Back to Journals » Journal of Multidisciplinary Healthcare » Volume 14
The Contrasting Perceptions and the Cause Regarding Patenting Technologies Between Academic Medical Researchers and Pharmaceutical Companies Based in Japan
Authors Sugimitsu K , Manome Y
Received 25 May 2021
Accepted for publication 2 July 2021
Published 12 July 2021 Volume 2021:14 Pages 1795—1805
DOI https://doi.org/10.2147/JMDH.S321834
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Kazunari Sugimitsu,1,2 Yoshinobu Manome2
1Graduate School of Innovation Management, Kanazawa Institute of Technology, Tokyo, Japan; 2Core Research Facilities, Jikei University School of Medicine, Tokyo, Japan
Correspondence: Kazunari Sugimitsu
Graduate School of Innovation Management, Kanazawa Institute of Technology, 1-3-4, Atago, Minato-ku, Tokyo, 105-0002, Japan
Tel +81 357770807
Fax +81 357772226
Email [email protected]
Background: The recent trend of pharmaceutical companies commercializing new objects as new drugs based on the findings of academic medical researchers, commonly categorizing them as “academic drug discovery” is increasingly gaining popularity in the pharmaceutical industry. Studies state that academic researchers based in universities have lower motivation to apply for patents. However, none of the studies evaluated the existence and extent of the “motivation for patent” in academic researchers, being lower than that of pharmaceutical companies. This study assesses two hypotheses; H1: academic medical researchers are less likely to believe that the patent system is necessary for pharmaceuticals, and thus have diminished interest in the commercialization of their research findings when compared to those in the pharmaceutical industry, H2: apprehension of the raison d’être of the patent system affects positive impressions on patents among academic medical researchers.
Methods: From February to March 2020, an anonymous survey was conducted among academic medical researchers, pharmaceutical industry professionals, and IP researchers based in Japan. Overall response rate was 27.4% (192/700). We conducted an analysis of variance for H1 and used the PLS-SEM model for H2 in order to verify the hypotheses.
Results: The results confirmed that the mean calculated from the responses of the academic medical researchers was significantly lower than the mean of pharmaceutical company personnel when responses to patenting an emerging technology or drug for the advancement of medicine were analyzed. In addition, we found that a causal relationship between academic medical researchers’ understanding of patents and their positive impressions on patents, depending on the degree to which they consider that the patent system is to encourage and promote new inventions.
Conclusion: We conclude that a contrasting perception of patents not only exists between academic medical researchers and pharmaceutical company personnel but also it is caused by their apprehension of patents. More efforts to promote the raison d’être of the patent system among academic medical researchers will enable them to view pharmaceutical patents in a more positive light. Through this study, the pertinence to promote academic drug discoveries has been uncovered.
Keywords: drug patent, intellectual property, industry university cooperation, drug development, academic drug discovery
Introduction
Academic medical researchers as well as those associated with pharmaceutical corporations play a significant role in launching new drugs for clinical applications.1 The industrialization of discoveries made by academic researchers is more common in pharmaceuticals than in other industries, and is commonly designated as “academic drug discovery”.2,3
In the United States of America, entrepreneurial ventures as well as universities play a major role in drug discovery. In stark comparison, in Japan, initiating a business start-up has been extremely rare.4 Therefore, it is expected that universities would play a more significant role in drug discovery than venture companies; however, this is not the case in reality whereby drug discovery by academic researchers is rather rare in Japan.5
Studies have shown that publication of research results is more important and prioritized than the acquisition of patents in academia, whereas in pharmaceutical companies, the acquisition of patents is given more importance than dissemination of the knowledge in the form of publication of research results.6–8
However, according to Patent Law in Japan as well as in other countries, inventions cannot be patented in principle if they are presented at an academic conference or published by submitting a paper before a patent application is filed. In this case, it will be difficult for pharmaceutical companies to invest in the discovery of the drug, which might lead to a delay in the supply of the drug. Hence, the motivation of medical researchers to patent is the key to realizing the production of new drugs through academic drug discovery.
Two schools of thoughts exist in the domain of pharmaceutical patents. According to Gaynes,9 in terms of Howard Florey, who shared the Nobel Prize in Physiology and Medicine in 1945 with Alexander Fleming, who discovered the world first antibiotic called penicillin, “Florey and others viewed patents as unethical for such a life-saving drug.” This is a typical negative view of pharmaceutical patents.
On the other hand, Kennedy et al stated that “Had Fleming protected (patented) penicillin, companies perhaps would have had incentive earlier to invest in developing and manufacturing the therapy and humankind could have had the treatment much earlier”.10 This would be a positive view of patents. Generally, the patent system is often thought of as a system for inventors to “monopolize” and “make money”.11,12
However, the value of the patent system, at least in the world of intellectual property (hereinafter simply referred to as “IP”) researchers, is to grant a monopoly to the inventors for a certain period of time, subsequently making it available to the wider public (eg, generic drugs) in order to ensure a return on the investment made to create a new invention. Numerous studies have shown that the academic community on IP considers that the raison d’être of the patent system provides incentive to promote new discoveries.13–19
In applying this “incentive theory” to pharmaceuticals it may be deemed that the patent system exists to encourage and promote the creation of new drugs.20,21 If this is the case, the system would be imperative for patients with incurable diseases waiting for a breakthrough or a long-awaited treatment modality following the discovery of a novel drug and therefore, the patent system may facilitate more positive outcomes.22
One critical question that arises here is the perception of patent rights and the patent system of academic medical researchers, especially compared with one of pharmaceutical industry-based personnel. To the best of the authors’ knowledge, there have been no previous attempts to study this issue, whereby the differences in the perspectives of these two study groups were documented systematically, let alone the cause of them.
For our study, the following two hypotheses were formulated, and a questionnaire-based survey was conducted among three study groups comprising of academic medical researchers, pharmaceutical industry personnel, and IP researchers, respectively who were based in Japan. The results were statistically analyzed to assess and evaluate any difference in the perception of patents and/or the patent system (hereafter simply referred to as “patents”) among the different study groups.
H1: Academic medical researchers are less likely to believe that the patent system is necessary for pharmaceuticals, and thus have diminished interest in the commercialization of their research findings when compared to those in the pharmaceutical industry.
H2: Apprehension of the raison d’être of the patent system affects to positive impressions on patents among academic medical researchers.
This study also identifies and enumerates the perceptions of patents harbored by academic researchers’ and underlying factors contributing to the perception. The results of this study may help in stimulating academic drug discovery.
Materials and Methods
Respondents and Methods
From February, 2020 to March, 2020, an anonymous survey was conducted in Japan among academic medical researchers, pharmaceutical industry professionals, and IP researchers, respectively.
Pre-existing literature in this domain was lacking and therefore we aimed for a sample size of 53 respondents in each group, which would achieve an α of 0.05 and β of 0.20 (or 80% power), with the expectation of a “medium” effect size as a result of the test and the purpose of the analysis of variance of the three groups.23
In context of the objective of this study, “academic medical researchers” is defined as those who belong to a medical department and are conducting research, but do not include hospital-based physicians or general practitioners. “Pharmaceutical company personnel” is defined as individuals associated with a pharmaceutical company which engages in research and development of drugs, and “IP researchers” is defined as researchers associated with a university with a specialization in IP.
For approaching the respondents, requests for the questionnaire were distributed to the members of the organization by e-mail via professors or persons in charge within the organization who had given their prior consent. Specifically, a clickable form with a link in the questionnaire request letter was included in the body of the e-mail, and once logged in, the questions were displayed on the web, and the individual were enabled to enter their responses on the form online. On the first page when they logged in, there was a description of this survey’s purposes and they were asked to click an online informed consent. If they did not, it was designed that they were not able to proceed to the next page with the questions.
One of the underlying reasons for adopting this method is that it is more efficient than paper-based questionnaire in terms of its distribution, collection, and data processing. In addition, the person in charge of the organization did not have access to view the responses of the respondents from the same organization, since the responses were collected directly from the respondents via the internet, eliminating potential result biases.
To view the response to the questionnaire authorization using a login and password was required. This prevented any unrelated person to accidentally or deliberately access the web page of the questionnaire to alter the response. In addition, prepared three tier password system was created and different passwords were used for academic medical researchers, pharmaceutical company personnel, and IP researchers, respectively, to maintain the integrity of the data collected.
For academic medical researchers, three medical schools were requested to fill in the survey form. The first medical school is Jikei University School of Medicine located in Tokyo, Japan. Through the manager of the Core Research Facilities in the university, we requested institutional researchers (n = 163) on the mailing list of users of the Core Research Facilities to respond to the questionnaire. The mailing list can be considered to be equivalent to random distribution because it includes all academic medical researchers on campus.
In order to avoid bias due to the unique circumstances of the university, we also sought the cooperation of two other medical schools. The first university is Sapporo Medical University in Hokkaido, located in eastern Japan. With the help of a professor at Sapporo Medical University, we used a mailing list for researchers at the university (n = 334) to request responses. We also requested another professor in the Department of Drug Discovery Medicine at Graduate School of Medicine in Kyoto University, located in the western part of Japan to respond to our request through the department’s mailing list (n = 32).
A total of 529 members from the three medical schools were asked to fill out the questionnaire. All of them used the university’s mailing list, so it is highly unlikely that responses from those outside the group who are unrelated or have different attributes could influence the responses. A total of 77 responses were received. The response rate was 14.6%.
Secondly, in light of the objectives of this study, we sought the cooperation of pharmaceutical companies involved in the development of the novel drugs. We requested Japan Pharmaceutical Manufacturers Association (JPMA) for assistance with the survey, which has a total of 72 research-based pharmaceutical companies enlisted as their members (as of August 30, 2020). Since, almost all pharmaceutical corporations in Japan with an active research and development division are enlisted with JPMA the results of this survey may be considered as unbiased. With the consent of the manager of the association, we requested the member companies to respond to our survey for random data collection. According to the head of JPMA, managers of 33 companies were registered on their mailing list. We requested each company manager to forward this survey to two more randomly selected colleagues in the company, bringing the total number of respondents to 99.
As with the academic medical researchers, it is highly unlikely that responses from people unrelated to the pharmaceutical industry or with different attributes could influence each other’s responses. A total of 59 responses were obtained from pharmaceutical industry personnel resulting in a response rate of 59.6%.
For the IP researchers, we requested the secretariat of Intellectual Property Association of Japan (the academic society for intellectual property in Japan) to distribute the e-mail to the members of the association belonging to one of the universities. The e-mail was distributed to 25 people. The e-mail was also distributed to all 29 faculty members without any bias, who specialize in IP at the university (Kanazawa Institute of Technology, Japan) to which one of the authors of this study is affiliated. In addition, the authors requested a total of 18 professors affiliated to the Meiji University (n = 7), Tokyo University of Science (n = 4), and Osaka Institute of Technology (n = 7), to distribute the questionnaire to their colleagues who specialize in IP.
Since, the specialized field of IP is unique and only limited number of universities in Japan are engaged in research of IP, it would be safe to assume that there is almost no bias. The possibility that the answers of unrelated or individuals of different attributes could influence the responses is very low, as is the case for academic medical researchers and pharmaceutical company personnel. We obtained responses from 56 IP researchers, and the response rate was 77.8%.
The overall recovery rate for the three study groups was 27.4% (192/700). All data obtained were handled such that individual data could not be identified, and efforts were made to protect the privacy of the data.
Survey Details
In each of the three study groups, data regarding the designation and educational background were collected as demographic characteristics. For academic medical researchers, data regarding the clinical experience and research field (basic medicine and/or clinical medicine) were also obtained additionally.
We selected six questions to verify the hypotheses with regard to the necessity (H1), the apprehension of, and positive impressions on patents (H2). For the study group comprising IP researchers no additional questions other than Q1 and Q6 were asked. This was maintained since they do not actively file patent applications, but rather, the patents are the subject of their research.
This study focused on answers of the following six questions:
Q1: Which and to what extent is your idea closer to A or B on the scale below?
A: Patent system is necessary for pharmaceuticals.
B: Patent system is NOT necessary for pharmaceuticals.
Q2: Which and to what extent is your idea closer to A or B on the scale below?
A: Filing patent application for new medicines should be more encouraged.
B: Patent application for new medicines should NOT be filed.
Q3: Which and to what extent is your idea closer to A or B on the scale below?
A: Patents should be obtained as long as they can be.
B: Patents should NOT be obtained even if they can be.
Q4: Have you ever learned about patents in detail?
1: NO
2: YES, I have read books about patents.
3: YES, I have attended a lecture about patents.
4: OTHER ()
Q5: Have you ever filed for the patent? Please fill in the number of applications. ()
Q6: What do you think about this perspective: Patent system exists as an incentive measure and a promoter for new inventions?
1. I do NOT agree at all.
2. I do not agree.
3. I agree.
4. I totally agree.
Q1, Q2, and Q3 consisted of Likert items assessing a statement on a 6-point Likert scale.
For question items containing two extreme options, A or B, a 6-point scale was adopted on intent, rather than the commonly used 5-point scale. For example, typically, as seen in Q1, this involves extreme options A: “Patent system is necessary for pharmaceuticals” and B: “Patent system is NOT necessary for pharmaceuticals.”
As shown in Figure 1, questions which included the two extremes were, from left to right, close to A, somewhat close to A, more or less close to A, more or less close to B, somewhat close to B, and close to B.
 |
Figure 1 6-point Likert scale used for Q1, Q2, and Q3, ranging from option A to option B. |
The underlying reason for adoption of a 6-point Likert scale was our agreement to a previous study, whereby: (The) obvious problem that arises if a middle response alternative is provided is that it is possible for respondents who are fatigued, or poorly motivated to complete the survey to select the middle alternative when they could, if pushed, give a directional response”.24
Next, in Q4, which gathered information about learning experience, the responses were divided into two groups, one with learning experience and one without learning experience, with a dummy variable. Specifically, option 1 was set to “0” and options 2 and 3 were set to “1.” In addition, option 4 was classified as 0 or 1, depending on the content of the individual entries. Q5 was treated as a quantitative variable, since it collected data regarding the number of patent applications filed. Furthermore, the question (Q6) which proposed a certain perspective and asked for approval or disapproval, was treated as a four-scale question on a Likert scale, since it was not possible for the respondent to assume a polarized opinion.
The question related to Hypothesis H1 was Q1. One of the items closely related to constructive concept “Apprehension of Patents” (Q6) corresponding to the cause in H2, was “What do you think about this perspective; Patent system exists as an incentive measure and a promoter for new inventions?” Two questions, “Learning Experience” (Q4) and “Filing Experience” (Q5), were added to this item.
Next, the following three questions related to the “positive impression on patents,” were finalized which corresponded to the results in hypothesis H2.
The first question Q1 (deals with variable of “Necessity”), the second question Q2 is: “Filing patent application for new medicines should be more encouraged or NOT” (deals with variable of “Evaluation”), and the third Q3 is: “Patents should be obtained as long as they can be or NOT” (as a variable name is ‘Future’).
Analytical Method
For Hypothesis H1, the two extremes of ideas were placed at both ends of a straight line as A and B (see Figure 1), with four more scales listed between them, and converted to scores (eg, 1 point for the closest to B and 6 point for the closest to A) by considering them as a 6-point Likert scale.
An analysis of variance was then conducted for comparing the responses of the three study groups.
For Hypothesis H2, we used the PLS-SEM model which allowed causal analysis, to confirm the causal relationship between the understanding of patents and the constructive concept “Positive Impressions on patents” for academic medical researchers.25
We used the traditional chi-square, the chi-square/degrees of freedom ratio (chi-square/gl), the Root Mean Square Residual (RMR), the Goodness-of-Fit Index (GFI), the Adjusted Goodness-of-Fit-Index (AGFI), the Comparative Fit Index (CFI), and the Root Mean Square Error of Approximation (RMSEA) as indices of SEM.
In addition, since the latent variable “Positive Impressions on patents” had been a constructive concept, a confirmatory factor analysis was performed to examine whether this concept converged to a single factor and verified its validity. Factors were confirmed using the unweighted least squares method using the Promax rotation method. Interpretation of the factors was based on factor loadings of 0.35 or more. Next, the reliability coefficient Cronbach’s alpha was calculated and the reliability of the scale was examined. The acceptable range of Cronbach’s alpha was set at 0.7 or more.
The significance level was set at 5% for all tests, and HAD and IBM SPSS Amos 26.0.0 (Japanese) statistical software was used in this study.26
Results
Data Cleaning
We did data cleaning by excluding invalid data. As a result, we excluded two data points from academic medical researchers’ group, six from group of pharmaceutical company personnel, and three from IP researchers. Therefore, responses from 75 academic medical researchers, 53 pharmaceutical company personnel, and 53 IP researchers were included in the final data set.
Demographic Characteristics
Characteristics of respondents are given in Table 1. Among all 75 above-mentioned respondents of academic medical researchers, 55 (73.3%) graduated from medical schools and 20 from school of medicine (26.7%). In terms of their present designation in the medical school, 20 held the post of professor or associate professor (26.7%), 35 were either assistant professors or instructors (46.6%), and 20 were doctoral students or others (26.7%). Out of the 75 above-mentioned respondents of academic medical researchers, 24 were providing medical care actively carrying out research (32.0%), 7 had clinical experience in the past (9.3%), and 44 dedicated themselves exclusively to research (58.6%). In addition, 29 conducted research only in basic medicine, 20 only in clinical medicine, and 25 in both basic and clinical medicine.
 |
Table 1 Demographic Characteristics |
On the other hand, among all 53 respondents of pharmaceutical company personnel, 23 graduated with a degree in medicine (43.4%), 10 in chemistry (18.9%), 5 in engineering (9.4%), 4 in agriculture (7.5%), 3 in biology (5.7%), 1 graduated from medical school (1.9%), and 7 in other disciplinary fields (13.2%). Additionally, two participants held executive position in pharmaceutical companies (3.8%), 15 worked as division chief (28.3%), 29 worked as section chief (54.7%), and 7 worked in miscellaneous positions (13.2%).
About the group of IP researchers, 17 out of all 53, were law graduates (32.1%), 14 graduated with a degree in engineering (26.4%), 9 graduated in science (17.0%), 4 graduated in economics (7.5%), 3 graduated in agriculture (5.7%), and 6 were awarded degrees in interdisciplinary fields (11.3%). In terms of academic appointments, 36 held a professorship or an associate professorship (67.9%), 7 of them were associate professors or instructors (13.2%), and other 10 were researchers without lectureship duties (18.9%).
Hypotheses: H1
The mean score of the response to the question about “Whether the patent system is a necessary system in medicine” for academic medical researchers was 4.893. Here a score of 1 point deemed the response to be unnecessary and 6 point represented most necessary. On the other hand, as shown in Figure 2, the mean score of the same question for the pharmaceutical company personnel was 5.736, and the mean score of the same question for the IP researchers was 5.698.
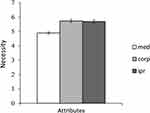 |
Figure 2 Bar graph of mean scores. Abbreviations: med, academic medical researchers; corp, pharmaceutical company personnel; ipr, IP researchers. |
An analysis of variance for the data of the responses of three different occupational groups- academic medical researchers, pharmaceutical company personnel and IP researchers - revealed that the main attribute significantly influencing the responses was that of the occupation of the respondent [F (2, 178) = 21.66, p = 0.000], as shown in Table 2.
 |
Table 2 Main Effect of Attributes |
The Holm–Bonferroni method was applied and the results of multiple comparisons showed that the above-mentioned mean score of the response to the question about “Whether the patent system is a necessary system in medicine” for academic medical researchers (M = 4.893) was significantly lower than that of pharmaceutical company personnel and IP researchers (pharmaceutical company personnel; M = 5.736; IP researchers; M = 5.698) as shown in Table 3 (pharmaceutical company personnel; t(178) = −5.657, Padj = 0.000;IP researchers: t(178) = −5.403, Padj = 0.000). There was no significant difference between pharmaceutical company personnel and IP researchers [t(178) = 0.234, n.s.].
 |
Table 3 Multiple Comparisons Adjusted with Holm’s Method |
Hypotheses: H2
We conducted a Covariance Structural Analysis of the relationship between the constructive concept “understanding of patents” and “Positive Impressions on patents” for a group of academic medical researchers, using a PLS model that allows us to confirm the cause-effect relationship.
As shown in Figure 3, the result was 0.65 for the standardized coefficient, confirming the existence of a causal relationship between the two concepts. In particular, the value of the path coefficient from “Apprehension (of patents)” was 0.92, confirming a strong influence.
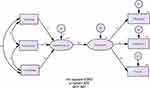 |
Figure 3 The hypothesized conceptual model with standardized path coefficients for H2. |
The model was considered adequate according to the following parameters: x2 = 2.764; x2/df = 0.461; RMR = 0.179; GFI = 0.988; AGFI = 0.959; CFI = 1.00; RMSEA = 0.00.
In addition, for confirmation, the factor analysis was also performed for three questions related to the constructive concept “Positive Impressions on patents” (Q1, Q2, and Q3). The results confirm that the factors converge as a single factor using a scree plot, as shown in Figure 4. Also, as shown in Table 4, the suitability of the conceptual model was confirmed. At the same time, we calculated the reliability coefficient Cronbach’s alpha, which was 0.837, and its acceptability was confirmed; since, its value exceeded the value of 0.7.
 |
Table 4 Factor Analysis |
 |
Figure 4 Scree plot. |
Discussion
As “academic drug discovery” becomes more important, both academic medical researchers and pharmaceutical corporations play a significant role in launching new drugs. However, existing literature6–8 shows that there might be a gap in “motivation to apply for patents” between academic researchers and pharmaceutical companies, and none of the studies assessed the existence and extent of this gap.
The aim of this study was to confirm the existence of difference in perception in the context of patenting technology between academic researchers and pharmaceutical companies based in Japan, as well as if any to find the cause of the difference.
We confirmed that the mean of academic medical researchers was significantly lower than the mean of pharmaceutical company personnel when asked whether they thought patent was necessary in medicine, while there was no difference between pharmaceutical company personnel and IP researchers.
The results support the hypothesis H1. A contrast in perception of the patent system exists when comparing between academic medical researchers and pharmaceutical company personnel, which needs to be resolved since academic researchers can potentially play a significant role in drug development which requires large investment.
More importantly, a causal relationship between academic medical researchers’ understanding of patents and their positive impressions on patents was confirmed, depending on the degree to which they consider that the patent system is to encourage and promote new inventions, which also simultaneously supports the hypothesis H2. This suggests that academic medical researchers are more likely to have positive impressions of the patent system if they are provided the opportunity and resources to gain knowledge of the spirit or legislative intent of patent law. However, in reality there are limited opportunities, lectures or resources on patents being shared by medical schools to disseminate information among academic researchers. Therefore, it is unrealistic to expect a comprehensive knowledge among them about the raison d’être of the patent system. Presumably from this perspective, Japan Agency for Medical Research and Development is now providing free educational materials for academic medical researchers to learn about the raison d’être of the patent system. The materials state that academic drug discovery is important in Japan for the mass production and supply of new drugs, and that in order to achieve this, academic medical researchers need to value patents as well as papers, and that if pharmaceutical companies hesitate to invest as a result of researchers prioritizing papers, it will ultimately be difficult to actually save patients.27
As mentioned above, collaboration between academic medical researchers and pharmaceutical companies is important for drug discovery, and since limited understanding of the patent system might impede drug discovery it would be pertinent to bridge this knowledge gap.
Limitations
One of the limitations of our study include small sample size. The sample studied just met the minimum sample size requirements which achieved an α of 0.05 and β of 0.20 (or 80% power). Moreover, the possibility of the existence of bias due to the small sample size could not be completely ruled out. The sample of the academic medical researchers was low, as only three specific schools responded to the survey. Secondly, this survey was conducted only in Japan, which restricts generalizations of our results to other nations. Thus, another limitation is that the result may depend on the selection of the medical schools/universities/academic research institutes/pharma companies chosen for the study and their area of research. Thirdly, the period of the survey, approximately 2 months, is rather small.
Furthermore, the limitations on the number of items that could be on the questionnaire to ensure that respondents were not overburdened lead us to omit questions on sociodemographic characteristics, such as gender and age that were hypothetically assumed to be irrelevant.
For much the same reason, we did not include questions about the technology/therapeutic area in which the medical school is researching or the pharmaceutical company considered for the study is working. Likewise, there were no questions on what stage of research these respondents are at and it has any bearing on academic drug discovery.
However, it is possible that these variables may have an effect on the responses registered by the participants of the study.
Future attempts to discern patent-based perceptions among study groups can increase the robustness of the study by including data from various medical schools, universities, pharmaceutical companies and academic research institutes all around the globe, by incorporating respondents’ other sociodemographic data like sex, age etc., research stage and involvement with drug discovery, or by expanding the survey period.
Despite of all the various limitations mentioned above, to the best of our knowledge our research survey is novel.
Conclusion
This study confirms that a stark difference in perception of patents exists between academic medical researchers and pharmaceutical industry-based personnel. To be more specific, a majority of the academic medical researchers tend to have a more negative view of pharmaceutical patents than pharmaceutical industry professionals.
Moreover, we first showed a causal relationship between academic medical researchers’ understanding of patents and their positive impressions of patents, depending on the extent to which they understand that the patent system is intended to encourage and promote invention.
Although, a majority of the academic medical researchers tend to view the patent system negatively, transformation of their perspective would occur if they are convinced that the raison d’être of the patent system provides incentive to promote new discoveries and thus it is essential to save patients who need the new drugs’ supply by collaborating with pharmaceutical companies.
Data Sharing Statement
No additional data are available.
Ethics Approval
This study does not involve human subjects and thus complies with the Declaration of Helsinki. Nevertheless, in consideration of the protection of personal information, the study was approved by the ethical review board at Jikei University School of Medicine, Japan, Ref. NO.31-318.
Acknowledgments
We would like to express our appreciation to the following persons and/or organizations for their cooperation in the completion of the survey used in this study: Prof. Masaho Ishino, Prof. Chikako Saotome, Prof. Shigeo Takakura, Prof. Jun Sugiura, Prof. Setsuko Asami, Japan Pharmaceutical Manufacturers Association and Intellectual Property Association of Japan. All possible errors in this paper are attributable to the authors.
Disclosure
The authors have no funding or conflicts of interest to disclose. The authors of this paper have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
References
1. FitzGerald GA. Drugs, industry, and academia. Science. 2008;320(5883):1563. doi:10.1126/science.1161006
2. Mansfield E. Academic research and industrial innovation. Res Policy. 1991;20(1):1–12. doi:10.1016/0048-7333(91)90080-A
3. Takebe T, Imai R, Ono S. The current status of drug discovery and development as originated in United States academia: the influence of industrial and academic collaboration on drug discovery and development. Clin Transl Sci. 2018;11(6):597–606. doi:10.1111/cts.12577
4. Kneller R. The importance of new companies for drug discovery: origins of a decade of new drugs. Nat Rev Drug Discov. 2010;9:867–882. doi:10.1038/nrd3251
5. Okuyama R, Tsujimoto M. Background and current status of academic drug discovery–analysis from standing points of industry, universities and government. J Jpn Soc Intellect Prod. 2017. Japanese.
6. Shunsuke T, Nobuyoshi K, Hiroyuki A, et al. Requirements for the license-out of drug candidates to pharmaceutical companies. Nihon Yakurigaku Zasshi. 2014;143(4):198–202. Japanese. doi:10.1254/fpj.143.198
7. Tanaka H, Aono T. Research on the exclusivity of pharmaceutical-related patents filed by national university corporations. Soc Res Pol Innovat Manag. 2008;263. Japanese.
8. Ramy A, Mohamed AR, Amr A. Knowledge management in the pharmaceutical industry between academic research and industry regulations. Knowledge Manag Res Pract. 2020;10:1–17. doi:10.1080/14778238.2020.1767517
9. Gaynes R. The discovery of Penicillin—new insights after more than 75 years of clinical use. Emerg Infect Dis. 2017;23(5):849–853. doi:10.3201/eid2305.161556
10. Kennedy JP, Wayne HW. How to Invent and Protect Your Invention: A Guide to Patents for Scientists and Engineers. New Jersey: John Wiley & Sons; 2012. doi:10.1002/9781118410103.fmatter
11. Bostyn S, Nicolas P. Patent= monopoly: a legal fiction; 2013. Available from: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2373471.
12. Sugimitsu K. Intellectual property as a marketing tool. JIPAJ. 2017;13(3):4–14.
13. Eisenberg RS. Patents and the progress of science: exclusive rights and experimental use. Univ Chicago Law Rev. 1989;56(3):1017–1086. doi:10.2307/1599761
14. Turner JS. The nonmanufacturing patent owner: toward a theory of efficient infringement. Cal L Rev. 1998;86(1):179–210. doi:10.2307/3481149
15. Caves RE, Whinston MD, Hurwirtz AM. Patent expiration, entry, and competition in the US pharmaceutical industry. Brookings Pap Econ Act. 1991;22(1991):1–66.
16. Kitch EW. The nature and function of the patent system. J Law Econ. 1977;20(2):265–290. doi:10.1086/466903
17. Ordover JA. A patent system for both diffusion and exclusion. J Econ Perspect. 1991;5(1):43–60. doi:10.1257/jep.5.1.43
18. United States. Federal trade commission. 2003 to promote innovation: the proper balance of competition and patent law and policy. DIANE Publishing; 2021. Available from: https://www.ftc.gov/sites/default/files/documents/reports/promote-innovation-proper-balance-competition-and-patent-law-and-policy/innovationrpt.pdf.
19. Ponchek T. Does the patent system promote scientific innovation-empirical analysis of patent forward citations. Alb LJ Sci Tech. 2015;25(2):289–338.
20. Cockburn I, Long G. The importance of patents to innovation: updated cross-industry comparisons with biopharmaceuticals. Expert Opin Ther Pat. 2015;25(7):739–742. doi:10.1517/13543776.2015.1040762
21. Grabowski HG, DiMasi JA, Long G. The roles of patents and research and development incentives in biopharmaceutical innovation. Health Aff (Millwood). 2015;34(2):302–310. doi:10.1377/hlthaff.2014.1047
22. Merges RP. Justifying Intellectual Property. Cambridge Massachusetts London, England: Harvard University Press; 2011:284.
23. Cohen J. Statistical Power Analysis for the Behavioral Sciences.
24. Sturgis P, Caroline R, Patten S. Middle alternatives revisited: how the neither/nor response acts as a way of saying “I don’t know?”. Sociol Methods Res. 2014;43(1):15–38. doi:10.1177/0049124112452527
25. Lowry PB, Gaskin J. Partial least squares (PLS) structural equation modeling (SEM) for building and testing behavioral causal theory: when to choose it and how to use it. IEEE Trans Prof Commun. 2014;57(2):123–146. doi:10.1109/TPC.2014.2312452
26. Shimizu H. Free statistical analysis software HAD: an introduction to its functions and a proposal for its use in statistical learning, education, and research practice. J Media Inf Commun. 2016;1:59–73. Japanese.
27. Japan Agency for Medical Research and Development. Intellectual property materials for medical researchers; 2021. Available from: https://www.amed.go.jp/chitekizaisan/chizai_kyouzai.html.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
