Back to Journals » Infection and Drug Resistance » Volume 15
The Correlations of Minimal Inhibitory Concentration Values of Anti-TB Drugs with Treatment Outcomes and Clinical Profiles in Patients with Multidrug-Resistant Tuberculosis (MDR-TB) in China
Authors Tang Q , Ke H, Sun WW, Zhang SJ, Fan L
Received 16 May 2022
Accepted for publication 20 August 2022
Published 7 September 2022 Volume 2022:15 Pages 5275—5287
DOI https://doi.org/10.2147/IDR.S374687
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Suresh Antony
Qin Tang, Hui Ke, Wen-wen Sun, Shao-jun Zhang, Lin Fan
Department of Tuberculosis, Shanghai Clinical Research Center for Tuberculosis, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, Shanghai Clinical Research Center for Tuberculosis, Shanghai Key Laboratory of Tuberculosis, Shanghai, 200433, People’s Republic of China
Correspondence: Lin Fan, Email [email protected]
Objective: It is a challenge to obtain satisfactory treatment outcomes for patients with multidrug-resistant/rifampicin-resistant tuberculosis (MDR/RR-TB); the study aims to correlate the Minimum Inhibitory Concentration (MIC) value of drugs with the outcome of patients with MDR/RR-TB to obtain an understanding for better regimens and optimal outcomes.
Methods: The patients diagnosed with MDR/RR-TB were retrospectively enrolled from January 1, 2018 to December 31, 2019, recorded clinical characteristics, MIC DST (Drug Susceptibility Test) results, and followed the treatment outcome. The data were analyzed on the correlations of MIC DST values with outcomes and clinical characteristics.
Results: A total of 276 patients with MDR/RR-TB were included, containing 98 cases (35.5%) with newly treated patients and 178 cases (64.5%) with re-treated patients. A total of 220 cases recorded treatment success (79.7%) and 49 cases recorded treatment failure or died. MIC values of isoniazid (H), moxifloxacin (Mfx), and ethionamide (Eto) in newly treated patients were lower than those in retreated patients, and resistance levels of Mfx and H were closely associated with the treatment outcome (P < 0.05) while those of other drugs had no close association with treatment outcome.
Conclusions: MIC values of some anti-TB drugs, such as fluoroquinolones (FQs) and H, can reflect the treatment outcome for patients with MDR/RR-TB, which can contribute to making regimens for better treatment outcomes.
Keywords: MIC value, resistance, MDR/RR-TB, newly treated, retreated, treatment outcome
Introduction
Tuberculosis (TB) remains a threat to global public health as one of the leading infectious diseases. The transmission of drug-resistant mycobacterium tuberculosis (MTB) exacerbates the challenge of TB control. According to the updated WHO report, TB was estimated at 9.9 million new cases and lead to 1.3 million deaths among the HIV-negative population in 2020, worldwide,1 MDR/RR-TB were referred to as MTB isolates at least resistant to H and rifampicin (R), the average treatment success rate was 59% globally in 2020 while it was varied among different regions under varied health conditions.
Making effective regimens for MDR/RR-TB patients is the key factor in obtaining a favorable outcome. Regimens containing novel anti-TB drugs such as bedaquiline can get a 91.2% sputum culture conversion rate at the end of treatment, a 71.3% success rate for MDR and XDR-TB patients was reported in 2017,2 the culture conversion rate reached 84.6% for MDR-TB, 83.9% for pre-XDR-TB and 86.6% for XDR-TB reported in China in 2021.3 Nix-TB regimen for XDR-TB or MDR-TB not responsive or not tolerant to treatment containing bedaquiline or pretomanid, and linezolid obtained 90% of the favorable outcome.4 Regimens containing delamanid reached 78.9% of the overall treatment success rate for MDR-TB patients.5 One study6 showed that the predictors of poor treatment outcomes for MDR-TB included resistance to second-line drugs, age, social or economic status, and so on, among which drug resistance was one of the most important factors. Therefore, having an accurate DST is essential to making efficient regimens for drug-resistant patients. However, the correlations of drug-resistant levels with treatment outcomes for patients with MDR/RR-TB remain imprecise.
DST methods usually contain traditional DST based on liquid or solid culture method (L-J or BACTEC MGIT system) as the phenotype method and molecular DST based on PCR and hybridization technique as genotype method (Xpert MTB/RIF, molecular linear probe hybridization, etc). The phenotype method is time-consuming, accurate, and can test at least 10 drugs while the genotype method is fast and accurate but only tests R (such as Xpert MTB/RIF) or H and R, and two second-line drug resistances (such as molecular linear probe hybridization). Therefore both DST methods have advantages and disadvantages. However, MIC culture and DST assays were applied in clinics and can provide more detailed information about the resistance level of each drug, MIC values from each drug are likely to be different among different patients with MDR-TB. A high level of H resistance or low level of H resistance was evaluated by MIC DST according to a MIC of >1 μg/mL as the cut-off value, which is the key issue to influence the efficacy of anti-TB regimens.7 Establishing the MIC distribution of some second-line drugs such as aminoglycosides and cyclic polypeptides antibiotics is important to improve the quality of drug susceptibility testing against MTB.8 Altogether, the MIC DST method is the high throughput methodology for MTB which can provide the routine breakpoint during DST and MIC determination.9 However, the decrease in dilution of Pyrazinamide (Z) MIC for Z-susceptible strains was not associated with sputum culture conversion and treatment outcome as previously reported.10 Therefore it is important to figure out the correlations of drug resistance degree with the treatment outcome and clinical profile of a patient with MDR-TB to make a better regimen and obtain the optimal outcome.
Except for Z, the correlation of MIC values for each drug with treatment outcome remains elusive. The present study was designed as a retrospective analysis, to follow up enrolled patients with MDR/RR-TB during all courses of treatment, and try to find some clues to guide making regimens for presuming the optimal outcome of patients with MDR/RR-TB.
Methods
Study Design and Patients
Patients diagnosed with MDR/RR-pulmonary TB were retrospectively enrolled from January 1, 2018 to December 31, 2019 in Shanghai Pulmonary Hospital, a pulmonary disease specialized hospital that admitted patients from four provinces and one municipality including Zhejiang, Anhui, Jiangsu, Jiangxi Province and Shanghai in eastern China. Included criteria were as follows: patients diagnosed as having MDR-TB/RR and HIV negative, confirmed by MTB culture of MIC DST and Xpert MTB/RIF, the DST results were repeated by the other respiratory sample if the DST results were not in accordance with clinics. Patients received chemotherapy for MDR/RR-TB in the hospital after consultation and confirmed by an expert group organized by Shanghai municipal CDC and received management in the county CDC nearby. MDR/RR-TB diagnosis based on MIC DST and Xpert MTB/RIF were performed according to the WHO guidelines.11 Excluded criteria were as follows: patients with HIV infection; patients infected with non-tuberculous mycobacteria (NTM); or patients switched to other hospitals after starting the regimen. Patients had been undergone MIC DST before receiving the treatment which was followed by a culture every three months during the whole course of treatment. All patients enrolled in the study were followed-up till one year after stopping the treatment.
MDR-TB refers to patients with a culture of MTB DST which is at least resistant to isoniazid (H) and rifampicin (R), XDR-TB is referred to for patients with a culture MTB DST resistant to at least H and R and additionally resistant to fluoroquinolones (FQs) or any second-line injectable agents (amikacin, kanamycin or capreomycin) according to the previous WHO guidelines. RR-TB is referred to for patients with a culture MTB DST resistant to R, RR-TB should include MDR-TB and XDR-TB.
Newly diagnosed MDR/RR-TB refers to patients without previous anti-TB treatment history or with less than one month of previous treatment history. Re-treated MDR/RR-TB refers to patients with previous anti-TB treatment history for more than one month, which included patients with a one-time treatment history and more than a one-time treatment history. The one-time treatment history should be referred to a patient with one-time drug-sensitive TB treatment history and having failed or relapsed, more than a one-time treatment history is referred to retreated patients having more than a one-time of TB treatment history and having failed or relapsed at the most recent treatment.
Ethical Approval
The present study was performed under the Declaration of Helsinki about ethical principles for research. This retrospective study was approved by The Ethics Committee of Shanghai Pulmonary Hospital (approval number K20-430). Since the study was retrospective, the written informed consent had been waived by the ethics committee, but it had been guaranteed that the privacy of patients enrolled was totally protected in this study.
MTB Culture and Identification
Sputum or BALF (bronchoalveolar lavage fluid) specimens were tested by MTB BACTEC MGIT 960 culture (Becton Dickinson, Cockeysville, MD, USA), which was performed by the manufacturer’s instructions. Positive culture isolates were validated by the MPT 64 antigen detection kit (Genesis, Hangzhou, China), and NTM was excluded from the study.
Minimum Inhibitory Concentration (MIC) DST of the Drugs
All positive MTB strains were tested by MIC DST and were confirmed by Xpert MTB/RIF (Cepheid, USA), Xpert MTB/RIF was performed according to the manufacturer protocol. MIC DST was performed using Myco TB system (MYCOTB; Trek Diagnostic Systems, ThermoFisher Scientific Inc., USA).12 Laboratory steps of MIC DST were performed by trained persons in biosafety cabinets according to the relevant guidelines. The TREK sensititre MIC plate had been settled by drugs with fixed concentration variation. For bacteria, M. tuberculosis H37Rv was used as a control strain. M. tuberculosis clinical isolates and H37Rv as control in Middlebrook 7H9 broth containing oleic acid-albumin-dextrose-catalase (OADC), vortexed for 30 s and get inoculation fluid with 1×105 cfu/mL (5×104~5×105cfu/mL). To each well of Sensititre Autoinoculator ®/AIM® inoculation MTB MIC plate was added 100 ul of inoculate bacteria fluid (finished adding bacteria fluid in each well were initially cultured in the BACTEC MGIT) 960 system, the bacteria were cultured at 37 °C for 1–7 days for proliferation. 100 μL of culture fluid vortex were inoculated within 30 minutes, covered by velum and then inoculated for 10 days at 35–37 °C in an aerobic environment and examined for the status of growing in control wells after 7–10 days. If the growth of the strain was insufficient to be performed by DST after being cultured for 10 days, it was re-incubated in the plate for up to an additional 11 days. The MIC value was referred to as the lowest drug concentration that inhibited the growth of bacteria. The critical concentration of MTB drug sensitivity test is based on CLSI M24-A2 and FDA-approved standards for drug susceptibility testing. Results can be read using the Vizion® System. The MIC of each strain to the drug higher than (≥) the cut-off drug concentration is referred to be resistant to this drug. The results of MIC DST were shown as quantified data with unit ug/mL. The cut-off concentration recommended were 4 µg/mL for Amikaxin (Ak), 25 µg/mL for Cycloserin (Cs), 5 µg/mL for Ethambutol (E), 0.2 µg/mL for H, 5 µg/mL for Kanamycin (Ka), 2 µg/mL for Aminosalicylic acid (PAS), 1 µg/mL for R, 0.5 µg/mL for moxifloxacin (Mfx), 2 ug/mL for ofloxacin (Ofx), 2 ug/mL for Streptomycin (Sm), 5 µg/mL for Eto.13
Making Regimen, Treatment, and Treatment Efficacy Evaluation
All included patients with MDR/RR-TB received regimens according to the principles of WHO and Chinese guideline recommendations in 2020 and 2019.14,15 All cases were given long-term regimens with a total course of 18–20 months which included injectable agents containing regimens or all-oral regimens if injectable agents were resistant.
Treatment outcomes for MDR-TB were evaluated according to WHO guidelines.16 The treatment outcomes were classified into “cured”, “completed treatment”, “failure”, “default” and “death”. The “cured” is patients who complete the treatment with consistently at least three negative culture results for the final 12 months of the treatment course and without evidence of failure;17 “completed treatment” was determined by bacterial negative conversion at the end of the treatment with less than three negative cultures. The “failure” was patients who had sputum culture-positive in the final 12 months of the treatment course or if any one of the final three cultures was positive or to be discontinued due to clinical or radiological adverse reactions or adverse events. “Death” was for patients who died for any reason during anti-TB treatment (ATT); “default” was a patient whose TB treatment was interrupted for at least two consecutive months for any reason. Favorable treatment outcomes were defined as the sum of “cured” and “treatment completed”, unfavorable outcomes included “failure”, “default” and “death”.18
Statistical Analysis
The statistical analysis was conducted with SPSS 18.0 (IBM Corp, Armonk, NY, USA) and GraphPad Prism (9.0). The baseline data were compared between the MIC values of patients with newly treated and retreated groups and between retreated patients at different times of treatment. Classification variables were described as frequency and percentage and compared using a chi-square test or Fisher's exact test. Continuous variables are described as medians and quartiles. When the data are normally distributed, the t-test was used to compare the mean values of continuous variables, otherwise, the Mann–Whitney test was used. Chi-square analysis was used to compare treatment outcomes including success and failure or death. A difference was considered as significant if the P-value was less than 0.05.
Results
Patients Characteristics
322 cases diagnosed as MDR/RR-TB were enrolled, 46 cases were excluded, including 5 cases due to incomplete record of treatment, 32 cases excluded due to being transferred into other hospitals, and 9 cases lacked culture MIC value results. Finally, a total of 276 cases diagnosed with MDR/RR-pulmonary TB were enrolled in the study with an average age of 41.6 (11–84) years, including males, 188 cases (68.1%) and females, 88 cases (31.9%). Among the 276 patients, there were 247 cases (89.5%) with MDR-TB, 100% cases with RR-TB, 44 cases (15.9%) with XDR-TB, 125 cases (45.3%) with pre-XDR-TB, 114 cases (41.3%) were pre-XDR-TB (FQs resistance), only 11 cases were pre-XDR-TB (second-line injectable agents resistance). Newly treated patients were 98 cases (35.5%) while re-treated patients were 178 cases (64.5%). For complications, coexisted pleural TB had 23 cases, tuberculous lymphadenitis 4 cases, diabetes mellitus 42 cases (15.2%), hepatitis 9 cases (3.3%), anemia 103 cases (37.3%), hypoproteinemia 26 cases (9.4%), hypertension 19 cases (6.9%), bronchiectasis 41 cases (14.9%), COPD 4 cases (1.4%), cardiopathy 5 cases (1.8%). Included flow diagram is shown in Figure 1, and detailed information about the characteristics of patients is given in Table 1.
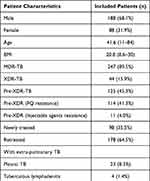 |
Table 1 Clinical Characteristics in All Included Patients with MDR/RR-TB (n = 276) |
 |
Figure 1 Included cases flow diagram. |
220 cases of enrolled patients got treatment success and 48 cases got treatment failure and 1 case died, 7 cases were loss of follow-up. The treatment success rate was 79.7% (220/276) in all patients and was 93.9% (92/98) in newly treatment patients which was significantly higher than 71.9% (128/178) in pretreated patients, p was 0.000 (χ2=18.58).
Comparison of MIC for Anti-TB Drugs Between Newly Treated and Retreated MDR-TB Patients
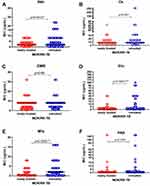
Among the 276 cases enrolled in the study, there were different MIC values of drugs between newly treated and retreated MDR-TB patients. The MIC value of H was significantly lower in newly treated patients (3.1±2.4) than that in retreated patients (5.5±6.8), p was 0.0012; MIC value of Cs seemedlower in newly treated patients (11.9±8.5) than in retreated patients (19.5±38.2) but had no statistical difference (p was 0.5); MIC value of E was similar between newly treated patients and retreated patients (p was 0.7); MIC value of ethionamide (Eto) was significantly lower in newly treated patients (3.2±7.9) than in retreated patients (13.0±23.7), p was 0.0001; MIC value of Mfx was significantly lower in newly treated patients (2.0±3.3) than in retreated patients (4.9±5.1), p was 0.0002; MIC value of PAS seemed lower in newly treated patients (3.6±18.1) than in retreated patients (9.4±29.2) but had no statistical significance (p was 0.08). It was summed up that H, Mfx, and Eto had statistically lower MIC values in newly treated patients than those in retreated patients while Cs, E, and PAS were similar MIC values in the two groups, see Figure 2A–F and Table 2.
 |
Table 2 Average MIC Value (Mean ± SD) Between Newly Treated and Retreated MDR-TB/RR-TB |
Comparison of MIC for Anti-TB Drugs Among Retreated MDR-TB Patients with Different Times of Treatment History
Among 178 retreated patients, there were differences of MIC value of H, Eto, Mfx between patients having one-time treatment history and patients having more than one-time treatment history while the MIC value of Cs and PAS had no statistical differences between the two groups, more specifically, MIC of H was statistically lower in retreated patients having a one-time treatment history (3.5±2.6) than that in retreated patients having more than a one-time treatment (4.5±3.7), p was 0.38; Eto MIC value was similarly lower in patients having a one-time treatment history (9.2±21.6) than that in patients having more than a one-time treatment history (21.2±33.0), p was 0.0008; Mfx MIC value was significantly lower in patients having a one-time treatment history (2.0±3.3) than that in patients having more than a one-time treatment history (4.4±5.2), p was 0.0002; MIC values of Cs and PAS seemed lower in patients having a one-time treatment history than those in patients having more than a one-time treatment history but had no statistical significance (P was 0.1, 0.2 respectively). See the data in Figure 3A–E.
The Correlation of MIC Among FQs, Injectable Agents, and Eto, Isoniazid
To further verify the possible correlation of MIC values among some drugs having close correlation, we made the analysis of correlation of MIC among FQs, injectable agents, Eto and H. The results showed that isoniazid had a moderate positive correlation of MIC with Eto, Pearson r was 0.34, P was <0.0001; kanamycin had a strong positive correlation of MIC with amikacin, Pearson r was 0.95, p was <0.0001; moxifloxacin had a strong positive correlation of MIC value with ofloxacin, Pearson r was 0.88, p was <0.0001, implying some of the strains resistant to isoniazid had a slight possibility of resistance to Eto while most strains resistant to Mfx or Ak had a great possibility of resistance to Ofx or Ka. These results can give a helpful hint to guide making regimen when you only have the DST results of some drugs. See Figure 4A–C.
The Correlation of Treatment Outcome with MIC Values of Drugs in MDR-TB Patients
Apart from 7 cases where there was a loss of follow-up during the treatment, 220 cases got treatment success (79.7%) and 49 cases finished the treatment with the outcome of failure or death (18.4%). To elucidate the correlation of the MIC value of each drug with the treatment outcome, the analysis was performed on patients with treatment success or failure and death. The results showed that patients with Cs MIC who varied from less than 8 ug/mL to more than 32 ug/mL had no statistically different outcomes; patients with Eto MIC who varied from less than 5 ug/mL to more than 40 ug/mL had no significant differences of treatment outcome no matter treatment success or failure or death, while patients with Mfx MIC with less than 1 ug/mL had a greater success rate than those patients with Mfx MIC more than 1 ug/mL, with increased Mfx MIC value beyond 1 ug/mL, the treatment outcome had no obvious differences among patients with varied Mfx MIC. Patients with an Mfx MIC value at 1 ≤ MIC ≤ 4 and ≥8 ug/mL had a greater rate of poor treatment outcome than those with Mfx MIC <1 ug/mL. Besides those patients, patients with H MIC value at 1 < MIC ≤ 4 ug/mL had poorer outcomes than those with H MIC <0.25 ug/mL. The data is shown in Table 3.
 |
Table 3 The Correlations of MIC Value with Treatment Outcome |
Discussion
The present study retrospectively recorded and made the analysis of the correlations between MIC values and treatment outcomes for each patient with MDR/RR-TB. The results showed the interesting findings that the MIC values of some drugs were closely associated with the treatment history of the patients, namely the times of the previous history of anti-TB treatment, some drugs had strong correlations of MIC DST values with treatment outcomes while some drugs did not.
We enrolled 276 patients who satisfied the inclusion criteria, the treatment success rate was 79.7%, and the treatment outcome was better in newly treated patients than in retreated patients (93.9% vs 71.9% in success rate), which was similar to the conclusion from our previous study.19 To excavate the underlying reasons for which aspects possibly influenced the outcome of DR-TB patients, we started with the MIC DST values and tried to find out the correlation based on the MIC values of drugs and the profile of treatment outcomes. We found that the MIC values of drugs from newly treated patients were lower than those from retreated patients, among which FQs, Ak, isoniazid, and Eto had statistically lower MIC values in newly treated patients while E, Cs, and PAS had seemed to have a lower MIC value in newly treated patients but there was no statistical significance. The lack of statistical significance was possibly due to the limited number of cases. The difference in MIC value of drugs, especially FQs, between newly treated patients and retreated patients had the consistency of the differences in the treatment outcome, implying resistance levels or extent of FQs, H, Eto or injectable agents might be able to influence the treatment outcomes for MDR/RR-TB due to different MIC values. FQs resistance is the essential factor to impact the outcome of MDR-TB patients, other reports had similar conclusions.20–22 The present study indicated MIC DST can help to give the hint on the outcome of MDR/RR-TB patients, especially FQs resistance extent since higher MIC values of Mfx had significantly increased poor treatment outcomes (rate of death and failure) as shown in Table 3.
Isoniazid (H), even though most of the patients enrolled in the study were MDR-TB, resistant to R, and susceptible to H had 25 cases occupied 9.1% (25/276), the resistance level for H reflected by MIC value was still an important factor to influence the treatment outcome of patients with MDR/RR-TB as the results show in Table 3. MIC value of H was lower in newly treated patients than in retreated patients, while it also was lower in retreated patients having a one-time treatment history than in patients with more than a one-time treatment history, implying H is likely to be the risk factor to impact treatment outcome for MDR/RR-TB, the previous study also mentions the risk of H resistance on poor treatment outcomes.23 Huyen et al made the analysis and found that katG315 mutations predicted poor outcomes and relapses while inhA promoter region mutations predicted relapses of H resistance TB.24 The other possible reason is that patients with resistance to R and susceptible to H in the present study were mostly found in newly treated patients who had better treatment outcomes than in retreated MDR/RR-TB patients. The important results indicated that H resistance level might be the underlying predicting factor for influencing the outcome of patients with MDR/RR-TB.
For other drugs in the present study, such as Cs, Eto and second-line injectable agents, the resistance levels presented by the MIC value did not have a close association with the outcome of patients, however, their MIC values were lower in newly treated patients than in retreated patients, and similarly lower in retreated patients having a one-time of treatment history than those having more than a one-time treatment. These results indicated that the resistance level presented by the MIC value had no obvious association with the treatment outcome for patients with MDR/RR-TB according to the general dose of Cs taken daily. Because one study25 pointed out that the dose of Cs, 250 to 500 mg once or twice daily, taken in the present study is not needed for the optimal PK/PD targets, that is possibly the reason why Cs MIC value was not found to be associated with outcomes in this study. Another study also pointed out that lower serum CS concentrations and delayed absorption were common in the Asian population.26 In consideration of adverse effects, especially psychological effects, a population pharmacokinetic model for Cs should be developed to decide the optimal doses of Cs to DST in vitro to guide the regimen making for clinics.27 For Eto and second-line injectable agents, we also did not found the correlation of their MIC values with the treatment outcome, which might be due to low dose or other unaccountable reasons.
The results in the present study also found strong correlations between two FQs agents, and two injectable agents, which were consistent with the high cross-resistance of different FQs and second-line injectable agents previously reported.28,29 The correlation analysis of MIC value with low degree correlation also reflected the cross-resistance of H and Eto.30
There were several limitations in the present study, firstly, this is a retrospective study, and the data bias by enrolled cases is likely to influence the analysis results. Secondly, the study did not record the MIC DST change if patients had culture reversion. Thirdly, the MIC DST for other core drugs such as linezolid, and clofazimine were not done due to the limited condition of the lab available.
Conclusion
MIC values of important drugs were significantly lower in newly treated MDR/RR-TB and retreated patients having a single history of treatment. FQs and H resistance levels can influence the treatment outcome while other drugs such as Cs and Eto did not.
Abbreviations
MTB, mycobacterium tuberculosis; MIC, minimal inhibitory concentration; H, isoniazid; R, rifampicin; Ak, amikacin; FQs, fluoroquinolones; Sm, streptomycin; Mfx, moxifloxacin; Ofx, ofloxacin; PAS, p-aminosalicylic acid; Cs, cycloserine; Pto, protionamide; Eto, ethionamide; PZA, pyrazinamide; LZD, linezolid; BDQ, bedaquiline; DST, drug sensitivity test; Cfz, clofazimine; E, ethambutol; CDC, Center for Disease Control and Prevention; MDR-TB, multidrug-resistant tuberculosis; RR-TB, resistant rifampicin tuberculosis; XDR-TB, extensively drug-resistant tuberculosis; ATT, anti-TB treatment.
Data Sharing Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the ethics committee of Shanghai Pulmonary Hospital, Tongji University School of Medicine (No. K20-430). Privacy protection was guaranteed for each included participant.
Acknowledgments
We thank all participants for their time and efforts.
Author Contributions
L.F. designed the research; L.F and Q.T. wrote the manuscript; Q.T. and H.K. included the patients and collected the clinical data; W.S. and S.Z. did the statistical work, All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Shanghai Science and technology committee fund (21Y11901000, 20ZR1446700), the National Natural Science Foundation of China (82170006), Clinical Research Foundation of Shanghai Pulmonary Hospital (SKPY2021003). The authors declare all the sources of funding including financial support in their manuscript. We confirmed the information in the above funding statement was accurate and by the funder’s requirement.
Disclosure
The authors have stated that they have no conflicts of interest.
References
1. World Health Organization. Global Tuberculosis Report. Geneva: World Health Organization; 2021 Licence: CC BY-NC-SA 30 IGO; 2021.
2. Borisov SE, Dheda K, Enwerem M. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: a multicentre study. Eur Respir J. 2017;49(5):1700387. doi:10.1183/13993003.00387-2017
3. Gao M, Gao J, Xie L, Wu G, Li L. Early outcome and safety of bedaquiline-containing regimens for treatment of MDR- and XDR-TB in China: a multicentre study. Clin Microbiol Infect. 2020;27(4):597–602. doi:10.1016/j.cmi.2020.06.004
4. Nimmo C, Naidoo K, O’Donnell M. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020;382(24):2376–2377.
5. Zhang Q, Liu Y, Tang S, Sha W, Xiao H. Clinical benefit of delamanid (OPC-67683) in the treatment of multidrug-resistant tuberculosis patients in China. Cell Biochem Biophys. 2013;67(3):957–963. doi:10.1007/s12013-013-9589-5
6. Javaid AAU, Ullah I, Masud H, et al. Predictors of poor treatment outcomes in multidrug-resistant tuberculosis patients: a retrospective cohort study. Clin Microbiol Infect. 2018;24(6):612–617. doi:10.1016/j.cmi.2017.09.012
7. Nagel S, Streicher EM, Klopper M, Warren RM, Van Helden PD. Isoniazid resistance and dosage as treatment for patients with tuberculosis. Curr Drug Metab. 2017;18(11):1030–1039. doi:10.2174/1389200218666171031121905
8. Jurgen P, Angeby K, Sturegard E, et al. Wild-type MIC distributions for aminoglycoside and cyclic polypeptide antibiotics used for treatment of Mycobacterium tuberculosis infections. J Clin Microbiol. 2010;48(5):1853. doi:10.1128/JCM.00240-10
9. Rampersad T, Makume M, Sobia P, Sturm AW. A high throughput methodology for susceptibility testing of Mycobacterium tuberculosis isolates. J Microbiol Methods. 2018;146:64. doi:10.1016/j.mimet.2018.02.001
10. Kuhlin J, Forsman LD, Manoj M, Nordvall MJ, Bruchfeld J. Genotypic resistance of pyrazinamide but not MIC is associated with longer time to sputum culture conversion in patients with multidrug-resistant tuberculosis. Clin Infect Dis. 2020;73(9):e3511–e3517.
11. Falzon D, Jaramillo E, Schünemann HJ, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J. 2011;38(3):516–528. doi:10.1183/09031936.00073611
12. Lee J, Armstrong DT, Ssengooba W, et al. Sensititre MYCOTB MIC plate for testing Mycobacterium tuberculosis susceptibility to first- and second-line drugs. Antimicrob Agents Chemother. 2014;58(1):11–18. doi:10.1128/AAC.01209-13
13. Wu X, Yang J, Tan G, Liu H, Yu F. Drug resistance characteristics of Mycobacterium tuberculosis isolates from patients with tuberculosis to 12 antituberculous drugs in China. Front Cell Infect Microbiol. 2019;9:345. doi:10.3389/fcimb.2019.00345
14. World Health Organization. WHO Operational Handbook on Tuberculosis. Module 4: Treatment - Drug-Resistant Tuberculosis Treatment. Geneva: World Health Organization; 2020. License: CC BY-NC-SA 3.0 IGO; 2020.
15. Chinese anti-Tuberculosis Association. Chemotherapy guidelines on drug-resistant Tuberculosis (2015). Chin Anti-TB J. 2019;37(5):421–469.
16. World Health Organization. Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis-2011 Update. Geneva, Switzerland: World Health Organization; 2011.
17. Lange C, van Leth F, Mitnick C, Dheda K, Günther G. Time to revise WHO-recommended definitions of MDR-TB treatment outcomes. Lancet Respir Med. 2018;6(4):246–248. doi:10.1016/S2213-2600(18)30104-8
18. Duan H, Chen X, Li Z, et al. Clofazimine improves clinical outcomes in multidrug-resistant tuberculosis: a randomized controlled trial. Clin Microbiol Infect. 2019;25(2):190–195. doi:10.1016/j.cmi.2018.07.012
19. Sun W, Wu Z, Ying Z, Fan X, Fan L. A highly effective and inexpensive standardized treatment of multidrug-resistant tuberculosis: a multicenter prospective study in China. BMC Infect Dis. 2021;21(1). doi:10.1186/s12879-021-06553-2
20. Ns A, Pks A, Us A, Aj A, Rg B. Fluoroquinolone drug resistance among MDR-TB patients increases the risk of unfavorable interim microbiological treatment outcome: an observational study. J Glob Antimicrob Resist. 2020;24:40–44.
21. Kurbatova EV, Gammino VM, Bayona J, et al. Predictors of sputum culture conversion among patients treated for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2012;16(10):1335–1343. doi:10.5588/ijtld.11.0811
22. Byeong-Ho J, Kyeongman J, Hye Y, et al. Outcomes of pulmonary MDR-TB: impacts of fluoroquinolone resistance and linezolid treatment. J Antimicrob Chemother. 2015;70(11):3127–3133. doi:10.1093/jac/dkv215
23. Ska B, Kj C, Ar A. Variations in rifampicin and isoniazid resistance-associated genetic mutations among drug naive and recurrence cases of pulmonary tuberculosis - ScienceDirect. Int J Infect Dis. 2020;103:56–61.
24. Huyen MN, Cobelens FG, Buu TN, et al. Epidemiology of isoniazid resistance mutations and their effect on tuberculosis treatment outcomes. Antimicrob Agents Chemother. 2013;57(8):3620–3627.
25. Alghamdi WA, Alsultan A, Al-Shaer MH, et al. Cycloserine population pharmacokinetics and pharmacodynamics in patients with tuberculosis. Antimicrob Agents Chemother. 2019;63(5). doi:10.1128/AAC.00055-19
26. Hung W, Yu M, Chiang Y, et al. Serum concentrations of cycloserine and outcome of multidrug-resistant tuberculosis in Northern Taiwan. Int J Tuberc Lung Dis. 2014;18(5):601–606. doi:10.5588/ijtld.13.0268
27. van der Galiën R, Boveneind-Vrubleuskaya N, Peloquin C, Skrahina A, Touw D, Alffenaar J. Pharmacokinetic modeling, simulation, and development of a limited sampling strategy of cycloserine in patients with multidrug-/extensively drug-resistant tuberculosis. Clin Pharmacokinet. 2020;59(7):899–910. doi:10.1007/s40262-020-00860-8
28. Mamatha HG, Shanthi V. Baseline resistance and Cross-resistance among fluoroquinolones in multi drug-resistant Mycobacterium tuberculosis isolates at a national reference laboratory. J Glob Antimicrob Resist. 2017;12:5–10.
29. Jugheli L, Bzekalava N, de Rijk P, Fissette K, Portaels F, Rigouts L. High level of cross-resistance between kanamycin, amikacin, and capreomycin among mycobacterium tuberculosis isolates from Georgia and a close relation with mutations in the rrs gene. Antimicrob Agents Chemother. 2009;53(12):5064–5068. doi:10.1128/AAC.00851-09
30. Malinga L, Brand J, Jansen V, Cassell G, Van D. Investigation of isoniazid and ethionamide cross-resistance by whole genome sequencing and association with poor treatment outcomes of multidrug-resistant tuberculosis patients in South Africa. Int J Mycobacteriol. 2016;5(Supplement 1):S36–S37. doi:10.1016/j.ijmyco.2016.11.020
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.