against E. coli, which showed stronger bacteriostatic effects than free erythromycin, possibly due to the increased cell membrane permeability enabled by TDN, which allowed erythromycin to enter the cell more efficiently.
Currently, Gram-negative bacteria are highly resistant to a wide range of drugs and are becoming increasingly resistant to most available antibiotics.82 The unique thick outer membrane of Gram-negative bacteria hinders the entry of most antibiotics into bacterial cell membrane. In addition, Gram-negative bacteria have an efflux pump that promotes the excretion of antibiotics in order to enhance their survival.83,84 To address this challenge, TDN’s excellent transmembrane ability makes it a promising delivery platform for antibiotics to enhance their bactericidal activity against Gram-negative bacteria. Future studies should focus on designing special aptamers and reducing the minimum inhibitory concentration (MIC) of antibiotics at the genetic level to directly eradicate drug-resistant bacteria.
The Combined Use of TDN with PNAs
PNAs are artificial oligonucleotide mimetics with a neutral peptidic backbone instead of a negatively charged phosphoribosyl backbone. As such, PNAs combine the properties of both peptides and nucleic acids and possess many superiorities: favorable stability,85,86 strong binding ability,87 and superior specificity,88 which make them effective for therapeutic applications. Previous studies have shown that antisense PNA oligomers can inhibit bacterial growth in micromolar concentrations by inhibiting the translation process.89 However, the hydrophobicity of the PNAs backbone causes poor water solubility of PNAs, making it difficult to deliver them to bacterial cells.90 Moreover, some bacteria can prevent the uptake of these peptides through gene mutations encoding translocated proteins.91 Therefore, it is necessary to develop novel vectors that can deliver PNAs without deleterious effects on mammalian cells.
In 2016, Readman et al92 developed a TDN nanoparticle platform incorporating a targeted anti-blaCTX-M-group 1 antisense PNA (PNA4), which has been previously demonstrated to restrain the expression of blaCTX-M-15 in Escherichia coli and partially restore cefotaxime (CTX) sensitivity in strains with reduced susceptibility phenotypes.93 The results showed that TDN-PNA4 potentiated the antibacterial activity of CTX in a dose-dependent manner, and the MIC was reduced. In contrast, no growth inhibitory effects were observed when treated with TDN vectors alone. Similarly, an antisense peptide nucleic acid (asPNA), targeting a specific ftsZ gene involved in bacterial cell division, was inserted into TDN by changing the short sequence on a single strand73,94 TDN-asPNA was easily taken up by MRSA cells and effectively inhibited MRSA growth in a dose-dependent manner by reducing ftsZ expression. However, the structure of TDN-asPNA was susceptible to degradation compared to naked TDN, which was possibly due to the imbalanced stress distribution between DNA duplexes.73,95 Therefore, how to improve cargo stability is still a problem that needs further study.
The Combined Use of TDN with AMPs
AMPs are a diverse group of bioactive oligopeptides that can combat drug-resistant bacteria by destroying the antimicrobial mechanism of bacterial cell wall.96,97 However, the antibacterial activities and stability of AMPs require further study.98 Recently, the antimicrobial peptide GL13K was coated onto TDN through electrostatic interaction, with a loading efficiency of approximately 95% at a 1:500 ratio of TDN/GL13K.74 TDN also reduced the susceptibility of GL13K to degradation in an extracellular environment rich in proteases of P. gingivalis. Furthermore, flow cytometry revealed that TDN improved the uptake of GL13K by E. coli and P. gingivalis, resulting in the deformation and disintegration of both bacterial strains. Another antibacterial peptide-like molecule, Histatin 5, was combined with TDN using the same electrostatic attraction method, which demonstrated excellent stability and enhanced bactericidal activity against Candida albicans.99 These findings suggest that TDN is a suitable carrier not only for loading AMPs for antibacterial applications but also for other types of protease-sensitive AMPs for various therapeutic applications. For instance, TDN could be used as a codelivery system for antibacterial AMPs and other therapeutics, such as anti-inflammatory or osteogenic AMPs for in vivo infectious models such as conjunctivitis, periodontitis, and infected bone defect repair.100,101 Further research will explore more ways to apply TDN/AMP complexes.
The Combined Use of TDN with ASOs
ASOs are short single-stranded DNA or RNA sequences that can block the transcription or translation of target genes.102 While ASOs have shown potential in developing gene-targeted therapeutics, natural ASOs are often degraded quickly in vivo, have low specificity, and can cause toxic side effects.103 As oligonucleotide drugs, ASOs can theoretically combine with TDN through complementary base pairing.
In 2020, Zhang et al developed an ASO-modified TDN delivery system by self-assembling four ssDNA and an ASO sequence.77 This system was stable and could easily penetrate the cell wall of S. mutans. When delivered into bacterial cells, the ASO sequence targeting multiple genes (gtfBCD, gbpB, and ftf) significantly reduced extracellular polysaccharide synthesis and inhibited biofilm formation. This represents the first attempt to combine ASOs with TDN in antibacterial applications, and these results suggest a promising treatment for chronic biofilm-mediated infections through early debridement. However, further investigation is needed to test this approach on different types of bacterial strains and biofilms.
TDN-Based Strategy for Bone Repair
Bone tissue defect is a commonly clinical practice challenge, affecting numerous people throughout the world.104 Bone tissue repair involves a complex physiological process that is activated early by an inflammatory immune reaction, which includes angiogenesis, osteogenic differentiation, biomineralization, and other multiple processes.105 Although bone tissue is continually undergoing remodeling and regeneration, in cases of severe bone damage or defects, external intervention is required to enhance the process. Currently, autologous or allogeneic bone grafting techniques are the most commonly used methods to deal with critical bone defects. However, these approaches are restricted by the size of harvestable grafts and increase the risk of surgical area morbidity, including infection and persistent post-operative pain.106 Bioactive materials-based bone tissue engineering technologies offer a new direction for repairing bone defects. In recent years, TDN has been shown to promote osteogenic differentiation of mesenchymal stem cells, enhance bone regeneration, and inhibit cartilage degradation in various in vitro and in vivo models.19,107,108 Additionally, TDN has been used as a delivery system for bioactive molecules to target bone tissue and enhance bone regeneration. These exciting findings suggest that TDN-based platforms hold great potential for the development of novel bone and cartilage tissue engineering strategies.
TDN Promote the Osteogenic Differentiation of Mesenchymal Stem Cells (MSCs)
MSCs, which are pluripotent stem cells capable of self-renewal and multidirectional differentiation, have been found to undergo osteogenic differentiation with the help of TDN, as demonstrated by previous studies on various MSCs, including adipose-derived stem cells (APSCs), dental pulp stem cells (DPSCs), and periodontal ligament stem cells (PDLSCs).33,109,110 PDLSCs, in particular, have attracted considerable attention for alveolar bone defect repair due to their accessibility through minimally invasive surgery and low immunogenicity.111 Recently, Zhou et al found that TDN facilitated osteogenic differentiation of PDLSCs by regulating the Wnt/β-catenin signaling pathway, suggesting that TDN may be useful for PDLSC-based bone tissue regeneration.33 Alveolar bone defect repair typically occurs in a chronic inflammatory and infectious condition.112 Recent evidence suggests TDN possess prominent anti-inflammatory and antioxidant activities mediated by regulating the macrophage response through suppression of MAPK phosphorylation.113 To further investigate TDN’s effects on PDLSCs in an inflammatory microenvironment, Zhou et al114 constructed an inflammatory model in vitro and in vivo induced by lipopolysaccharide and silk ligature. PCR assays showed that TDN treatment enhanced the gene expression levels of ALP and Runx2, while the expression of osteogenic proteins, such as OPN and Runx2, increased after exposure to TDN (Figure 4A and B). Additionally, TDN significantly reduced ROS production and LPS-induced inflammatory cytokines like TNF-α, IL-6, and IL-1β in PDLSCs. In vivo experiments demonstrated that TDN inhibited the destruction of periodontal tissue in periodontitis (Figure 4C–E).114 In summary, TDN was found to promote osteogenic differentiation of PDLSCs in both normal and inflammatory conditions. These findings suggest that TDN may be useful as a prophylactic or therapeutic agent in an inflammatory bone defect model.
 |
Figure 4 TDNs protect alveolar bone under inflammatory conditions. (A) Analysis of RUNX2 and OPN expression levels in PDLSCs exposed to TDNs and LPS using Western blotting, with statistical evaluation of results. Data are presented as means ± standard deviations (n = 3). ***P < 0.001. (B) Immunofluorescence staining imaging of OPN proteins. Scale bars are 50 μm. (C) Schematic diagram depicting the rat periodontitis experiment. (D) The images of H&E staining at 5×magnification and 20×magnification. The area surrounded by yellow solid line was cementum, and the area surrounded by yellow dotted line was the cementum absorbed by inflammation. The yellow arrow indicated the cementum in the yellow line. (E) The photographs of TRAP staining at 5×magnification and 20×magnification. The red arrow indicated the osteoclasts. The tFNA is equal to TDN. (D) dentin; (C) cementum. (A–E) Reproduced from Zhou M, Gao S, Zhang X, et al. The protective effect of tetrahedral framework nucleic acids on periodontium under inflammatory conditions. Bioactive Materials. 2021;6(6):1676–1688. Copyright 2020, with permission from Elsevier.114 Abbreviations: MR/DR, mesial root/distal root; AB, alveolar ridge; PDL, periodontal ligament. |
TDN-Based Strategy for Bone Tissue Repair
In recent decades, nucleic acid-based gene therapy has emerged as a promising therapeutic strategy for bone tissue repair and regeneration.115 Specifically, gene therapy employs nucleic acid therapeutics such as ASOs, siRNAs, miRNAs, CRISPR-Cas9, etc. to activate or inhibit key signaling pathways in specific organisms.116–118 Among these, miRNAs are a class of evolutionarily conserved non-coding short RNA molecules that play a vital role in regulating various physiological and pathological activities, including cell proliferation and differentiation, as well as vascular growth and invasion at the posttranscriptional level of gene expression.119 However, the instability of miRNAs has been a limiting factor as they are vulnerable to degradation by RNA enzymes.120 To address this issue, TDN has been developed as a promising nanomaterial for efficient gene delivery, particularly for promoting bone tissue repair and regeneration.
Recently, Li et al developed a versatile TDN-based transport system for delivering miR-2861 to promote bone regeneration by targeting histone deacetylase 5 (HDAC5) expression in BMSCs.35 The system employed four ssDNAs with sticky ends self-assembled to a sticky-end modified TDN, which acted as a “truck” to deliver double-stranded miRNA with paired sticky ends. The TDN/miR complex was easily able to penetrate the cytomembrane in BMSCs after incubation for 12 hours, leading to darker blue-purple precipitates and denser mineralized nodules observed by alkaline phosphatase staining and alizarin red staining, respectively (Figure 5A and B). In addition, a femoral defect model was constructed to assess the effect of TDN/miR-2861 on bone regeneration in vivo. The representative images from micro-CT indicated a completely recuperated appearance after topical injection of the TDN-miR-2861 complex for 2 weeks (Figure 5C and E). The histological analysis further demonstrated the regenerative tissue was much closer to a natural bone tissue (Figure 5D, F and G). To extend the release time and enhance the bone repair, a dual delivery heparin lithium hydrogel system was also constructed to transit lithium and TDN- miR-335-5 complex to treat challenging bone lesions associated with steroid-associated osteonecrosis (SAON).121 The results showed that the injectable poly-porous heparin lithium hydrogel encapsulating the TDN-miR-335-5 complex facilitated the bone defect repair in the early stage of SAON.122 Li et al also integrated TDN carriers encapsulated Clindamycin (CLI) and BMSCs in a 3D bioprinted methylacrylylated gelatin (GelMA) scaffold for treating infected bone defects. The hydrogel provided a 3D extracellular matrix-like environment for sustained-release of transplanted cells or antibiotics, and interconnected holes facilitating vascularization and bone ingrowth.123
 |
Figure 5 Using TDN to deliver osteogenic miRNA for bone regeneration. (A) Osteogenic differentiation was assessed by alkaline phosphatase detection and the statistical analysis results at day 5 are displayed. Scale bars are 200 μm. Data are presented as means ± standard deviations (n = 3). **P < 0.01. (B) Osteogenic differentiation at day 14 detected using alizarin red S staining and the statistical analysis results. Scale bars are 100 μm. Data are presented as means ± standard deviations (n = 3). **P < 0.01. (C) 3D reconstruction of the femur at day 14. (D) Representative images of Masson staining at 5×magnification. (E) The statistical analysis of the results of BV/TV, Tb.N, Tb.Sp, and Tb.Th (C). (F and G) The statistical analysis of collagen volume fraction at day 7 and day 14 (D). Data are presented as mean ± standard deviation (SD) (n = 5). *p < 0.05, **p < 0.01. (A–G) Reproduced from Li S, Liu Y, Tian T, et al. Bioswitchable delivery of microRNA by framework nucleic acids: application to bone regeneration. Small. 2021;17(47): e2104359. Copyright 2021, with permission from Wiley-VCH GmbH.35 |
TDN-Based Strategy for Cartilage Repair and Regeneration
Articular cartilage (AC) lacks vascellum, nerves, and lymphatic vessels, which restricts its self-repair when damaged, making it a significant challenge in clinical research and regenerative medicine.124–126 Traditional clinical treatments, such as conservative drug therapy and articular replacement have their own limitations. However, in recent years, tissue engineering techniques based on seed cells, biomaterials, and appropriate microenvironments have shown remarkable potential in cartilage regeneration. In this section, we summarize the current applications of TDN-based nanostructures for repairing and regenerating cartilage.
TDN Regulates Chondrocyte Function
Chondrocytes have become a popular choice for cell-based cartilage regeneration strategies;127 however, their utility is limited by the scarcity of donor sites and the low yield of isolated cells. Furthermore, chondrocytes are prone to dedifferentiation and the loss of their characteristic phenotype during in vitro expansion, which presents a challenge for their efficacy in tissue engineering.128 This issue is compounded in elderly patients, as chondrocytes in this population exhibit reduced proliferation and differentiation potential, significantly impacting their function in cartilage tissue engineering.129,130 To improve the performance of chondrocytes, Shao et al19 investigated the effects of TDN on chondrocytes and the results indicated that TDN at 250 nM concentration significantly promoted the proliferation of chondrocytes (Figure 6A). The motility ability of chondrocytes was also evaluated using a wound healing assay, which showed a significant increase in migratory capacity after treatment with TDN (Figure 6B and C).45 Additionally, immunofluorescent staining revealed that TDN helped maintain the relevant round and stereoscopic morphology of chondrocytes, indicating that TDN preserved the chondrocyte phenotype more effectively (Figure 6D and E).
 |
Figure 6 TDNs regulate chondrocyte performance. (A) Proliferation of chondrocytes detected by real-time cell analysis (RTCA). (B) The effect of TDNs on Chondrocyte migration through wound healing assays. (C) The statistical analysis of migrated cells. Data are presented as mean±SD (n=3). *P<0.05. (B and C) Reproduced from Shi S, Lin S, Shao X, Li Q, Tao Z, Lin Y. Modulation of chondrocyte motility by tetrahedral DNA nanostructures. Cell Prolif. 2017;50(5). Copyright 2017, with permission from John Wiley & Sons Ltd.45 (D) The fluorescent images of cytoskeleton in chondrocytes. Scale bars are 25 µm. (E) Changes of cell area distribution after exposure to TDNs. (A, D and E) Reproduced from Shao X, Lin S, Peng Q, et al. Tetrahedral DNA nanostructure: a potential promoter for cartilage tissue regeneration via regulating chondrocyte phenotype and proliferation. Small. 2017;13(12). Copyright 2017, with permission from Wiley VCH.19 |
TDN Promote the Repair and Regeneration of Cartilage
Osteoarthritis (OA) is thought to be the most prevalent chronic joint disease that causes pain and disability in the adult population.131,132 Chondrocytes are the only cell type present in articular cartilage, and their dysfunction breaks the balance between synthesis and degradation of extracellular matrix (ECM) molecule, leading to the degenerative and progressive pathological changes in OA.133 TDN, a promising therapeutic agent for tissue healing, has been shown to exert antiapoptotic and antioxidative effects.53,134 Moreover, TDN has demonstrated potential therapeutic value in OA by inhibiting IL-1β-stimulated apoptosis and oxidative stress in chondrocytes.108 To this end, Shi et al developed an injectable TDN/wogonin system by intercalating wogonin into double-stranded DNA groove, which exhibited remarkable therapeutic efficacy in reducing inflammatory cytokine and matrix metalloproteinases (MMP1, MMP3, MMP13) expression, and increasing chondrogenic marker contents and the level of related inhibitory protein of MMPs in chondrocytes. The TDN/wogonin complex also led to a smoother and better-structured cartilage surface after injection in the rat knee-joint. (Figure 7A).25 Similarly, Li et al proposed a synovial mesenchymal stem cell (SMSCs)-based tissue engineering strategy, which combined TDN with chitosan (CS) hydrogel/3D-printed poly(ε-caprolactone) (PCL) hybrid scaffold encapsulated with SMSCs, and showed great potential as an effective method for cartilage tissue regeneration (Figure 7B).135 In this study, a hybrid scaffold consisting of chitosan (CS) hydrogel and 3D-printed poly(ε-caprolactone) (PCL) was utilized to encapsulate synovial membrane-derived stem cells (SMSCs) possessing excellent chondrogenic differentiation capacity.136,137 The resulting scaffold was then implanted into rabbit articular cartilage defects. To further enhance the regeneration process, TDN was injected into the articular cavity, which electrostatically bound to the CS hydrogel, providing an optimal microenvironment for the proliferation and chondrogenic differentiation of the SMSCs. As a result, the regeneration of the cartilage defect was significantly promoted.
 |
Figure 7 TDN-based strategy for cartilage repair and regeneration. (A) Schematic diagram of the effects of TDN/wogonin complex on osteoarthritis. The middle section showed the synthesis of TDN/wogonin complex. The upper section showed the TDN/wogonin complex alleviated the inflammation of chondrocytes in OA. The lower section showed the TDN/wogonin complex alleviated OA in the rat model. Reproduced from Sirong S, Yang C, Taoran T, et al. Effects of tetrahedral framework nucleic acid/wogonin complexes on osteoarthritis. Bone Res. 2020;8:6. Copyright 2020, with permission from Springer Nature.25 (B) Schematic diagram of TDN-based strategy for articular repair and regeneration. In this study, a cartilage regenerative system was assembled based on a chitosan (CS) hydrogel/3D-printed poly(ε‐caprolactone) (PCL) hybrid containing synovial mesenchymal stem cells and recruiting Tetrahedral DNA nanostructure (TDN) injected into the articular cavity. Reproduced from Li P, Fu L, Liao Z, et al. Chitosan hydrogel/3D-printed poly(ε-caprolactone) hybrid scaffold containing synovial mesenchymal stem cells for cartilage regeneration based on tetrahedral framework nucleic acid recruitment. Biomaterials. 2021;278:121,131. Copyright 2021, with permission from Elsevier Ltd.135 |
Though these TDN-based strategies proposed novel therapies for cartilage repair and regeneration, their effectiveness remains uncertain, and there are several challenges to overcome. One such challenge is finding the optimal balance between the rapid degradation of TDN and the slow rate of tissue regeneration. As a result, there is a need for continuous TDN injections, which increase the risk of intra-articular infection. Nevertheless, TDN-based injective agents remain a promising approach for treating cartilage defects and osteoarthritis. However, further research is necessary to address these concerns and fully realize their potential as a viable treatment option. By overcoming these obstacles, TDN-based therapies could offer a much-needed solution to the growing problem of cartilage damage and degeneration.
Prospects and Challenges
TDN-based nanostructures have been widely utilized in a variety of biomedical applications such as antibacterial therapy, biosensing, anticancer, and tissue engineering. Despite these promising applications, the development of TDN still faces certain challenges and obstacles that must be addressed before it can be successfully implemented in medical practice. Firstly, most studies on TDN are conducted in vitro or on small animals, which may not accurately reflect the complexities of the human body. Therefore, it is imperative to further confirm the clinical effects of TDN through similar studies on multiple large animals. Additionally, long-term cytotoxicity and biological availability of TDN should be investigated in depth to uncover any unknown risks or benefits. Secondly, the high cost of TDN synthesis may limit mass production and large-scale clinical application. To address this issue, DNA origami technology can be used to fold specific regions of long-stranded DNA into the desired structure, potentially enabling low-cost, simple, and efficient fabrication of mirror structures of TDN that possess greater serum stability and longer physical retention time. Finally, chemically modified TDN must meet the necessary requirements for in vivo application, including biostability, suitable pharmacodynamic and pharmacokinetic properties. Addressing these challenges will require continued research and development of TDN and other DNA nanomaterials with real therapeutic value.
Conclusion
In this review, we discuss the synthetic and biological characteristics of TDN, as well as their applications in antibacterial therapy, bone and cartilage tissue repair and regeneration. TDN possess several advantages, including simple self-assembly, high synthesis efficiency, stable microstructure, admirable biocompatibility, good stability, and abundant functional modification sites. Additionally, the unique spatial structure and small size of TDN enable them to enter various types of cells without the need for transfection agents. TDN-based nanocarriers provide a novel alternative for antimicrobial therapy. TDN not only improve the local concentration of antibiotics and their affinity to bacteria but also provide novel antimicrobial modalities that can directly kill drug-resistant bacteria and decrease the MIC of antibiotics at the genetic level. Furthermore, due to the physicochemical nature of DNA, TDNs are easily editable and exhibit prominent regulation ability on cellular behavior, including increased cell proliferation, migration, osteogenic differentiation ability, anti-inflammatory and ROS-scavenging ability, and chondrocyte phenotype maintaining ability. These properties make TDN suitable for use as a bioactive nanomaterial or a targeted nanocarrier for therapeutic molecules in bone tissue repair and regeneration.
Abbreviations
TDN, tetrahedral DNA nanostructure; ssDNA, single DNA strands; WHO, World Health Organization; MRSA, Methicillin-resistant Staphylococcus aureus; P. gingivalis, Porphyromonas gingivalis; E. coli, Escherichia coli; PAGE, polyacrylamide gel electrophoresis; AFM, atomic force microscopy; TEM, transmission electron microscopy; DLS, dynamic light scattering; FRET, fluorescence resonance energy transfer; PNAs, peptide nucleic acids; AMPs, antimicrobial peptides; ASOs, antisense oligonucleotides; MIC, minimum inhibitory concentration; PBP2, penicillin-binding protein 2; PNA4, anti-blaCTX-M-group 1 antisense PNA; CTX, cefotaxime;, asPNA, antisense peptide nuclear acid; His-5, Histatin 5; MSCs, mesenchymal stem cells; APSCs, adipose-derived stem cells; DPSCs, dental pulp stem cells; PDLSCs, periodontal ligament stem cells; TNF-α, tumor necrosis factor-alpha; IL-6, interleukin-6; IL-1β, interleukin-1beta; siRNA, small interfering RNA; miRNA, microRNA; HDAC5, histone deacetylase 5; BMSCs, bone marrow mesenchymal stem cells; SAON, steroid-associated osteonecrosis; CLI, Clindamycin; GelMA, methylacrylylated gelatin; AC, articular cartilage; OA, osteoarthritis; ECM, extracellular matrix; MMP, matrix metalloproteinase; SMSCs, synovial mesenchymal stem cell; CS, chitosan; PCL, poly(ε-caprolactone); Ref, reference.
Consent for Publication
The image information quoted in this article has been applied to the relevant publishers and has been approved for use.
Funding
This work was supported by National Natural Science Foundation of China (82071096, 82001006, 81871490), Science and Technology Commission of Shanghai Municipality (21490711700, 21DZ2294600), the Interdisciplinary Program of Shanghai Jiao Tong University (YG2021ZD12, YG2022ZD014), Program of Shanghai Academic/Technology Research Leader (20XD1433100, 19XD1434500), Double Hundred Plan (20191819), CAMS Innovation Fund for Medical Sciences (CIFMS) (2019-I2M-5-037), Shanghai Sailing Program (19YF1425800) and Postdoctoral Scientific Research Foundation of Shanghai Ninth People’s Hospital.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Contera S, Bernardino de la Serna J, Tetley TD. Biotechnology, nanotechnology and medicine. Emerg Top Life Sci. 2020;4(6):551–554. doi:10.1042/ETLS20200350
2. Seeman NC. Nucleic acid junctions and lattices. J Theor Biol. 1982;99(2):237–247. doi:10.1016/0022-5193(82)90002-9
3. Goodman RP, Schaap IA, Tardin CF, et al. Endocytosis. Science. 2005;310(5754):1661–1665. doi:10.1126/science.1120367
4. Bhatia D, Mehtab S, Krishnan R, Indi SS, Basu A, Krishnan Y. Icosahedral DNA nanocapsules by modular assembly. Angew Chem Int Ed Engl. 2009;48(23):4134–4137. doi:10.1002/anie.200806000
5. Fujibayashi K, Hariadi R, Park SH, Winfree E, Murata S. Toward reliable algorithmic self-assembly of DNA tiles: a fixed-width cellular automaton pattern. Nano Lett. 2008;8(7):1791–1797. doi:10.1021/nl0722830
6. Andersen ES, Dong M, Nielsen MM, et al. Self-assembly of a nanoscale DNA box with a controllable lid. Nature. 2009;459(7243):73–76. doi:10.1038/nature07971
7. Chen JH, Seeman NC. Synthesis from DNA of a molecule with the connectivity of a cube. Nature. 1991;350(6319):631–633. doi:10.1038/350631a0
8. Goodman RP, Berry RM, Turberfield AJ. The single-step synthesis of a DNA tetrahedron. Chem Commun. 2004;12:1372–1373.
9. Tian Y, Wang T, Liu W, et al. Prescribed nanoparticle cluster architectures and low-dimensional arrays built using octahedral DNA origami frames. Nat Nanotechnol. 2015;10(7):637–644. doi:10.1038/nnano.2015.105
10. Zhang C, Su M, He Y, et al. Conformational flexibility facilitates self-assembly of complex DNA nanostructures. Proc Natl Acad Sci U S A. 2008;105(31):10665–10669. doi:10.1073/pnas.0803841105
11. Rothemund PW, Ekani-Nkodo A, Papadakis N, Kumar A, Fygenson DK, Winfree E. Design and characterization of programmable DNA nanotubes. J Am Chem Soc. 2004;126(50):16344–16352. doi:10.1021/ja044319l
12. Rothemund PW. Folding DNA to create nanoscale shapes and patterns. Nature. 2006;440(7082):297–302. doi:10.1038/nature04586
13. Han D, Pal S, Nangreave J, Deng Z, Liu Y, Yan H. DNA origami with complex curvatures in three-dimensional space. Science. 2011;332(6027):342–346. doi:10.1126/science.1202998
14. Lin Y, Li Q, Wang L, et al. Advances in regenerative medicine applications of tetrahedral framework nucleic acid-based nanomaterials: an expert consensus recommendation. Int J Oral Sci. 2022;14(1):51. doi:10.1038/s41368-022-00199-9
15. Shih WM, Quispe JD, Joyce GF. A 1.7-kilobase single-stranded DNA that folds into a nanoscale octahedron. Nature. 2004;427(6975):618–621. doi:10.1038/nature02307
16. Ding H, Li J, Chen N, et al. DNA nanostructure-programmed like-charge attraction at the cell-membrane interface. ACS Cent Sci. 2018;4(10):1344–1351. doi:10.1021/acscentsci.8b00383
17. Peng Q, Shao XR, Xie J, et al. Understanding the biomedical effects of the self-assembled tetrahedral DNA nanostructure on living cells. ACS Appl Mater Interfaces. 2016;8(20):12733–12739. doi:10.1021/acsami.6b03786
18. Liu N, Zhang X, Li N, et al. Tetrahedral framework nucleic acids promote corneal epithelial wound healing in vitro and in vivo. Small. 2019;15(31):e1901907. doi:10.1002/smll.201901907
19. Shao X, Lin S, Peng Q, et al. Tetrahedral DNA nanostructure: a potential promoter for cartilage tissue regeneration via regulating chondrocyte phenotype and proliferation. Small. 2017;13(12). doi:10.1002/smll.201602770
20. Li H, Han M, Weng X, Zhang Y, Li J. DNA-tetrahedral-nanostructure-based entropy-driven amplifier for high-performance photoelectrochemical biosensing. ACS Nano. 2021;15(1):1710–1717. doi:10.1021/acsnano.0c09374
21. Zhang T, Tian T, Zhou R, et al. Design, fabrication and applications of tetrahedral DNA nanostructure-based multifunctional complexes in drug delivery and biomedical treatment. Nat Protoc. 2020;15(8):2728–2757. doi:10.1038/s41596-020-0355-z
22. Mathur D, Rogers KE, Díaz SA, et al. Determining the cytosolic stability of small DNA nanostructures in cellula. Nano Lett. 2022;22(12):5037–5045. doi:10.1021/acs.nanolett.2c00917
23. Xiao D, Li Y, Tian T, et al. Tetrahedral framework nucleic acids loaded with aptamer AS1411 for siRNA delivery and gene silencing in malignant melanoma. ACS Appl Mater Interfaces. 2021;13(5):6109–6118. doi:10.1021/acsami.0c23005
24. Zhao D, Liu M, Li J, et al. Angiogenic aptamer-modified tetrahedral framework nucleic acid promotes angiogenesis in vitro and in vivo. ACS Appl Mater Interfaces. 2021;13(25):29439–29449. doi:10.1021/acsami.1c08565
25. Sirong S, Yang C, Taoran T, et al. Effects of tetrahedral framework nucleic acid/wogonin complexes on osteoarthritis. Bone Res. 2020;8:6. doi:10.1038/s41413-019-0077-4
26. Wiraja C, Zhu Y, Lio DCS, et al. Framework nucleic acids as programmable carrier for transdermal drug delivery. Nat Commun. 2019;10(1):1147. doi:10.1038/s41467-019-09029-9
27. Chai H, Tang Y, Miao P. Tetrahedral DNA supported walking nanomachine for ultrasensitive miRNA detection in cancer cells and serums. Anal Chem. 2022;94(28):9975–9980. doi:10.1021/acs.analchem.2c02288
28. Zhu J, Guo Z, Cui J, Miao P. Partial collapse of DNA tetrahedron for miRNA assay with duplex-specific nuclease-assisted amplification. Analyst. 2023;148(3):512–515. doi:10.1039/D2AN01889F
29. Yang F, Li Q, Wang L, Zhang G-J, Fan C. Framework-nucleic-acid-enabled biosensor development. ACS Sens. 2018;3(5):903–919. doi:10.1021/acssensors.8b00257
30. Alexandrov K, Vickers CE. In vivo protein-based biosensors: seeing metabolism in real time. Trends Biotechnol. 2023;41(1):19–26. doi:10.1016/j.tibtech.2022.07.002
31. Chai H, Wang M, Tang L, Miao P. Ultrasensitive electrochemical detection of miRNA coupling tetrahedral DNA modified gold nanoparticles tags and catalyzed hairpin assembly. Anal Chim Acta. 2021;1165:338543. doi:10.1016/j.aca.2021.338543
32. Ma W, Zhan Y, Zhang Y, et al. An intelligent DNA nanorobot with in vitro enhanced protein lysosomal degradation of HER2. Nano Lett. 2019;19(7):4505–4517. doi:10.1021/acs.nanolett.9b01320
33. Zhou M, Liu N, Zhang Q, et al. Effect of tetrahedral DNA nanostructures on proliferation and osteogenic differentiation of human periodontal ligament stem cells. Cell Prolif. 2019;52(3):e12566. doi:10.1111/cpr.12566
34. Ma W, Zhan Y, Zhang Y, Xie X, Mao C, Lin Y. Enhanced neural regeneration with a concomitant treatment of framework nucleic acid and stem cells in spinal cord injury. ACS Appl Mater Interfaces. 2020;12(2):2095–2106. doi:10.1021/acsami.9b19079
35. Li S, Liu Y, Tian T, et al. Bioswitchable delivery of microRNA by framework nucleic acids: application to bone regeneration. Small. 2021;17(47):e2104359. doi:10.1002/smll.202104359
36. Afewerki S, Bassous N, Harb S, et al. Advances in dual functional antimicrobial and osteoinductive biomaterials for orthopaedic applications. Nanomedicine. 2020;24:102143. doi:10.1016/j.nano.2019.102143
37. Baker CE, Moore-Lotridge SN, Hysong AA, et al. Bone fracture acute phase response-a unifying theory of fracture repair: clinical and scientific implications. Clin Rev Bone Miner Metab. 2018;16(4):142–158. doi:10.1007/s12018-018-9256-x
38. Lu H, Liu Y, Guo J, Wu H, Wang J, Wu G. Biomaterials with antibacterial and osteoinductive properties to repair infected bone defects. Int J Mol Sci. 2016;17(3):334. doi:10.3390/ijms17030334
39. Depypere M, Morgenstern M, Kuehl R, et al. Pathogenesis and management of fracture-related infection. Clin Microbiol Infect. 2020;26(5):572–578. doi:10.1016/j.cmi.2019.08.006
40. Xia J, Gao J, Tang W. Nosocomial infection and its molecular mechanisms of antibiotic resistance. Biosci Trends. 2016;10(1):14–21. doi:10.5582/bst.2016.01020
41. Schilcher K, Horswill AR. Staphylococcal biofilm development: structure, regulation, and treatment strategies. Microbiol Mol Biol Rev. 2020;84(3). doi:10.1128/MMBR.00026-19
42. Arciola CR, Campoccia D, Ehrlich GD, Montanaro L. Biofilm-based implant infections in orthopaedics. Adv Exp Med Biol. 2015;830:29–46.
43. Goodman RP, Schaap IA, Tardin CF, et al. Rapid chiral assembly of rigid DNA building blocks for molecular nanofabrication. Science. 2005;310(5754):1661–1665.
44. Zhang T, Tian T, Lin Y. Functionalizing framework nucleic-acid-based nanostructures for biomedical application. Adv Mater. 2021;2021;e2107820.
45. Shi S, Lin S, Shao X, Li Q, Tao Z, Lin Y. Modulation of chondrocyte motility by tetrahedral DNA nanostructures. Cell Prolif. 2017;50(5):e12368. doi:10.1111/cpr.12368
46. Zhou M, Zhang T, Zhang B, et al. A DNA nanostructure-based neuroprotectant against neuronal apoptosis via inhibiting toll-like receptor 2 signaling pathway in acute ischemic stroke. ACS Nano. 2021;16(1):1456–1470.
47. Lin S, Zhang Q, Zhang T, et al. Tetrahedral DNA nanomaterial regulates the biological behaviors of adipose-derived stem cells via DNA methylation on Dlg3. ACS Appl Mater Interfaces. 2018;10(38):32017–32025. doi:10.1021/acsami.8b12408
48. Ma W, Shao X, Zhao D, et al. Self-assembled tetrahedral DNA nanostructures promote neural stem cell proliferation and neuronal differentiation. ACS Appl Mater Interfaces. 2018;10(9):7892–7900. doi:10.1021/acsami.8b00833
49. Ma W, Xie X, Shao X, et al. Tetrahedral DNA nanostructures facilitate neural stem cell migration via activating RHOA/ROCK2 signalling pathway. Cell Prolif. 2018;51(6):e12503. doi:10.1111/cpr.12503
50. Yao Y, Wen Y, Li Y, et al. Tetrahedral framework nucleic acids facilitate neurorestoration of facial nerves by activating the NGF/PI3K/AKT pathway. Nanoscale. 2021;13(37):15598–15610. doi:10.1039/D1NR04619E
51. Gao S, Wang Y, Li Y, et al. Tetrahedral framework nucleic acids reestablish immune tolerance and restore saliva secretion in a Sjögren’s syndrome mouse model. ACS Appl Mater Interfaces. 2021;13(36):42543–42553. doi:10.1021/acsami.1c14861
52. Renehan AG, Booth C, Potten CS. What is apoptosis, and why is it important? BMJ. 2001;322(7301):1536–1538. doi:10.1136/bmj.322.7301.1536
53. Qin X, Li N, Zhang M, et al. Tetrahedral framework nucleic acids prevent retina ischemia-reperfusion injury from oxidative stress via activating the Akt/Nrf2 pathway. Nanoscale. 2019;11(43):20667–20675. doi:10.1039/C9NR07171G
54. Walsh AS, Yin H, Erben CM, Wood MJ, Turberfield AJ. DNA cage delivery to mammalian cells. ACS Nano. 2011;5(7):5427–5432. doi:10.1021/nn2005574
55. Kim KR, Kim DR, Lee T, et al. Drug delivery by a self-assembled DNA tetrahedron for overcoming drug resistance in breast cancer cells. Chem Commun. 2013;49(20):2010–2012. doi:10.1039/c3cc38693g
56. Liang L, Li J, Li Q, et al. Single-particle tracking and modulation of cell entry pathways of a tetrahedral DNA nanostructure in live cells. Angew Chem Int Ed Engl. 2014;53(30):7745–7750. doi:10.1002/anie.201403236
57. Zhang Q, Jiang Q, Li N, et al. DNA origami as an in vivo drug delivery vehicle for cancer therapy. ACS Nano. 2014;8(7):6633–6643. doi:10.1021/nn502058j
58. Wei M, Li S, Yang Z, Cheng C, Li T, Le W. Tetrahedral DNA nanostructures functionalized by multivalent microRNA132 antisense oligonucleotides promote the differentiation of mouse embryonic stem cells into dopaminergic neurons. Nanomedicine. 2021;34:102375. doi:10.1016/j.nano.2021.102375
59. Zhang M, Zhang X, Tian T, et al. Anti-inflammatory activity of curcumin-loaded tetrahedral framework nucleic acids on acute gouty arthritis. Bioact Mater. 2022;8:368–380. doi:10.1016/j.bioactmat.2021.06.003
60. Tian TR, Xiao DX, Zhang T, et al. A framework nucleic acid based robotic nanobee for active targeting therapy. Adv Funct Mater. 2021;31(5):2007342. doi:10.1002/adfm.202007342
61. Li J, Pei H, Zhu B, et al. Self-assembled multivalent DNA nanostructures for noninvasive intracellular delivery of immunostimulatory CpG oligonucleotides. ACS Nano. 2011;5(11):8783–8789. doi:10.1021/nn202774x
62. He P, Han W, Bi C, et al. Many birds, one stone: a smart nanodevice for ratiometric dual-spectrum assay of intracellular MicroRNA and multimodal synergetic cancer therapy. ACS Nano. 2021;15(4):6961–6976. doi:10.1021/acsnano.0c10844
63. Lee H, Lytton-Jean AK, Chen Y, et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat Nanotechnol. 2012;7(6):389–393. doi:10.1038/nnano.2012.73
64. Qin X, Xiao L, Li N, et al. Tetrahedral framework nucleic acids-based delivery of microRNA-155 inhibits choroidal neovascularization by regulating the polarization of macrophages. Bioact Mater. 2022;14:134–144. doi:10.1016/j.bioactmat.2021.11.031
65. Leigh DA. Antibacterial activity and pharmacokinetics of clindamycin. J Antimicrob Chemother. 1981;7(Suppl A):3–9. doi:10.1093/jac/7.suppl_A.3
66. Li Y, Gao S, Shi S, et al. Tetrahedral framework nucleic acid-based delivery of resveratrol alleviates insulin resistance: from innate to adaptive immunity. Nanomicro Lett. 2021;13(1):86. doi:10.3847/1538-4357/ac06c8
67. Huang Y, Huang W, Chan L, Zhou B, Chen T. A multifunctional DNA origami as carrier of metal complexes to achieve enhanced tumoral delivery and nullified systemic toxicity. Biomaterials. 2016;103:183–196. doi:10.1016/j.biomaterials.2016.06.053
68. Ozhalici-Unal H, Armitage BA. Fluorescent DNA nanotags based on a self-assembled DNA tetrahedron. ACS Nano. 2009;3(2):425–433. doi:10.1021/nn800727x
69. Erben CM, Goodman RP, Turberfield AJ. Single-molecule protein encapsulation in a rigid DNA cage. Angew Chem Int Ed Engl. 2006;45(44):7414–7417. doi:10.1002/anie.200603392
70. Agudelo D, Bourassa P, Bérubé G, Tajmir-Riahi HA. Review on the binding of anticancer drug doxorubicin with DNA and tRNA: structural models and antitumor activity. J Photochem Photobiol B. 2016;158:274–279. doi:10.1016/j.jphotobiol.2016.02.032
71. Ijäs H, Shen B, Heuer-Jungemann A, et al. Unraveling the interaction between doxorubicin and DNA origami nanostructures for customizable chemotherapeutic drug release. Nucleic Acids Res. 2021;49(6):3048–3062. doi:10.1093/nar/gkab097
72. Setyawati MI, Kutty RV, Tay CY, Yuan X, Xie J, Leong DT. Novel theranostic DNA nanoscaffolds for the simultaneous detection and killing of Escherichia coli and Staphylococcus aureus. ACS Appl Mater Interfaces. 2014;6(24):21822–21831. doi:10.1021/am502591c
73. Zhang Y, Ma W, Zhu Y, et al. Inhibiting methicillin-resistant Staphylococcus aureus by tetrahedral DNA nanostructure-enabled antisense peptide nucleic acid delivery. Nano Lett. 2018;18(9):5652–5659. doi:10.1021/acs.nanolett.8b02166
74. Liu Y, Sun Y, Li S, et al. Tetrahedral framework nucleic acids deliver antimicrobial peptides with improved effects and less susceptibility to bacterial degradation. Nano Lett. 2020;20(5):3602–3610. doi:10.1021/acs.nanolett.0c00529
75. Sun Y, Liu Y, Zhang B, et al. Erythromycin loaded by tetrahedral framework nucleic acids are more antimicrobial sensitive against Escherichia coli (E. coli). Bioact Mater. 2021;6(8):2281–2290. doi:10.1016/j.bioactmat.2020.12.027
76. Sun Y, Li S, Zhang Y, et al. Tetrahedral framework nucleic acids loading ampicillin improve the drug susceptibility against methicillin-resistant Staphylococcus aureus. ACS Appl Mater Interfaces. 2020;12(33):36957–36966. doi:10.1021/acsami.0c11249
77. Zhang Y, Xie X, Ma W, et al. Multi-targeted antisense oligonucleotide delivery by a framework nucleic acid for inhibiting biofilm formation and virulence. Nanomicro Lett. 2020;12(1):74. doi:10.1007/s40820-020-0409-3
78. Bispo PJM, Sahm DF, Asbell PA. A systematic review of multi-decade antibiotic resistance data for ocular bacterial pathogens in the United States. Ophthalmol Ther. 2022;11(2):503–520. doi:10.1007/s40123-021-00449-9
79. Kalan L, Wright GD. Antibiotic adjuvants: multicomponent anti-infective strategies. Expert Rev Mol Med. 2011;13:e5. doi:10.1017/S1462399410001766
80. Skov R, Varga A, Matuschek E, et al. EUCAST disc diffusion criteria for the detection of mecA-mediated β-lactam resistance in Staphylococcus pseudintermedius: oxacillin versus cefoxitin. Clin Microbiol Infect. 2020;26(1):122.e121–122.e126. doi:10.1016/j.cmi.2019.05.002
81. Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol Rev. 2008;32(2):234–258. doi:10.1111/j.1574-6976.2008.00105.x
82. Hawkey PM, Warren RE, Livermore DM, et al. Treatment of infections caused by multidrug-resistant gram-negative bacteria: report of the British society for antimicrobial chemotherapy/healthcare infection society/British infection association joint working party. J Antimicrob Chemother. 2018;73(suppl_3):iii2–iii78. doi:10.1093/jac/dky027
83. Roth N, Käsbohrer A, Mayrhofer S, Zitz U, Hofacre C, Domig KJ. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: a global overview. Poult Sci. 2019;98(4):1791–1804. doi:10.3382/ps/pey539
84. Baron SA, Rolain JM. Efflux pump inhibitor CCCP to rescue colistin susceptibility in mcr-1 plasmid-mediated colistin-resistant strains and gram-negative bacteria. J Antimicrob Chemother. 2018;73(7):1862–1871. doi:10.1093/jac/dky134
85. Hyrup B, Nielsen PE. Peptide nucleic acids (PNA): synthesis, properties and potential applications. Bioorg Med Chem. 1996;4(1):5–23. doi:10.1016/0968-0896(95)00171-9
86. Demidov VV, Potaman VN, Frank-Kamenetskii MD, et al. Stability of peptide nucleic acids in human serum and cellular extracts. Biochem Pharmacol. 1994;48(6):1310–1313. doi:10.1016/0006-2952(94)90171-6
87. Nielsen PE. PNA technology. Mol Biotechnol. 2004;26(3):233–248. doi:10.1385/MB:26:3:233
88. Demidov VV, Frank-Kamenetskii MD. Two sides of the coin: affinity and specificity of nucleic acid interactions. Trends Biochem Sci. 2004;29(2):62–71. doi:10.1016/j.tibs.2003.12.007
89. Narenji H, Gholizadeh P, Aghazadeh M, Rezaee MA, Asgharzadeh M, Kafil HS. Peptide nucleic acids (PNAs): currently potential bactericidal agents. Biomed Pharmacother. 2017;93:580–588. doi:10.1016/j.biopha.2017.06.092
90. Równicki M, Wojciechowska M, Wierzba AJ, et al. Vitamin B(12) as a carrier of peptide nucleic acid (PNA) into bacterial cells. Sci Rep. 2017;7(1):7644. doi:10.1038/s41598-017-08032-8
91. Quijano E, Bahal R, Ricciardi A, Saltzman WM, Glazer PM. Therapeutic peptide nucleic acids: principles, limitations, and opportunities. Yale J Biol Med. 2017;90(4):583–598.
92. Readman JB, Dickson G, Coldham NG. Tetrahedral DNA nanoparticle vector for intracellular delivery of targeted peptide nucleic acid antisense agents to restore antibiotic sensitivity in cefotaxime-resistant Escherichia coli. Nucleic Acid Ther. 2017;27(3):176–181. doi:10.1089/nat.2016.0644
93. Readman JB, Dickson G, Coldham NG. Translational inhibition of CTX-M extended spectrum β-lactamase in clinical strains of Escherichia coli by synthetic antisense oligonucleotides partially restores sensitivity to cefotaxime. Front Microbiol. 2016;7:373. doi:10.3389/fmicb.2016.00373
94. Haydon DJ, Stokes NR, Ure R, et al. An inhibitor of FtsZ with potent and selective anti-staphylococcal activity. Science. 2008;321(5896):1673–1675. doi:10.1126/science.1159961
95. Zhang T, Cui W, Tian T, Shi S, Lin Y. Progress in biomedical applications of tetrahedral framework nucleic acid-based functional systems. ACS Appl Mater Interfaces. 2020;12(42):47115–47126. doi:10.1021/acsami.0c13806
96. Chen X, Han J, Cai X, Wang S. Antimicrobial peptides: sustainable application informed by evolutionary constraints. Biotechnol Adv. 2022;60:108012. doi:10.1016/j.biotechadv.2022.108012
97. Gong T, Fu J, Shi L, Chen X, Zong X. Antimicrobial peptides in gut health: a review. Front Nutr. 2021;8:751010. doi:10.3389/fnut.2021.751010
98. Almaaytah A, Mohammed GK, Abualhaijaa A, Al-Balas Q. Development of novel ultrashort antimicrobial peptide nanoparticles with potent antimicrobial and antibiofilm activities against multidrug-resistant bacteria. Drug Des Devel Ther. 2017;11:3159–3170. doi:10.2147/DDDT.S147450
99. Zhang B, Qin X, Zhou M, et al. Tetrahedral DNA nanostructure improves transport efficiency and anti-fungal effect of histatin 5 against Candida albicans. Cell Prolif. 2021;54(5):e13020. doi:10.1111/cpr.13020
100. Luo Y, Song Y. Mechanism of antimicrobial peptides: antimicrobial, anti-inflammatory and antibiofilm activities. Int J Mol Sci. 2021;22(21):11401. doi:10.3390/ijms222111401
101. Cheng Q, Zeng K, Kang Q, et al. The antimicrobial peptide LL-37 promotes migration and odonto/osteogenic differentiation of stem cells from the apical papilla through the Akt/Wnt/β-catenin signaling pathway. J Endod. 2020;46(7):964–972. doi:10.1016/j.joen.2020.03.013
102. Bennett CF. Therapeutic antisense oligonucleotides are coming of age. Annu Rev Med. 2019;70(1):307–321. doi:10.1146/annurev-med-041217-010829
103. Chi X, Gatti P, Papoian T. Safety of antisense oligonucleotide and siRNA-based therapeutics. Drug Discov Today. 2017;22(5):823–833. doi:10.1016/j.drudis.2017.01.013
104. Turnbull G, Clarke J, Picard F, et al. 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater. 2018;3(3):278–314. doi:10.1016/j.bioactmat.2017.10.001
105. Roddy E, DeBaun MR, Daoud-Gray A, Yang YP, Gardner MJ. Treatment of critical-sized bone defects: clinical and tissue engineering perspectives. Eur J Orthop Surg Traumatol. 2018;28(3):351–362. doi:10.1007/s00590-017-2063-0
106. García-Gareta E, Coathup MJ, Blunn GW. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone. 2015;81:112–121. doi:10.1016/j.bone.2015.07.007
107. Shao XR, Lin SY, Peng Q, et al. Effect of tetrahedral DNA nanostructures on osteogenic differentiation of mesenchymal stem cells via activation of the Wnt/β-catenin signaling pathway. Nanomedicine. 2017;13(5):1809–1819. doi:10.1016/j.nano.2017.02.011
108. Shi SR, Tian TR, Li YJ, et al. Tetrahedral framework nucleic acid inhibits chondrocyte apoptosis and oxidative stress through activation of autophagy. ACS Appl Mater Interfaces. 2020;12(51):56782–56791. doi:10.1021/acsami.0c17307
109. Shi S, Peng Q, Shao X, et al. Self-assembled tetrahedral DNA nanostructures promote adipose-derived stem cell migration via lncRNA XLOC 010623 and RHOA/ROCK2 signal pathway. ACS Appl Mater Interfaces. 2016;8(30):19353–19363. doi:10.1021/acsami.6b06528
110. Zhou M, Liu NX, Shi SR, et al. Effect of tetrahedral DNA nanostructures on proliferation and osteo/odontogenic differentiation of dental pulp stem cells via activation of the notch signaling pathway. Nanomedicine. 2018;14(4):1227–1236. doi:10.1016/j.nano.2018.02.004
111. Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149–155. doi:10.1016/S0140-6736(04)16627-0
112. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038. doi:10.1038/nrdp.2017.38
113. Zhang Q, Lin S, Shi S, et al. Anti-inflammatory and antioxidative effects of tetrahedral DNA nanostructures via the modulation of macrophage responses. ACS Appl Mater Interfaces. 2018;10(4):3421–3430. doi:10.1021/acsami.7b17928
114. Zhou M, Gao S, Zhang X, et al. The protective effect of tetrahedral framework nucleic acids on periodontium under inflammatory conditions. Bioact Mater. 2021;6(6):1676–1688. doi:10.1016/j.bioactmat.2020.11.018
115. Zhuang Y, Cui W. Biomaterial-based delivery of nucleic acids for tissue regeneration. Adv Drug Deliv Rev. 2021;176:113885. doi:10.1016/j.addr.2021.113885
116. García-Sánchez D, González-González A, García-García P, et al. Effective osteogenic priming of mesenchymal stem cells through LNA-ASOs-mediated Sfrp1 gene silencing. Pharmaceutics. 2021;13(8):1277. doi:10.3390/pharmaceutics13081277
117. Son J, Kim J, Lee K, et al. DNA aptamer immobilized hydroxyapatite for enhancing angiogenesis and bone regeneration. Acta Biomater. 2019;99:469–478. doi:10.1016/j.actbio.2019.08.047
118. Jiang W, Zhu P, Huang F, et al. The RNA methyltransferase METTL3 promotes endothelial progenitor cell angiogenesis in mandibular distraction osteogenesis via the PI3K/AKT pathway. Front Cell Dev Biol. 2021;9:720925. doi:10.3389/fcell.2021.720925
119. Zhou X, Cao H, Yuan Y, Wu W. Biochemical signals mediate the crosstalk between cartilage and bone in osteoarthritis. Biomed Res Int. 2020;2020:5720360. doi:10.1155/2020/5720360
120. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–222. doi:10.1038/nrd.2016.246
121. Li D, Yang Z, Luo Y, Zhao X, Tian M, Kang P. Delivery of MiR335-5p-pendant tetrahedron DNA nanostructures using an injectable heparin lithium hydrogel for challenging bone defects in steroid-associated osteonecrosis. Adv Healthc Mater. 2022;11(1):e2101412. doi:10.1002/adhm.202101412
122. Yang Z, Yi P, Liu Z, et al. Stem cell-laden hydrogel-based 3D bioprinting for bone and cartilage tissue engineering. Front Bioeng Biotechnol. 2022;10:865770. doi:10.3389/fbioe.2022.865770
123. Li J, Lai Y, Li M, et al. Repair of infected bone defect with clindamycin-tetrahedral DNA nanostructure complex-loaded 3D bioprinted hybrid scaffold. Chem Eng J. 2022;435:134855. doi:10.1016/j.cej.2022.134855
124. Veronesi F, Giavaresi G, Tschon M, Borsari V, Nicoli Aldini N, Fini M. Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev. 2013;22(2):181–192. doi:10.1089/scd.2012.0373
125. Qi C, Liu J, Jin Y, et al. Photo-crosslinkable, injectable sericin hydrogel as 3D biomimetic extracellular matrix for minimally invasive repairing cartilage. Biomaterials. 2018;163:89–104. doi:10.1016/j.biomaterials.2018.02.016
126. Cui P, Pan P, Qin L, et al. Nanoengineered hydrogels as 3D biomimetic extracellular matrix with injectable and sustained delivery capability for cartilage regeneration. Bioact Mater. 2023;19:487–498. doi:10.1016/j.bioactmat.2022.03.032
127. Niemeyer P, Albrecht D, Andereya S, et al. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: a guideline by the working group “clinical tissue regeneration” of the German Society of Orthopaedics and Trauma (DGOU). Knee. 2016;23(3):426–435. doi:10.1016/j.knee.2016.02.001
128. Ponticiello MS, Schinagl RM, Kadiyala S, Barry FP. Gelatin-based resorbable sponge as a carrier matrix for human mesenchymal stem cells in cartilage regeneration therapy. J Biomed Mater Res. 2000;52(2):246–255. doi:10.1002/1097-4636(200011)52:2<246::AID-JBM2>3.0.CO;2-W
129. Giannoni P, Pagano A, Maggi E, et al. Autologous chondrocyte implantation (ACI) for aged patients: development of the proper cell expansion conditions for possible therapeutic applications. Osteoarthritis Cartilage. 2005;13(7):589–600. doi:10.1016/j.joca.2005.02.015
130. Yin L, Wu Y, Yang Z, et al. Characterization and application of size-sorted zonal chondrocytes for articular cartilage regeneration. Biomaterials. 2018;165:66–78. doi:10.1016/j.biomaterials.2018.02.050
131. Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115–2126. doi:10.1016/S0140-6736(11)60243-2
132. Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376–387. doi:10.1016/S0140-6736(14)60802-3
133. Kim JH, Jeon J, Shin M, et al. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell. 2014;156(4):730–743. doi:10.1016/j.cell.2014.01.007
134. Lin SY, Zhang Q, Li SH, et al. Antioxidative and angiogenesis-promoting effects of tetrahedral framework nucleic acids in diabetic wound healing with activation of the Akt/Nrf2/HO-1 pathway. ACS Appl Mater Interfaces. 2020;12(10):11397–11408. doi:10.1021/acsami.0c00874
135. Li P, Fu L, Liao Z, et al. Chitosan hydrogel/3D-printed poly(ε-caprolactone) hybrid scaffold containing synovial mesenchymal stem cells for cartilage regeneration based on tetrahedral framework nucleic acid recruitment. Biomaterials. 2021;278:121131. doi:10.1016/j.biomaterials.2021.121131
136. De bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–1942. doi:10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P
137. Jones BA, Pei M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng Part B Rev. 2012;18(4):301–311. doi:10.1089/ten.teb.2012.0002
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

